Abstract
Background and Objective
Age- and sex-related differences in body composition could affect the pharmacokinetic parameters of administered drugs. The purpose of this post hoc analysis was to investigate the influences of age and sex on the pharmacokinetics of lacosamide.
Methods
This post hoc analysis used pharmacokinetic data taken at steady state from (i) two phase I studies of oral lacosamide in healthy adult subjects (n = 66), and (ii) a population pharmacokinetic analysis carried out using data from two phase III studies of adjunctive oral lacosamide in adults (n = 565) with focal epilepsy taking 1–3 concomitant anti-epileptic drugs. Phase I data were stratified by age and sex as ‘younger female’ (aged 18–40 years), ‘younger male’ (aged 18–45 years) or ‘elderly male/female’ (aged ≥65 years), then normalized by body weight (lean body weight or fat-free mass), height or volume of distribution, and analysed using non-compartmental analysis. Population pharmacokinetic data were stratified by sex and analysed using a one-compartment model.
Results
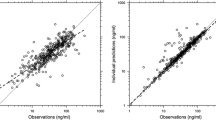
Minor numerical differences between lacosamide exposure [the area under the concentration–time curve at steady state over the dosage interval (AUCτ,ss)] and the maximum plasma concentration at steady state (C max,ss) in subjects of different ages or sexes were noted. The differences could be explained by a scaling factor between the drug applied and the plasma concentration. Following normalization by lean body weight or volume of distribution, an analysis of relative bioavailability resulted in 90 % confidence intervals of the ratios for AUCτ,ss and C max,ss for age (elderly to younger) or sex (male to female) falling within the range accepted for equivalence (80–125 %); without normalization, the 90 % confidence intervals were outside this range. Minor numerical differences in lacosamide plasma concentrations were noted in the comparison between male and female patients (aged 16–71 years) with focal epilepsy. Simulations using different body weights demonstrated a minimal effect of body weight on lacosamide plasma concentrations in adult patients with focal epilepsy.
Conclusion
Age and sex had no relevant effects on the rates of absorption and elimination of lacosamide in this post hoc analysis, as the minor numerical differences could be explained by the main scaling factor for body weight or volume of distribution. The pharmacokinetic profile of lacosamide was unaffected by age or sex in adults with focal epilepsy.



Similar content being viewed by others
References
Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523.
Perucca E. Pharmacokinetic variability of new antiepileptic drugs at different ages. Ther Drug Monit. 2005;27(6):714–7.
Italiano D, Perucca E. Clinical pharmacokinetics of new-generation antiepileptic drugs at the extremes of age: an update. Clin Pharmacokinet. 2013;52(8):627–45.
Perucca E, Berlowitz D, Birnbaum A, Cloyd JC, Garrard J, Hanlon JT, et al. Pharmacological and clinical aspects of antiepileptic drug use in the elderly. Epilepsy Res. 2006;68(Suppl 1):S49–63.
Klotz U. The elderly—a challenge for appropriate drug treatment. Eur J Clin Pharmacol. 2008;64(3):225–6.
Bossingham MJ, Carnell NS, Campbell WW. Water balance, hydration status, and fat-free mass hydration in younger and older adults. Am J Clin Nutr. 2005;81(6):1342–50.
Fliser D, Bischoff I, Hanses A, Block S, Joest M, Ritz E, et al. Renal handling of drugs in the healthy elderly: creatinine clearance underestimates renal function and pharmacokinetics remain virtually unchanged. Eur J Clin Pharmacol. 1999;55(3):205–11.
Lackner TE, Cloyd JC, Thomas LW, Leppik IE. Antiepileptic drug use in nursing home residents: effect of age, gender, and comedication on patterns of use. Epilepsia. 1998;39(10):1083–7.
Leppik IE. Treatment of epilepsy in the elderly. Curr Treat Options Neurol. 2008;10(4):239–45.
Haegele KD, Huebert ND, Ebel M, Tell GP, Schechter PJ. Pharmacokinetics of vigabatrin: implications of creatinine clearance. Clin Pharmacol Ther. 1988;44(5):558–65.
Stefan H, May TW, Pfäfflin M, Brandt C, Füratsch N, Schmitz B, et al. Epilepsy in the elderly: comparing clinical characteristics with younger patients. Acta Neurol Scand. 2014;129(5):283–93.
UCB Pharma. Vimpat® (lacosamide) EPAR product information. Brussels, Belgium. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000863/WC500050338.pdf. Accessed 17 Feb 2015.
UCB Inc. Vimpat® (lacosamide tablets, injection, oral solution) US prescribing information. Smyrna. http://www.vimpat.com/pdf/vimpat_PI.pdf. Accessed Feb 17 2015.
