Abstract
Background
DA-3031 is a newly developed pegylated filgrastim, a recombinant human granulocyte colony-stimulating factor, that is expected to have an extended duration of action compared with non-modified filgrastim.
Objective
This study evaluated the tolerability, pharmacokinetics, and pharmacodynamics of DA-3031 in humans, and compared them with filgrastim.
Methods
The study was conducted in 48 healthy male Korean subjects. Forty subjects received subcutaneous single doses of 1.8, 3.6, 6, or 18 mg of DA-3031 or placebo in a dose block-randomized, double-blind, dose-escalation design. The remaining eight subjects were given subcutaneous doses of 100 μg/m2 of filgrastim daily for 5 days. Serial blood samples were collected for pharmacokinetic and pharmacodynamic analyses up to 312 h after the administration of DA-3031 and up to 264 h following the first administration of filgrastim.
Results
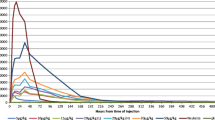
DA-3031 reached its peak plasma concentration at 6.0–48.0 h and was eliminated mono-exponentially. The pharmacokinetic parameters of DA-3031 increased with dose in a non-linear fashion. Absolute neutrophil count (ANC) levels increased with the dose of DA-3031, although the extent of the increase in ANC decreased at higher dose levels. DA-3031 resulted in similar ANC changes in the 3.6 to 6 mg dose range as 100 μg/m2 of filgrastim. The most frequent adverse event was back pain, which was observed after both DA-3031 and filgrastim administration.
Conclusions
DA-3031 showed non-linear pharmacodynamic and pharmacokinetic profiles and an extended duration of action compared with non-modified filgrastim, without unexpected toxicities in healthy subjects.





Similar content being viewed by others
References
Souza LM, Boone TC, Gabrilove J, et al. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232(4746):61–5.
Frampton JE, Lee CR, Faulds D. Filgrastim: a review of its pharmacological properties and therapeutic efficacy in neutropenia. Drugs. 1994;48(5):731–60.
Welte K, Gabrilove J, Bronchud MH, et al. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996;88(6):1907–29.
Yowell SL, Blackwell S. Novel effects with polyethylene glycol modified pharmaceuticals. Cancer Treat Rev. 2002;28(Suppl A):3–6.
Kuwabara T, Kobayashi S, Sugiyama Y. Pharmacokinetics and pharmacodynamics of a recombinant human granulocyte colony-stimulating factor. Drug Metab Rev. 1996;28(4):625–58. doi:10.3109/03602539608994020.
Lord BI, Woolford LB, Molineux G. Kinetics of neutrophil production in normal and neutropenic animals during the response to filgrastim (r-metHu G-CSF) or filgrastim SD/01 (PEG-r-metHu G-CSF). Clin Cancer Res. 2001;7(7):2085–90.
Molineux G. Pegylation: engineering improved biopharmaceuticals for oncology. Pharmacotherapy. 2003;23(8 Pt 2):3S–8S.
Molineux G. The design and development of pegfilgrastim (PEG-rmetHuG-CSF, Neulasta). Curr Pharm Des. 2004;10(11):1235–44.
Kinstler O, Molineux G, Treuheit M, et al. Mono-N-terminal poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev. 2002;54(4):477–85.
Yang BB, Kido A. Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet. 2011;50(5):295–306. doi:10.2165/11586040-000000000-00000.
Johnston E, Crawford J, Blackwell S, et al. Randomized, dose-escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapy. J Clin Oncol. 2000;18(13):2522–8.
Yang BB, Lum PK, Hayashi MM, et al. Polyethylene glycol modification of filgrastim results in decreased renal clearance of the protein in rats. J Pharm Sci. 2004;93(5):1367–73. doi:10.1002/jps.20024.
Zamboni WC. Pharmacokinetics of pegfilgrastim. Pharmacotherapy. 2003;23(8 Pt 2):9S–14S.
van Der Auwera P, Platzer E, Xu ZX, et al. Pharmacodynamics and pharmacokinetics of single doses of subcutaneous pegylated human G-CSF mutant (Ro 25-8315) in healthy volunteers: comparison with single and multiple daily doses of filgrastim. Am J Hematol. 2001;66(4):245–51. doi:10.1002/ajh.1052.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–32.
Krippendorff BF, Kuester K, Kloft C, et al. Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis. J Pharmacokinet Pharmacodyn. 2009;36(3):239–60. doi:10.1007/s10928-009-9120-1.
Roskos LK, Lum P, Lockbaum P, et al. Pharmacokinetic/pharmacodynamic modeling of pegfilgrastim in healthy subjects. J Clin Pharmacol. 2006;46(7):747–57. doi:10.1177/0091270006288731.
Wang YM, Krzyzanski W, Doshi S, et al. Pharmacodynamics-mediated drug disposition (PDMDD) and precursor pool lifespan model for single dose of romiplostim in healthy subjects. AAPS J. 2010;12(4):729–40. doi:10.1208/s12248-010-9234-9.
Ulich TR, del Castillo J, Souza L. Kinetics and mechanisms of recombinant human granulocyte-colony stimulating factor-induced neutrophilia. Am J Pathol. 1988;133(3):630–8.
Shin KH, Kim TE, Lim KS, et al. Pharmacokinetic and pharmacodynamic properties of a new long-acting granulocyte colony-stimulating factor (HM10460A) in healthy volunteers. BioDrugs. 2013;27(2):149–58. doi:10.1007/s40259-013-0010-0.
Acknowledgments
Sponsored by Dong-A Pharmaceutical Co., Seoul, Republic of Korea, this study was designed and performed by qualified investigators of the Department of Clinical Pharmacology and Therapeutics of Seoul National University Hospital. None of the authors have any conflicts of interest to disclose regarding the content of this article. Li Young Ahn, Kwang-Hee Shin, and Hyewon Jeon received training program grants from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A070001, Korea National Enterprise for Clinical Trials).
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov identifier: NCT00959777.
Rights and permissions
About this article
Cite this article
Ahn, L.Y., Shin, KH., Lim, K.S. et al. Relationship Between Absolute Neutrophil Count Profiles and Pharmacokinetics of DA-3031, a Pegylated Granulocyte Colony-Stimulating Factor (Pegylated-G-CSF): A Dose Block-Randomized, Double-Blind, Dose-Escalation Study in Healthy Subjects. Clin Drug Investig 33, 817–824 (2013). https://doi.org/10.1007/s40261-013-0130-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0130-9