Abstract
Objectives and Background
The objective of this study was to investigate the image quality-improving and heart rate-lowering effects of landiolol hydrochloride (a short-acting β1-adrenergic receptor blocker) on coronary computed tomography angiography (CCTA). During CCTA, β-adrenergic receptor blockers have been commonly used to lower heart rate and improve image quality.
Methods
A total of 258 subjects suspected of having ischemic cardiac disease and requiring CCTA were randomized to either a landiolol hydrochloride 0.125 mg/kg group or placebo group to study the efficacy and safety of landiolol hydrochloride in a multicenter, double-blind, randomized parallel study. The primary endpoint was the diagnosable proportion (proportion of subjects whose coronary stenosis was diagnosable).
Results
The diagnosable proportions about the reconstruction images at mid-diastole were 68.2 and 38.2 % in the landiolol hydrochloride and placebo group, respectively, indicating significant superiority of landiolol hydrochloride over placebo (p < 0.0001). The diagnosable proportions about the optimal reconstruction images were 81.4 and 54.2 % in the landiolol hydrochloride and placebo group, respectively, indicating significant superiority of landiolol hydrochloride over placebo (p < 0.0001). The mean heart rate-lowering effect was first observed soon after administration of landiolol hydrochloride, was most marked at 3–5 min, and disappeared 30 min after completion of administration. The mean heart rate-lowering proportion at that time was −19.1 ± 8.1 % and −5.9 ± 9.7 % in the landiolol hydrochloride and placebo groups, respectively, showing a significantly higher proportion in the landiolol hydrochloride group.
Conclusions
Landiolol hydrochloride was confirmed to significantly and rapidly lower heart rate after intravenous injection, suggesting that it is a safe and useful agent for improving the image quality of CCTA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Coronary computed tomography (CT) angiography (CCTA) is a non-invasive method of diagnosing the existence and extent of coronary stenosis [1, 2]. Single- and multicenter studies have shown CCTA to be useful, with a very high negative predictive value [3, 4]. However, it has been reported that CT image quality is lowered in patients with a high heart rate, requiring administration of β-adrenergic receptor blockers to lower the heart rate and improve image quality by increasing the relative time resolution during CCTA [1, 2]. In fact, many clinical and other types of studies of CCTA have reported the administration of β-adrenergic receptor blockers to lower the heart rate during CCTA [3, 4].
One recent study reported high diagnostic capability without the use of β-adrenergic receptor blockers, thanks to shortening of the imaging time and improvement in time resolution [5]. However, those results were obtained only using a specific model, such as dual-source CT, in an updated facility, and thus common CT equipment used in clinical practice still requires the use of β-adrenergic receptor blockers to lower the heart rate during CCTA. Furthermore, since many techniques to reduce the volume of exposure to radiation are applicable only at low heart rates, it is essential to lower the heart rate to reduce such exposure volume [6, 7].
Injectable or oral β-adrenergic receptor blockers, which not only take more than 1 h to become effective but also have long half-lives [2.3 h for injection (propranolol), and 2.8 (metoprolol) to 3.9 h (propranolol) for tablets], constraining patients for a longer time, were widely used in previous studies. Therefore, there has been a desire for short-acting β-adrenergic receptor blockers, in order to achieve safer and more efficient inspection. The pharmacokinetic profile of landiolol hydrochloride shows high β1 selectivity as well as a very short half-life (3.97 min) [8].
However, no study has examined the efficacy and safety of short-acting β-adrenergic receptor blockers in CCTA in a placebo-controlled, double-blind manner. In the present study, the efficacy and safety of the short-acting β1-adrenergic receptor blocker landiolol hydrochloride (ONO-1101) 0.125 mg/kg at CCTA were assessed using 64-row CT in common clinical use and 320-row CT equipped with the maximum number of detectors. In addition, a higher diagnosable proportion can be achieved by improving the image quality. The diagnosable proportion has been reported to be improved at a heart rate not higher than 65 beats/min. Therefore, we investigated the relationship between the diagnosable proportion and heart rate in order to confirm the image quality-improving effect of administration of landiolol hydrochloride.
