Abstract
Background
HM10460A is a newly developed recombinant human granulocyte colony-stimulating factor with long-lasting characteristics. This factor is expected to be used for chemotherapy-related neutropenic conditions.
Objective
The aim of the present study was to evaluate the pharmacokinetics and pharmacodynamics of HM10460A following subcutaneous administration to healthy Korean subjects.
Methods
A randomized, double-blind, placebo-controlled, escalating single-dose study was conducted in 40 healthy Korean subjects. The subjects were allocated to single-dose groups of 5, 15, 45, 135 or 350 μg/kg, or placebo. Serial blood samples for pharmacokinetic/pharmacodynamic analyses were collected up to 22 days, and urine samples for pharmacokinetic analysis were collected up to 3 days after subcutaneous administration of HM10460A. The serum and urine concentrations were analyzed by enzyme-linked immunosorbent assay.
Results
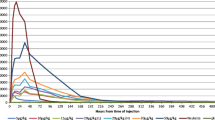
Most of the serum concentrations in the 5 and 15 μg/kg dosing groups were below the lower limit of quantification (LLOQ). The median times to the peak concentration (Tmax) of HM10460A in the 45, 135, and 350 μg/kg dosing groups were 8.0, 14.0, and 24.0 h, respectively. The mean ± standard deviation values of the dose-normalized maximum concentration (Cmax) and dose-normalized area under the concentration-time curve (AUClast) for the 45, 135, and 350 μg/kg dosing groups were 14.13 ± 6.37, 66.19 ± 38.71, and 34.65 ± 19.69 μg/L/mg, respectively, and 265.0 ± 124.1, 2144 ± 1232, and 1386 ± 701.2 μg h/L/mg, respectively. The concentrations of HM10460A in the urine were below the LLOQ in all of the subjects. In all of the dosing groups, the area under the effect-time curve (AUEClast) of both the absolute neutrophil count (ANC) and the CD34+ cell count increased as the dose increased.
Conclusion
HM10460A showed dose-dependent pharmacokinetic characteristics, and the systemic exposure of HM10460A was positively correlated with the ANC and CD34+ cell counts.





Similar content being viewed by others
References
Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78(11):2791–808.
Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC, et al. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232(4746):61–5.
Nagata S, Tsuchiya M, Asano S, Kaziro Y, Yamazaki T, Yamamoto O, et al. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature. 1986;319(6052):415–8.
Symann M. Hematopoietic growth factors as supportive therapy for cancer- and chemotherapy-induced conditions. Curr Opin Oncol. 1991;3(4):648–55.
Morstyn G, Campbell L, Souza LM, Alton NK, Keech J, Green M, et al. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988;1(8587):667–72.
Gabrilove JL, Jakubowski A, Scher H, Sternberg C, Wong G, Grous J, et al. Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. New Eng J Med. 1988;318(22):1414–22.
Kuwabara T, Kobayashi S, Sugiyama Y. Pharmacokinetics and pharmacodynamics of a recombinant human granulocyte colony-stimulating factor. Drug Metab Rev. 1996;28(4):625–58.
Ballestrero A, Boy D, Gonella R, Miglino M, Clavio M, Barbero V, et al. Pegfilgrastim compared with filgrastim after autologous peripheral blood stem cell transplantation in patients with solid tumours and lymphomas. Ann Hematol. 2008;87(1):49–55.
Molineux G, Kinstler O, Briddell B, Hartley C, McElroy P, Kerzic P, et al. A new form of Filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp Hematol. 1999;27(12):1724–34.
Renwick W, Pettengell R, Green M. Use of filgrastim and pegfilgrastim to support delivery of chemotherapy: twenty years of clinical experience. Biodrugs. 2009;23(3):175–86.
Veronese FM, Mero A. The impact of PEGylation on biological therapies. Biodrugs. 2008;22(5):315–29.
Kuwabara T, Uchimura T, Kobayashi H, Kobayashi S, Sugiyama Y. Receptor-mediated clearance of G-CSF derivative nartograstim in bone marrow of rats. Am J Physiol. 1995;269(1 Pt 1):E1–9.
Kuwabara T, Uchimura T, Takai K, Kobayashi H, Kobayashi S, Sugiyama Y. Saturable uptake of a recombinant human granulocyte colony-stimulating factor derivative, nartograstim, by the bone marrow and spleen of rats in vivo. J Pharmacol Exp Ther. 1995;273(3):1114–22.
