Abstract
Cannabis is one of the oldest psychotropic drugs and its anticonvulsant properties have been known since the last century. The aim of this reveiw was to analyze the efficacy of cannabis in the treatment of epilepsy in adults and children. In addition, a description of the involvement of the endocannabinoid system in epilepsy is given in order to provide a biochemical background to the effects of endogenous cannabinoids in our body. General tolerability and adverse events associated with cannabis treatment are also investigated. Several anecdotal reports and clinical trials suggest that in the human population cannabis has anticonvulsant properties and could be effective in treating partial epilepsies and generalized tonic-clonic seizures, still known as “grand mal.” They are based, among other factors, on the observation that in individuals who smoke marijuana to treat epilepsy, cessation of cannabis use precipitates the re-emergence of convulsive seizures, whereas resuming consumption of this psychotropic drug controls epilepsy in a reproducible manner. In conclusion, there is some anecdotal evidence for the potential efficacy of cannabis in treating epilepsy. Though there has been an increased effort by patients with epilepsy, their caregivers, growers, and legislators to legalize various forms of cannabis, there is still concern about its efficacy, relative potency, availability of medication-grade preparations, dosing, and potential short- and long-term side effects, including those on prenatal and childhood development.


Similar content being viewed by others
References
Ben Amar M, L´eonard L. Chapter 16: Cannabis. In: L´eonard L, Ben Amar M, editors. Les Psychotropes: Pharmacologie et Toxicomanie. Montreal: Les Presses de l’Universit´e de Montr´eal; 2002. p. 571–627.
O’Shaugnessy WB. On the preparations of the Indian hemp, or gunjah (Cannabis indica): their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Transactions of Medical and Physical Society of Bengal, 1838–1840. p. 421–461.
Robson P. Therapeutic aspects of cannabis and CBss. BrJ Psychiatry. 2001;178:107–15.
Carter GT, Weijdt P, Kyashna-Tocha M, Abrams DI. Medicinal cannabis: rational guidelines for dosing. Drugs. 2004;75:464–70.
Fankhauser M. Chapter 4: history of cannabis in Western medicine. In: Grotenhermen F, Russo R, editors. Cannabis and CBss: pharmacology, toxicology and therapeutic potential. New York: The Haworth Integrative Healing Press; 2002. p. 37–51.
Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25.
Mirken B. Marijuana on the state. Lancet. 2004;364:842.
Hoey J. Marijuana: federal smoke clears a little. CMAJ. 2001;164:1397.
Health Canada. Medical Use of Marihuana. Stakeholder Statistics. Ottawa: Office of Cannabis Medical Access, Health Canada; 2005. p. 2.
Gorter RW, Butorac M, Pulido Cobian E, van der Sluis W. Medical use of cannabis in the Netherlands. Neurology. 2005;64:917–9.
Health Canada CIHR. Medical Marihuana Research Program. Ottawa: Health Canada and Canadian Institutes of Health Research; 1999. p. 7.
Health Canada [website]. Medical use of marijuana. Ottawa: Health Canada; 2015. Available from: http://www.hc-sc.gc.ca/dhp-mps/marihuana/index-eng.php. Accessed 10 July 2015.
Collège des médecins du Québec. Guidelines concerning the prescription of dried cannabis for medical purposes. Montreal: Collège des médecins du Québec; 2014. Available from: http://www.cmq.org/en/MedecinsMembres/DossierMembreFormulaires/~/media/Files/Cannabis/Guidelinesprescription-cannabis.pdf?111402. Accessed 10 July 2015.
Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–27.
McKim WA. Drugs and behavior. An introduction to behavioral pharmacology, 4th ed. Upper Saddle River: Prentice-Hall; 2000. p. 400.
Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631.
Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19:17078–106.
Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, Molinari M, Maccarrone M. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci. 2009;29:4564–70.
Ross RA. Allosterism and cannabinoid CB(1) receptors: the shape of things to come. Trends Pharmacol Sci. 2007;28:567–72.
Galve-Roperh I, Rueda D, Gómez del Pulgar T, Velasco G, Guzmán M. Mechanism of extracellular signal-regulated kinase activation by the CB(1) cannabinoid receptor. Mol Pharmacol. 2002;62:1385–92.
Sánchez C, de Ceballos ML, Gomez del Pulgar T, Rueda D, Corbacho C, Velasco G, Galve-Roperh I, Huffman JW, Ramón y Cajal S, Guzmán M. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001;61:5784–89.