Cawello W, Bökens H, Nickel B, Andreas JO, Halabi A. Tolerability, pharmacokinetics, and bioequivalence of the tablet and syrup formulations of lacosamide in plasma, saliva, and urine: saliva as a surrogate of pharmacokinetics in the central compartment. Epilepsia. 2013;54(1):81–8.
Cawello W, Boekens H, Bonn R. Absorption, disposition, metabolic fate and elimination of the anti-epileptic drug lacosamide in humans: mass balance following intravenous and oral administration. Eur J Drug Metab Pharmacokinet. 2012;37(4):241–8.
Hoy SM. Lacosamide: a review of its use as adjunctive therapy in the management of partial-onset seizures. CNS Drugs. 2013;27(12):1125–42.
Cawello W, Nickel B, Eggert-Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50(4):459–71.
Cawello W, Bonn R. No pharmacokinetic interaction between lacosamide and valproic acid in healthy volunteers. J Clin Pharmacol. 2012;52(11):1739–48.
Cawello W, Rosenkranz B, Schmid B, Wierich W. Pharmacodynamic and pharmacokinetic evaluation of coadministration of lacosamide and an oral contraceptive (levonorgestrel plus ethinylestradiol) in healthy female volunteers. Epilepsia. 2013;54(3):530–6.
Cawello W, Mueller-Voessing C, Fichtner A. Pharmacokinetics of lacosamide and omeprazole coadministration in healthy volunteers: results from a phase I, randomized, crossover trial. Clin Drug Investig. 2014;34(5):317–25.
Cawello W, Mueller-Voessing C, Andreas JO. Effect of lacosamide on the steady-state pharmacokinetics of digoxin: results from a phase I, multiple-dose, double-blind, randomised, placebo-controlled, crossover trial. Clin Drug Investig. 2014;34(5):327–34.
Stockis A, van Lier JJ, Cawello W, Kumke T, Eckhardt K. Lack of effect of lacosamide on the pharmacokinetic and pharmacodynamic profiles of warfarin. Epilepsia. 2013;54(7):1161–6.
Thomas D, Scharfenecker U, Nickel B, Doty P, Cawello W, Horstmann R. Lacosamide has low potential for drug–drug interaction [abstract]. Eur J Neurol. 2007;14(Suppl 1):211–2.
Schiltmeyer B, Cawello W, Kropeit D, Horstmann R Population pharmacokinetics of the new antileptic drug lacosamide in healthy subjects with different age and gender. 14th PAGE Meeting; Pamplona, Spain; 16–17 June 2005. http://www.page-meeting.org/default.asp?abstract=743. Accessed 27 Jan 2015.
Chung S, Sperling MR, Biton V, Krauss G, Hebert D, Rudd GD, et al. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia. 2010;51(6):958–67.
Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, Rosenow F, Doty P, Hebert D, et al. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50(3):443–53.
Jorquera F, Almar MM, Jimeno A, Gonzalez-Sastre M, Gonzalez-Gallego J. Assessment of antipyrine kinetics from saliva or plasma: influence of age. J Pharm Biomed Anal. 1995;13(9):1141–5.
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051–65.
Markoula S, Teotonio R, Ratnaraj N, Duncan JS, Sander JW, Patsalos PN. Lacosamide serum concentrations in adult patients with epilepsy: the influence of gender, age, dose, and concomitant antiepileptic drugs. Ther Drug Monit. 2014;36(4):494–8.
Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76.
Beyreuther BK, Freitag J, Heers C, Krebsfänger N, Scharfenecker U, Stohr T. Lacosamide: a review of preclinical properties. CNS Drug Rev. 2007;13(1):21–42 (Spring).
Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol. 2009;8(11):1019–30.
Acknowledgments
Editorial support was provided by Karen Burrows of Evidence Scientific Solutions (Horsham, UK) and funded by UCB Pharma. Publication coordination was provided by Barbara Pelgrims, PhD, of UCB Pharma (Brussels, Belgium). The studies included in this analysis were funded by UCB Pharma (Monheim am Rhein, Germany). Carina Schaefer, Willi Cawello and Jan-Peer Elshoff are employees of UCB Pharma (Monheim am Rhein, Germany). Josef Waitzinger is a retired employee of Nusivan (Neu-Ulm, Germany).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schaefer, C., Cawello, W., Waitzinger, J. et al. Effect of Age and Sex on Lacosamide Pharmacokinetics in Healthy Adult Subjects and Adults with Focal Epilepsy. Clin Drug Investig 35, 255–265 (2015). https://doi.org/10.1007/s40261-015-0277-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0277-7