Abstract
Background
β-Adrenoceptor antagonists (β-blockers) have been reported to be effective for regulation of heart rate (HR) and restoring sinus rhythm in postoperative atrial fibrillation and atrial flutter, as well as in the prevention of those arrhythmias after open-heart surgery.
Objectives
The objectives of this study were to evaluate the dose-dependent effects of landiolol, an ultra-short-acting β1-blocker, as well as the effectiveness and safety of the drug in suppressing supraventricular tachyarrhythmias (SVT) in postoperative patients.
Methods
Landiolol was administered as a four-dose titration regimen (LL, L, M, and H doses) to postoperative patients who developed SVT. The titration sequence began with a 1-min loading infusion at a rate of 0.015 mg/kg/min, followed by a 10-min continuous infusion at 0.005 mg/kg/min (the LL dose). Infusions at progressively higher doses followed in sequence until 20 % reduction in HR was achieved. The L dose was a 1-min loading infusion at 0.03 mg/kg/min, followed by a 10-min continuous infusion at 0.01 mg/kg/min. The M dose was a 1-min loading infusion at 0.06 mg/kg/min, followed by a 10-min continuous infusion at 0.02 mg/kg/min. The H dose was a 1-min loading infusion at 0.125 mg/kg/min, followed by a 10-min continuous infusion at 0.04 mg/kg/min. The patient was then observed for 30 min to determine the cardiovascular responses to withdrawal of the medication. After completion of this follow-up period, additional maintenance infusion for up to 6 h was permitted if considered necessary by the investigator.
Results
A total of 108 patients were enrolled in this study. The cumulative improvement rates (percentage of patients obtaining ≥20 % reduction in HR) were 11.4, 32.4, 63.1, and 87.3 % at the LL, L, M, and H doses, respectively, demonstrating the dose-dependent effectiveness of landiolol. Additional infusion for up to 6 h was conducted in 16 patients. HR was maintained between 95.5 and 116.8 beats/min during the maintenance period (mean 259.8 min). Landiolol was generally well tolerated, although one patient with sick sinus syndrome developed an approximately 5-s cardiac arrest.
Conclusions
The overall results, including those pertaining to patient safety, demonstrate that landiolol is effective and useful for the treatment of postoperative SVT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Catecholamines play a role in development of postoperative supraventricular tachyarrhythmias (SVT) [1–4], which frequently occur after open-heart surgery and resection of esophageal cancer [5–10] in the early postoperative period [8, 9]. Because persistence of SVT increases cardiac burden and aggravates hemodynamics, it is important to restore patients to a normal heart rate (HR). β-adrenoceptor antagonists (β-blockers) have been reported to be effective for regulation of HR and restoring sinus rhythm in postoperative atrial fibrillation and atrial flutter [11–15], as well as in the prevention of those arrhythmias after open-heart surgery [16, 17]. Landiolol is a new, ultra-short-acting, highly selective β1-blocker [18] with an elimination half-life of 4 min in human blood [19]; its effectiveness for treatment of various types of tachyarrhythmias has been confirmed [20]. In Japan, this drug has been approved for emergency treatment of SVT (sinus tachycardia, atrial fibrillation, and atrial flutter) during surgery [21, 22]. This study was performed to investigate the effectiveness and safety of landiolol for treatment of postoperative SVT.
2 Methods
This study was conducted in accordance with the ethical principles of the Helsinki Declaration and Good Clinical Practice. Written informed consent was obtained from all patients and/or their representatives prior to enrollment in the study, and the study protocol was approved by the Institutional Review Board at each hospital. The study was conducted in Japan between December 1996 and December 1997.
2.1 Study Population
The study population consisted of patients between 20 and 80 years old who developed postoperative SVT, including atrial fibrillation, atrial flutter, paroxysmal supraventricular tachycardia, and sinus tachycardia. Patients were enrolled when the cause of SVT (e.g., hypovolemia, abnormal electrolyte levels, pyrexia, or pain) could be identified, but the tachyarrhythmia still remained even after removal of the underlying cause. In addition, patients enrolled in the study met one or both of the following criteria: sinus tachycardia with an HR of more than 120 beats/min and continued for more than 3 min, and SVT with an HR of more than 100 beats/min that continued for more than 1 min. Exclusion criteria included acute myocardial infarction (within 1 month after onset), severe heart failure (New York Heart Association [NYHA] functional classes III and IV), atrioventricular block (grade II or higher), or sick sinus syndrome (SSS). Patients were also excluded if they had been prescribed tri- or tetracyclic psychotropic agents; had a medical history of liver, kidney, heart, or vascular diseases; had a drug allergy; or were pregnant or might become pregnant during the study.