2 Methods
2.1 Study Population
Prior to CCTA, Japanese subjects aged 20 years or over who were suspected of having ischemic cardiac disease were selected based on symptoms recorded by a physician, physical examination, standard 12-lead ECG, chest X-ray, or echocardiography findings. The patients included in the study were those who (1) presented with stable angina syndromes and were referred for clinically indicated CCTA; and (2) had a heart rate of 70–90 beats/min on admission to the CT room and immediately before administration of a nitrate vasodilator drug.
Patients were excluded from the present study if they had a cardiac pacemaker, defibrillator, or both implanted; had undergone coronary-artery bypass surgery; had systolic blood pressure <110 mmHg before CCTA; had atrial fibrillation or extrasystoles at imaging; were pregnant, lactating, or possibly pregnant or desiring to become pregnant during the study period; or the use of β-adrenergic receptor blockers or non-ionic contrast media was contraindicated. The concomitant use of the following drugs was prohibited: non-dihydropyridine calcium channel antagonists, antiarrhythmic agents, sympathomimetic agents, and biguanide antidiabetic agents. However, the concomitant use of β-adrenergic receptor blockers or dihydropyridine calcium channel antagonists for conditions such as hypertension or angina was allowed.
The appropriateness of the study was reviewed and accepted by the Institutional Review Board at each study center before initiating the study. This study was conducted in accordance with the ethical principles in the Declaration of Helsinki, and in compliance with the Pharmaceutical Affairs Law and the Ordinance on Standards for Implementation of Clinical Studies on Drugs (MHW Ordinance No. 28) in Japan. Prior to the study, written informed consent was obtained from all patients upon confirming that they had understood the study content.
2.2 Study Design
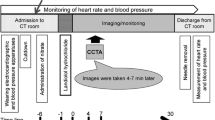
The present study was a multicenter, double-blind, randomized, parallel-group study, which was conducted at 33 study centers in Japan. The ClinicalTrials.org identifier was NCT00924586. The eligible subjects were randomized (permuted-block randomization) to one of two groups: the landiolol hydrochloride group (treated with intravenous landiolol hydrochloride 0.125 mg/kg) and placebo group (treated with intravenous placebo containing physiological saline) at a ratio of 1:1 before CCTA. As shown in Fig. 1, the subjects received the study drug as a bolus injection over 1 min after receiving a nitrate drug (nitroglycerin 0.3 mL was administered sublingually), and underwent CCTA 4–7 min after administration of the study drug. The study period was from June 2009 to February 2010.
2.3 Endpoints
The primary endpoint was the diagnosable proportion (proportion of subjects whose coronary stenosis was diagnosable in reconstructed images). The secondary endpoints were the degree and duration of the drug effect on heart rate and blood pressure, percutaneous oxygen saturation (SpO2), ECG parameters, and adverse events. Heart rate (Holter ECG), blood pressure, and SpO2 were monitored before initiation of the study (baseline: measured on the day of CCTA), on admission to the CT room, immediately before administration of the nitrate drug, immediately before administration of the study drug, every minute from 0 to 10 min after completion of administration of the study drug, and at 15 and 30 min after completion of administration of the study drug. Additionally, 12-lead ECG and laboratory values were assessed before initiation of the study (baseline) and within 3 days after completion of administration of the study drug. Adverse events were followed from the initiation of study drug administration until the end of the monitoring period.
3 Coronary Computed Tomography Angiography
3.1 Image Acquisition
CCTA was carried out between 4 and 7 min after completion of study drug administration. The reason for this timing of CCTA is that heart rate is reported to be lowest from 4 to 7 min after intravenous administration of landiolol hydrochloride [8]. The CT equipment used was a SOMATOM Sensation Cardiac 64 (Siemens), Aquilion™ 64 and Aquilion™ ONE (Toshiba Medical Systems Co.), LightSpeed® VCT (GE Medical Systems, Inc.), and Brilliance 64 (Philips Electronics), yielding four models of 64-row CT and one 320-row CT. Table 1 shows the imaging conditions for each type of CT. The rotation speed of the X-ray tube was set to the maximum for each type of equipment. A non-ionic contrast medium, iopamidol (370 mgI/mL), was rapidly injected intravenously at 3–4.5 mL/s using a 2-channel injector, followed by infusion of 20–30 mL saline.