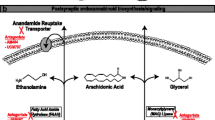
Schmid HHO, Schmid PC, Natarajan V. N-Acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29:1–43.
Jin XH, Uyama T, Wang J, Okamoto Y, Tonai T, Ueda N. cDNA cloning and characterization of human and mouse Ca(2+)-independent phosphatidylethanolamine N-acyltransferases. Biochim Biophys Acta. 2009;1791:32–8.
Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–305.
Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–72.
Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci. 2006;103:13345–50.
Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9.
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7.
Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281:36569–78.
Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem. 2005;280:11082–92.
Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–8.
Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci. 2002;99:10819–24.
Navia-Paldanius D, Savinainen JR, Laitinen JT. Biochemical and pharmacological characterization of human α/β-hydrolase domain containing 6 (ABHD6) and 12 (ABHD12). J Lipid Res. 2012;53:2413–24.
Ueda N, Tsuboi K, Uyama T. Metabolism of endocannabinoids and related N-acylethanolamines: canonical and alternative pathways. FEBS J. 2013;280:1874–94.
Ueda N, Tsuboi K, Uyama T. N-Acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA). Prog Lipid Res. 2010;49:299–315.
Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev. 2011;111:5899–921.
Romigi A, Bari M, Placidi F, Marciani MG, Malaponti M, Torelli F, Izzi F, Prosperetti C, Zannino S, Corte F, Chiaramonte C, Maccarrone M. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia. 2010;51:768–72.
Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, Watanabe M. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16:264–77.
Wallace MJ, Blair RE, Falenski KW, Martin BR, De Lorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–37.
Fezza F, Marrone MC, Avvisati R, Di Tommaso M, Lanuti M, Rapino C, Mercuri NB, Maccarrone M, Marinelli S. Distinct modulation of the endocannabinoid system upon kainic acid-induced in vivo seizures and in vitro epileptiform bursting. Mol Cell Neurosci. 2014;62:1–9.
Citraro R, Russo E, Ngomba RT, Nicoletti F, Scicchitano F, Whalley BJ, Calignano A, De Sarro G. CB1 agonists, locally applied to the cortico-thalamic circuit of rats with genetic absence epilepsy, reduce epileptic manifestations. Epilepsy Res. 2013;106:74–82.
Rizzo V, Ferraro G, Carletti F, Lonobile G, Cannizzaro C, Sardo P. Evidences of cannabinoids-induced modulation of paroxysmal events in an experimental model of partial epilepsy in the rat. Neurosci Lett. 2009;462:135–9.
Clement AB, Hawkins EG, Lichtman AH, Cravatt BF. Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J Neurosci. 2003;23:3916–23.
Deshpande LS, Blair RE, Ziobro JM, Sombati S, Martin BR, DeLorenzo RJ. Endocannabinoids block status epilepticus in cultured hippocampal neurons. Eur J Pharmacol. 2007;558:52–9.
Carletti F, Gambino G, Rizzo V, Ferraro G, Sardo P. Cannabinoid and nitric oxide signaling interplay in the modulation of hippocampal hyperexcitability: study on electrophysiological and behavioral models of temporal lobe epilepsy in the rat. Neuroscience. 2015;303:149–59.
Shubina L, Aliev R, Kitchigina V. Attenuation of kainic acid-induced status epilepticus by inhibition of endocannabinoid transport and degradation in guinea pigs. Epilepsy Res. 2015;111:33–44.
Karanian DA, Karim SL, Wood JT, Williams JS, Lin S, Makriyannis A, Bahr BA. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J Pharmacol Exp Ther. 2007;322:1059–66.
Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C. Lutz B CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–8.
Naidoo V, Karanian DA, Vadivel SK, Locklear JR, Wood JT, Nasr M, Quizon PM, Graves EE, Shukla V, Makriyannis A, Bahr BA. Equipotent inhibition of fatty acid amide hydrolase and monoacylglycerol lipase—dual targets of the endocannabinoid system to protect against seizure pathology. Neurotherapeutics. 2012;9:801–13.
Mechoulam R. Chapter 1: the pharmacohistory of Cannabis sativa. In: Mechoulan R, editor. CBss as therapeutic agents. Boca Raton: CRC Press; 1986. p. 1–19.
Consroe P, Wood JC, Buchsbaum H. Anticonvulsant nature of marihuana smoking. JAMA. 1975;234:306–7.
Ellison JM, Gelwan E, Ogletree J. Complex partial seizure symptoms affected by marijuana abuse. J Clin Psychiatry. 1990;51:439–40.