Spunt SL, Irving H, Frost J, Sender L, Guo M, Yang BB, et al. Phase II, randomized, open-label study of pegfilgrastim-supported VDC/IE chemotherapy in pediatric sarcoma patients. J Clin Oncol. 2010;28(8):1329–36.
Dumont JA, Low SC, Peters RT, Bitonti AJ. Monomeric Fc fusions: impact on pharmacokinetic and biological activity of protein therapeutics. Biodrugs. 2006;20(3):151–60.
EMEA/CHMP/BMWP/31329/2005. Annex to guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues, Guidance on similar medicinal products containing recombinant granulocyte-colony stimulating factor. In: European Medicines Agency. Evaluation of Medicines for Human Use. 2006.
Drew E, Merzaban JS, Seo W, Ziltener HJ, McNagny KM. CD34 and CD43 inhibit mast cell adhesion and are required for optimal mast cell reconstitution. Immunity. 2005;22(1):43–57.
Lubenau H, Bias P, Maly AK, Siegler KE, Mehltretter K. Pharmacokinetic and pharmacodynamic profile of new biosimilar filgrastim XM02 equivalent to marketed filgrastim Neupogen: single-blind, randomized, crossover trial. Biodrugs. 2009;23(1):43–51.
Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48(5):1267–81.
U.S.Department of Health and Human Services, Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. National Institutes of Health, National Cancer Institute. 2009 May 28:112.
Johnston E, Crawford J, Blackwell S, Bjurstrom T, Lockbaum P, Roskos L, et al. Randomized, dose-escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapy. J Clin Oncol. 2000;18(13):2522–8.
van Der Auwera P, Platzer E, Xu ZX, Schulz R, Feugeas O, Capdeville R, et al. Pharmacodynamics and pharmacokinetics of single doses of subcutaneous pegylated human G-CSF mutant (Ro 25–8315) in healthy volunteers: comparison with single and multiple daily doses of filgrastim. Am J Hematol. 2001;66(4):245–51.
Yang BB, Lum PK, Hayashi MM, Roskos LK. Polyethylene glycol modification of filgrastim results in decreased renal clearance of the protein in rats. J Pharm Sci. 2004;93(5):1367–73.
Avalos BR. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood. 1996;88(3):761–77.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–32.
Gluckman E, Socie G, Yver A, Esperou H, Devergie A, Stern A. Transient cyclic neutropenia following GM-CSF in a patient with chronic granulocytic leukemia transplanted with HLA-identical T cell-depleted donor bone marrow. Bone Marrow Transplant. 1989;4(5):591–2.
Godbout C, Bilodeau R, Van Rooijen N, Bouchard P, Frenette J. Transient neutropenia increases macrophage accumulation and cell proliferation but does not improve repair following intratendinous rupture of Achilles tendon. J Orthop Res. 2010;28(8):1084–91.
Donahue RE, Tuschong L, Bauer TR Jr, Yau YY, Leitman SF, Hickstein DD. Leukocyte integrin activation mediates transient neutropenia after G-CSF administration. Blood. 2011;118(15):4209–14.
Author contributions:
Kwang-Hee Shin participated in the study design, conduct of the study, data analysis and interpretation of the data. Drs Kyung-Sang Yu, Sang-Goo Shin, and In-Jin Jang participated in the study design, data analysis and review of the manuscript. Drs Tae-Eun Kim and Kyoung Soo Lim contributed to the data interpretation and review of the manuscript. Drs Seo-Hyun Yoon and Joo Youn Cho contributed to the review of the bio-analysis data and manuscript. Drs Sei-Eun Kim and Kyung-Mi Park participated in the study design.
Disclosures:
This study was sponsored by Hanmi Pharmaceutical Co., Ltd., Seoul, Korea. Drs. Sei-Eun Kim, and Kyung-Mi Park are employers of Hanmi Pharmaceutical Co., Ltd. Other authors have no conflicts of interest to disclose.
Investigational site:
Clinical Trials Center, Seoul National University Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, KH., Kim, TE., Lim, K.S. et al. Pharmacokinetic and Pharmacodynamic Properties of a New Long-Acting Granulocyte Colony-Stimulating Factor (HM10460A) in Healthy Volunteers. BioDrugs 27, 149–158 (2013). https://doi.org/10.1007/s40259-013-0010-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-013-0010-0