3.2 Image Reconstruction
Image reconstruction followed the retrospective ECG-gated reconstruction method at each study center, with a slice thickness for reconstruction of 0.5–0.75 mm [0.75 mm for Siemens (64), 0.5 mm for Toshiba (64) and Toshiba (320), 0.625 mm for GE (64), and 0.67 mm for Philips (64)]. Image reconstruction was performed with the optimal conditions at each study center and was performed for acquisition of phase images starting at mid-diastole (70 % of the R–R interval) at each study center. In addition, the primary endpoint was used for the reconstruction images at mid-diastole.
3.3 Image Analysis
At the core laboratory, volume-rendering images, curved multi-planar reformation (MPR) images, interactive oblique MPR images, thin MIP images, and cross-sectional images were prepared using the images reconstructed at the image analysis center of a third party. All images of each of 15 coronary segments based on the American Heart Association Classification were assessed and classified by the Central Coronary Visualization Judgment Committee, consisting of three independent radiodiagnostic specialists, as image quality score: Score 1 (motion artifact(s) present and impossible to diagnose), Score 2 (motion artifact(s) present but diagnosable), and Score 3 (no motion artifact and diagnosable). The validity of this assessment (comparison with coronary angiographic findings) has already been confirmed by our phase II study [9].
Preparation of images as well as assessment of the diagnosable proportion were performed using a workstation, Aquarius.NET Server (client PC networked with Aquarius.NET Server), of the same model.
3.4 Radiation Dose Measurement
The effective dose for a non-enhanced scan and CCTA was estimated from the dose-length product and a conversion coefficient (k × 0.014 mSv/[mGy × cm]) for the chest as the investigated anatomical region [10].
4 Statistical Analysis
The sample size was set so that significant differences could be detected at a probability of 90 % or higher when the difference in image quality scores of 2 and 3 between the landiolol hydrochloride and placebo groups was 23.0 % or more. Thus, the required number of subjects was set at 97 per group, and the sample size was fixed at 105–115 subjects per group, since about 5–15 % of subjects might discontinue or drop out. The number of subjects to be analyzed by each type of CT equipment was set at ten or more per group.
Subject background and CCTA conditions were analyzed to determine the inter-group uniformity by Chi-squared (χ 2) test or Wilcoxon rank-sum test. The diagnosable proportion, as the primary endpoint, was compared between the landiolol hydrochloride and placebo groups by χ 2 test, and heart rate was examined by t test. A p value of <0.05 was considered statistically significant.
5 Results
5.1 Subjects
A total of 258 subjects were enrolled in this study, and 130 and 128 were randomly allocated to the landiolol hydrochloride group and placebo group, respectively. During the study period, one subject each in the landiolol hydrochloride group and the placebo group discontinued the study [because of a decrease in blood pressure before administration of the study drug (landiolol hydrochloride group) and withdrawal of consent (placebo group)]. Therefore, 256 subjects received the study drug. Two subjects who did not receive the study drug were excluded from the analysis set. Further, one subject in each of the two groups discontinued the study because of CT malfunction, but they received the study drug and were therefore included in the analysis set. The analysis set of the primary endpoint was thus composed of 129 subjects in the landiolol hydrochloride group and 127 subjects in the placebo group, totaling 256 subjects (Fig. 2).
5.2 Baseline Characteristics
The background factors and CCTA conditions of subjects enrolled in the present study are summarized in Table 2. Heart rate (mean ± standard deviation) immediately before administration of the study drug was 77.6 ± 9.5 and 76.9 ± 8.9 beats/min in the landiolol hydrochloride and placebo groups, respectively. The number of subjects in the landiolol hydrochloride and placebo groups by CT model was 77 and 74 for Siemens (64), 13 and 14 for Toshiba (64), 14 and 12 for Toshiba (320), 12 and 12 for GE (64), and 13 and 15 for Philips (64), respectively. The number (%) of subjects with concomitant use of oral β-adrenergic receptor blockers was 13 (11.8 %) and 11 (10 %) in the landiolol hydrochloride and placebo groups, respectively, showing no significant difference between the two groups (p = 0.0555). There was no bias in background factors and imaging conditions between the treatment groups (Table 2).