Grinspoon L, Bakalar JB. Marihuana. The Forbidden Medicine. New Haven: Yale University Press; 1997. p. 296.
Gurley RJ, Aranow R, Katz M. Medicinal marijuana: a comprehensive review. J Psychoactive Drugs. 1998;30:137–47.
Carlini EA, Mechoulam R, Lander N. Anticonvulsant activity of four oxygenated cannabidiol derivatives. Res Commun Chem Pathol Pharmacol. 1975;12:1–15.
Ames FR, Cridland S. Anticonvulsant effect of cannabidiol. S Afr Med J. 1985;69:14.
Cortesi M, Fusar-Poli P. Potential therapeutical effects of cannabidiol in children with pharmacoresistant epilepsy. Med Hypotheses. 2007;68:920–1.
Luszczki JJ, Czuczwar P, Cioczek-Czuczwar A, Czuczwar SJ. Arachidonyl- 2′-chloroethylamide, a highly selective CBs CB1 receptor agonist, enhances the anticonvulsant action of valproate in the mouse maximal electroshock- induced seizure model. Eur J Pharmacol. 2006;547:65–74.
Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wölfel B, Dodt HU, Zieglgänsberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–66.
Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42:1266–72.
Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967;28:474–5.
Cunha JM, Carlini EA, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers 2 and epileptic patients. Pharmacology. 1980;21:175–85.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;3:CD009270.
Davis JP, Ramsey HH. Anti-epileptic action of marijuana active substances. Fed Proc. 1949;8:167.
Feeney DM. Marihuana use among epileptics. JAMA. 1976;235:1105.
Mechoulam R, Carlini EA. Toward drugs derived from cannabis. Naturwissenschaften. 1978;65:174–9.
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556–63.
Trembly B, Sherman M. Double-blind clinical study of cannabidiol as a secondary anticonvulsant. In: Marijuana ’90 International Conference on Cannabis and Cannabinoids; 1990 July 8–11, section 2. Kolympari: International Association for CBs Medicines; 1990. p. 5.
Dos Santos RG, Hallak JE, Leite JP, Zuardi AW, Crippa JA. Phytocannabinoids and epilepsy. J Clin Pharm Ther. 2015;40:135–43.
Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–51.
Yamaori S, Ebisawa J, Okushima Y, et al. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011;88:730–6.
Jiang R, Yamaori S, Okamoto Y, et al. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet. 2013;28:332–8.
Macleod J, Oakes R, Copello A, Crome I, Egger M, Hickman M, Oppenkowski T, Stokes-Lampard H, Davey Smith G. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet. 2004;363:1579–88.
Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–3.
Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29:574–7.
Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015;10(373):1048–58.
Devinsky O, et al. Epidiolex (Cannabidiol) in treatment resistant epilepsy, American Epilepsy Society Annual Meeting, Poster, December 7, 2015.
Lorentzos MS, Webster R. Cannabinoids for paediatric epilepsy: weeding out the issues. J Paediatr Child Health. 2015;51:476–7.
Szaflarski JP, Bebin EM. Cannabis, cannabidiol, and epilepsy–from receptors to clinical response. Epilepsy Behav. 2014;41:277–82.
Ng SKC, Brust JCM, Hauser WA, Susser M. Illicit drug use and the risk of new-onset seizures. Am J Epidemiol. 1990;132:47–57.
Lorenz R. Experiences with THC-treatment in children and adolescents. In: IACM 2nd Conference on CBss in Medicine. Koeln: International Association for Cannabis as Medicine; 2003. p. 6.
Gross DW, Hamm J, Ashowrth NL, Quigley D. Marijuana use and epilepsy. Neurology. 2004;62:2095–7.
Mortati K, Dworetzky B, Devinsky O. Marijuana: an effective treatment in partial epilepsy? A case report and review of the literature. Rev Neurol Dis. 2007;4:103–6.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Financial disclosure
The authors have no financial relationships to disclose with regard to this article.
Conflict of interest
AV, MC, MM and FF have no conflicts of interest.
Additional information
A. Verrotti, M. Maccarrone and F. Fezza are equally senior authors.
Rights and permissions
About this article
Cite this article
Verrotti, A., Castagnino, M., Maccarrone, M. et al. Plant-Derived and Endogenous Cannabinoids in Epilepsy. Clin Drug Investig 36, 331–340 (2016). https://doi.org/10.1007/s40261-016-0379-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0379-x