5.3 Heart Rate Evaluation
As shown in Fig. 3 and Table 3, heart rate at CCTA was 62.6 ± 8.5 beats/min in the landiolol hydrochloride group, which was significantly lower than the value of 72.9 ± 12.0 beats/min in the placebo group (t test: p < 0.0001). The heart rate-lowering rate calculated as percentage change from the baseline to CCTA was −19.1 ± 8.1 % in the landiolol hydrochloride group, which was significantly greater than the value of −5.9 ± 9.7 % in the placebo group (t test: p < 0.0001).
In the landiolol hydrochloride group, the percentage of subjects with the lowest heart rate by 3, 5, and 7 min after completion of study drug administration was 32.8, 67.2, and 75.0 %, respectively, showing a rapid reduction. The heart rate then rapidly recovered toward the baseline value, showing no significant difference from that of the placebo group at 30 min after completion of study drug administration.
5.4 Blood Pressure Evaluation
As shown in Table 3, mean systolic blood pressure at CCTA was 125.1 ± 20.7 and 132.7 ± 20.7 mmHg in the landiolol hydrochloride and placebo groups, respectively, showing a significant difference between the two groups (t test: p < 0.05). However, the percentage change of blood pressure from immediately before administration of the study drug until the timepoint of CCTA was −2.6 ± 9.3 and 1.7 ± 10.2 % in the landiolol hydrochloride and placebo groups, respectively, showing that the lowering proportion of blood pressure was limited and it had recovered to the baseline value at 30 min after administration of the study drug (Fig. 4).
Mean ± standard deviation changes in blood pressure. Mean blood pressure showed a significant difference between the two groups, but the lowering proportion was limited, as shown in Table 3. CCTA coronary computed tomography angiography, CT computed tomography, *p < 0.05 vs. placebo (t test)
5.5 Radiation Dose Evaluation
The radiation dose (mSv) of each type of CT equipment was 4.9 ± 1.0, 13.3 ± 1.7, 2.6 ± 0.59, 15.9 ± 6.0, and 12.9 ± 1.6 for Siemens (64), Toshiba (64), Toshiba (320), GE (64), and Philips (64), respectively.
5.6 Image Quality Score
As shown in Table 4, an image quality score of 2 or 3 about the reconstruction images at mid-diastole in the analysis by subject was observed in 68.2 % (75/110 subjects; 95 % CI 59.5–76.9) and 38.2 % (42/110 subjects; 95 % CI 29.1–47.3) of subjects in the landiolol hydrochloride and placebo groups, respectively, showing that the image quality score was significantly higher in the landiolol hydrochloride group than in the placebo group (χ 2 test: p < 0.0001). A score of 2 or 3 about the reconstruction images at mid-diastole in the analysis by coronary vessel was observed in 87.8 % (360/410 vessels; 95 % CI 84.6–91.0) and 74.8 % (308/412 vessels; 95 % CI 70.6–79.0) of subjects in the landiolol hydrochloride and placebo groups, respectively, showing that the image quality score was significantly higher in the landiolol hydrochloride group than in the placebo group (χ2 test: p < 0.0001). A score of 2 or 3 about the reconstruction images at mid-diastole in the analysis by coronary segment was observed in 92.9 % (1,169/1,259 segments; 95 % CI 91.4–94.3) and 84.5 % (1,084/1,283 segments; 95 % CI 82.5–86.5) of subjects in the landiolol hydrochloride and placebo groups, respectively, which was significantly higher in the landiolol hydrochloride group than in the placebo group (χ2 test: p < 0.0001).
An image quality score of 2 or 3 about the optimal reconstruction images in the analysis by subject was observed in 81.4 % (96/118 subjects; 95 % CI 74.3–88.4) and 54.2 % (64/118 subjects; 95 % CI 45.2–63.2) of subjects in the landiolol hydrochloride and placebo groups, respectively, showing that the image quality score was significantly higher in the landiolol hydrochloride group than in the placebo group (χ 2 test: p < 0.0001). A score of 2 or 3 about the optimal reconstruction images in the analysis by coronary vessel was observed in 94.3 % (413/438 vessels; 95 % CI 92.1–96.5) and 84.3 % (371/440 vessels; 95 % CI 80.9–87.7) of subjects in the landiolol hydrochloride and placebo groups, respectively, showing that the image quality score was significantly higher in the landiolol hydrochloride group than in the placebo group (χ 2 test: p < 0.0001). A score of 2 or 3 about the optimal reconstruction images in the analysis by coronary segment was observed in 97.9 % (1,326/1,354 segments; 95 % CI 97.2–98.7) and 93.9 % (1,270/1,353 segments; 95 % CI 92.6–95.1) of subjects in the landiolol hydrochloride and placebo groups, respectively, which was significantly higher in the landiolol hydrochloride group than in the placebo group (χ 2 test: p < 0.0001).
In subgroup analysis by CT model, the proportion of subjects with image quality scores of 2 and 3 about the optimal reconstruction images was 84.7 % for Siemens (64), 80.0 % for Toshiba (64), 92.9 % for Toshiba (320), 63.6 % for GE (64), and 63.6 % for Philips in the landiolol hydrochloride group, and 52.9 % for Siemens (64), 53.8 % for Toshiba (64), 66.7 % for Toshiba (320), 54.5 % for GE (64), and 50.0 % for Philips in the placebo group. These results show that the diagnosable proportion for coronary stenosis by subject was numerically superior in the landiolol hydrochloride group to that in the placebo group, with imaging by any of the CT models tested.
In subgroup analysis of the presence (+) or absence (−) of oral β-adrenergic receptor blockers, the proportion of subjects with image quality scores of 2 and 3 was 81.7 % for β-adrenergic receptor blockers (−), 78.6 % for β-adrenergic receptor blockers (+) in the landiolol hydrochloride group, and 55.1 % for β-adrenergic receptor blockers (−) and 45.5 % for β-adrenergic receptor blockers (+) in the placebo group.
5.7 Relationship Between Diagnosable Proportion and Heart Rate
As shown in Fig. 5, although the diagnosable proportion was only 55.3 % (at heart rate: 65–69 beats/min), it increased to 76.5 % (at heart rate: 60–64 beats/min), 83.3 % (at heart rate: 55–59 beats/min), and 94.7 % (at heart rate: 54 beats/min or less), showing a positive correlation between the diagnosable proportion and heart rate (Spearman rank correlation, r = 0.5088).
5.8 Safety
No subject died and no serious adverse reaction that required termination of study drug administration occurred during the study period. The incidence of adverse reactions and of decreased blood pressure did not differ significantly between the two groups (χ 2 test: p = 0.1299 and p = 0.9911). In addition, a mild decrease in blood pressure was observed in one subject each in the landiolol hydrochloride and placebo groups. In the subject in the landiolol hydrochloride group, blood pressure (systolic blood pressure/diastolic blood pressure) was 92/53 mmHg at baseline and decreased to 76/44 mmHg at 8 min after completion of administration, but it returned to the baseline value 1 min later without treatment; these changes were of no clinical concern.
6 Discussion
In the present study, injection of landiolol hydrochloride was found to be effective to rapidly lower the heart rate soon after administration. The results confirmed that this drug can be administered to patients upon confirming the heart rate immediately before CCTA, in contrast to oral agents requiring administration 1–2 h before CCTA. In addition, the study drug, with a half-life of only 4 min, did not have a prolonged β-blocking effect after CCTA and lowered the heart rate only during CCTA (Fig. 3); therefore, safety does not need to be monitored for a long period after CCTA. In fact, in clinical practice using oral agents, patients must attend the hospital to take a β-blocking agent 1–2 h before the initiation of CCTA and to monitor the heart rate to determine whether it meets the conditions for CCTA. This means it takes several hours before starting CCTA. In the case of this study drug, in contrast, administration is possible immediately before CCTA, allowing early completion of imaging. Thus, this drug appears to increase the efficiency of CCTA.
On the other hand, while bradyarrhythmia and hypotension induced by the β1-blocking effect and bronchoconstriction and peripheral circulatory disorder induced by the β2-blocking effect are known adverse reactions of β-adrenergic receptor blockers, the primary adverse reactions to the study drug are likely to be bradyarrhythmia and hypotension, because of the high selectivity of this drug for β1-adrenergic receptors (β1/β2: 251/1) [11]. In the present study, no subject developed bradyarrhythmia, and the mild decrease in blood pressure observed in one subject in the landiolol hydrochloride group rapidly resolved without requiring any clinical treatment. Furthermore, this drug was shown to lower the heart rate only during CCTA (until approximately 30 min) and not to have a prolonged effect after the completion of CCTA, confirming its safety.
Meijboom et al. [12] and Marano et al. [13] confirmed the high diagnostic performance of CCTA in multivendor, multicenter clinical studies using other CT models. In the present study using 64-row CTs from Siemens, Toshiba, GE, and Philips as well as a maximal 320-row CT from Toshiba, which are widely used in Japan, CCTA was performed only in subjects with a pre-CT heart rate as high as 70–90 beats/min, confirming the efficacy and safety of injection of the short-acting β1-adrenergic receptor blocker landiolol hydrochloride. Consequently, the improvement in the diagnosable proportion by this drug was considered not to be affected by the CT equipment model and concomitant use of β-adrenergic receptor blockers and dihydropyridine calcium channel antagonists.
Methods to reduce the radiation dose include automated exposure control, step and shoot, electrocardiographically controlled tube current modulation, and tube voltage. The present study employed only the automated exposure control method. The radiation dose in the present study, however, was lower than that reported by Hausleiter et al. [7] in a multivendor, multicenter (50 study sites: 21 university hospitals and 29 community hospitals) clinical study.
There have been many reports that the diagnosable proportion at CCTA is improved at a heart rate of 65 beats/min [14, 15]. Therefore, efforts have been devoted to control the heart rate to no higher than 65 beats/min. However, in this study it was demonstrated that approximately one-quarter of the subjects were affected by motion artifacts at a heart rate of below 60–64 beats/min, suggesting that a further reduction of heart rate is necessary to achieve sufficient image quality. In the future, it is necessary to upgrade CT equipment for faster processing, or to further lower the heart rate by using other β-blocking agents.
In summary, landiolol hydrochloride was suggested to be useful as a β-blocking agent in order to improve the diagnosable proportion at CCTA, since it decreased the effect of motion artifacts induced by heart rate at CCTA, and did not exhibit a prolonged β-blocking effect after the examination. Furthermore, administration of landiolol hydrochloride showed a positive correlation between the image quality score and heart rate.
7 Study Limitations
In the present study, the high-definition CT (GE), immunofluorescent CT (Philips), and Dual Source CT (Siemens), CT models currently commercially available in Japan, were not used. Calcium scoring was not employed as an inclusion or exclusion criterion in the present study, which excluded subjects whose heart rate was higher than 90 beats/min before CCTA (regardless of the heart rate immediately before administration of the study drug) and subjects anticipated to develop arrhythmia during CCTA.
8 Conclusions
Landiolol hydrochloride administered at CCTA significantly improved image quality and produced a rapid reduction and then rapid recovery of heart rate, but the lowering proportion of blood pressure was limited, suggesting that it is a safe and useful drug for effectual control of heart rate.
References
Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation. 2008;118:586–606.
American College of Cardiography Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, Budoff MJ, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;2010(121):2509–43.
Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–23.
Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–36.
Ropers U, Ropers D, Pflederer T, et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J Am Coll Cardiol. 2007;50:2393–8.
Husmann L, Valenta I, Gaemperil O, et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008;29:191–7.
Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–7.
Nakashima M, Kanemaru M. Phase I study of ONO-1101, a new ultra short acting β1-blocking agent in healthy volunteers [in Japanese]. J Clin Ther Med. 2000;16:1531–56.
Jinzaki M, Hirano M, Hara K, Suzuki T, Yamashina A, Ikari Y, et al. A randomized, double-blind, placebo-controlled, phase II dose-finding study of the short acting β1-blocker, landiolol hydrochloride, in patients with suspected ischemic cardiac disease. Int J Cardiovasc Imaging. 2013;29:7–20.
Bongartz G, Golding SJ, Jurik AG, et al. European Guidelines for Multislice Computed Tomography: Appendix C. Funded by the European Commission; 2004. Contract number FIGM-CT2000-20078-CT-TIP. http://www.msct.eu/CT_Quality_Criteria.htm.
Iguchi S, Iwamura H, Nishizaki M, et al. Development of a highly cardioselective ultra short-acting β-blocker, ONO-1101. Chem Pharm Bull (Tokyo). 1992;40:1462–9.
Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–44.
Marano R, De Cobelli F, Floriani I, et al: NIMISCAD Study Group. Italian multicenter, prospective study to evaluate the negative predictive value of 16- and 64-slice MDCT imaging in patients scheduled for coronary angiography (NIMISCAD-Non Invasive Multicenter Italian Study for Coronary Artery Disease). Eur Radiol. 2009;19:1114–23.
Nikolaou K, Knez A, Rist C, et al. Accuracy of 64-MDCT in the diagnosis of ischemic heart disease. AJR Am J Roentgenol. 2006;187:111–7.
Pugliese F, Mollet NR, Runza G, et al. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16:575–82.
Acknowledgments
This study was supported by a grant from Ono Pharmaceutical Co., Ltd., Osaka, Japan, the manufacturer of landiolol hydrochloride. Masaharu Hirano, Kazuhiro Hara, Yuji Ikari, Masahiro Jinzaki, Misako Iino, Chikuma Hamada, and Sachio Kuribayashi received consulting fees from Ono Pharmaceutical Co., Ltd.
We gratefully acknowledge the contributions of the members of the Landiolol Hydrochloride Study Group (listed below) to this study, as well as of Mr. Hiroshi Higashino, Mr. Masahiro Higashi, and Mr. Teruhito Kido (Central Coronary Visualization Judgment Committee).
Principal investigators: Hiroshi Inoue, Toyama-city, Toyama; Masashi Iwabuchi, Kitakyusyu-city, Fukuoka; Ikutaro Okada, Kita-ku, Kyoto; Yukio Ozaki, Toyoake-city, Aichi; Shigeo Kakinoki, Otaru-city, Hokkaido; Masaki Kawamura, Yokkaichi-city, Mie; Koji Kubota, Hakusan-city, Ishikawa; Toshiro Kurosawa, Machida-city, Tokyo; Taishi Sasaoka, Kitamoto-city, Saitama; Hideyuki Sakai, Ota-ku, Tokyo; Kenei Shimada, Nishi-ku, Osaka; Michihisa Jogasaki, Kagoshima-city, Kagoshima; Hideaki Jinouchi, Kumamoto-city, Kumamoto; Shoji Suzuki, Kasama-city, Ibaraki; Jun Tateishi, Amagasaki-city, Hyogo; Syozo Tanaka, Yao-city, Osaka; Masahiro Tamashiro, Tomigusuku-city, Okinawa; Yuichi Tsunoda, Higashiokitama-gun, Yamagata; Tamotsu Tejima, Shibuya-ku, Tokyo; Shigeru Nakamura, Nishikyo-ku, Kyoto; Yawara Nijima, Takasaki-city, Gunma; Hiroyuki Ninuma, Morioka-city, Iwate; Masato Baden, Takarazuka-city, Hyogo; Yoshikazu Hiasa, Komatsujima-city, Tokushima; Yuji Hiraoka, Yamashina-ku, Kyoto; Hiroyuki Fujinaga, Tokushima-city, Tokushima; Toshikazu Funazaki, Kawaguchi-city, Saitama; Hiroshi Maeda, Inushima-gun, Ibaraki; Yoichi Matsunaga, Kiyose-city, Tokyo; Takashi Murou, Abeno-ku, Osaka; Masao Moroi, Meguro-ku, Tokyo, Japan.
Ono Pharmaceutical clinical development team: Mitsunobu Tanimoto, Tatsuaki Okamura, Hiroshi Inose, Akira Tsuchiya (data manager), Masahiro Yoshizaki (statistician), and Shinichi Kikawa.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
ClinicalTrials.gov identifier: NCT00924586.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hirano, M., Yamashina, A., Hara, K. et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Study of the Short-Acting β1-Adrenergic Receptor Blocker Landiolol Hydrochloride for Coronary Computed Tomography Angiography in Japanese Patients with Suspected Ischemic Cardiac Disease. Clin Drug Investig 34, 53–62 (2014). https://doi.org/10.1007/s40261-013-0149-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0149-y