2.2 Study Design
The study design is shown in Fig. 1. The study was designed as a prospective, open-label study, and consisted of a dose-titration treatment period and a follow-up period without any landiolol infusion. After titration trials, a maintenance period of up to 6 h was allowed after completion of the 30-min follow-up period. This maintenance dose was continued when the investigator decided that additional infusion was necessary. Patients were bed-rested prior to and during the study. Landiolol was administered at a loading dose for 1 min, immediately followed by continuous infusion for 10 min using an infusion pump. Intravenous continuous administration was initiated at the LL dose (1-min loading infusion at a rate of 0.015 mg/kg/min, followed by continuous intravenous infusion at 0.005 mg/kg/min for 10 min). If a reduction of HR ≥20 % from the baseline was not achieved after the 11-min administration period, then the dose was increased to the L dose (1-min loading infusion at 0.03 mg/kg/min, followed by continuous intravenous infusion at 0.01 mg/kg/min for 10 min). Subsequently, if the target HR was not achieved, the dose was escalated to the M dose (1-min loading infusion at 0.06 mg/kg/min, followed by continuous intravenous infusion at 0.02 mg/kg/min for 10 min), and then to the H dose (1-min loading infusion at 0.125 mg/kg/min, followed by continuous intravenous infusion at 0.04 mg/kg/min for 10 min). If the target HR was achieved, the treatment was terminated after the 11-min drug administration period. By contrast, if the target HR was not achieved, even at the H dose, administration was discontinued, and treatment was changed to a different available therapy. From the standpoint of ensuring patient safety, landiolol administration was discontinued when blood pressure decreased by ≥20 % from the baseline blood pressure and reached a value of ≤90/60 mmHg, or when HR was reduced to ≤60 beats/min. After completion of the study period, the patient was observed for 30 min for follow-up, during which no infusion was performed. The decision to proceed with an additional period of up to 6 h of landiolol administration, or to switch to alternative therapy, was made during the follow-up period, based on the patient’s condition.
2.3 Maintenance Period of Up to 6 h
After completion of the 30-min follow-up, the patient entered a maintenance period of 6 h or less, based on their condition. During this period, the dose could be changed within a range between 0.005 and 0.04 mg/kg/min in order to maintain an acceptable HR. Administration was discontinued, and treatment was switched to an alternative therapy, under the following circumstances: blood pressure decreased by ≥20 % from the baseline and reached a value of ≤90/60 mmHg, or the HR was significantly reduced (≤60 beats/min), even when the dose was reduced to 0.005 mg/kg/min; or no effect on HR was observed, even when the dose was increased to 0.04 mg/kg/min.
2.4 Concomitant Drugs and Therapies
No patients received intravenous administration of antiarrhythmic agents such as procainamide, disopyramide, mexiletine, lidocaine, propafenone, propranolol, verapamil, or diltiazem, either before or during the study. During landiolol administration, initiation of the following drugs was prohibited: drugs that may affect HR, such as other β-blockers; calcium-channel blockers other than nifedipine, nicardipine, and nilvadipine; antiarrhythmic sympathetic agents (dopamine and dobutamine); and drugs under investigation. However, concomitant administration of these drugs was acceptable if they had been administered by continuous infusion before initiation of landiolol administration. Concomitant administration of digitalis was allowed only when steady state and constant HR were confirmed.
2.5 Clinical Measurements
Before the study, the following aspects of patient background were collected: demographic characteristics, anthropometry, site and details of surgery, underlying diseases and complications, past medical history, classification of cardiac function (NYHA), central venous pressure, pulmonary capillary wedge pressure, and course of treatment of SVT. In addition, electrocardiogram (ECG) and laboratory tests were performed. A 12-lead ECG was recorded before the study, unless it was an emergency situation, and at least a two-lead ECG was recorded for a minimum of 10 s during the study period; the RR interval, PQ interval, QRS duration, QT interval, and corrected QT interval (QTc) were measured. Blood pressures (systolic blood pressure [SBP] and diastolic blood pressure [DBP]), HR, and ECG were measured at specified time points before and during the study, during the 30-min follow-up period, during the additional infusion period of up to 6 h, and after completion of the additional infusion period.
2.6 Endpoints
When the targeted HR at any dose was achieved, the outcome was designated as ‘improved’. Improvement was defined as an HR reduction of more than 20 %. The percentages of patients obtaining the target HR were calculated by the following equation:
In this study, the cumulative improvement rate was designated as the primary endpoint in investigating the dose-dependent effectiveness of landiolol in patients with postoperative SVT. By contrast, the relative improvement rate, as well as HR, blood pressure, and ECG, were considered as secondary endpoints. Cardiac functions, including the cardiac output and cardiac index, were also evaluated.
2.7 Safety Variables
Subject symptom and objective findings (HR, blood pressure, ECG, laboratory test) were monitored and defined as adverse drug reactions (ADRs) by the site investigators.
2.8 Statistical Analysis
Summary statistics (percentages, means, standard deviation, maximum, minimum, etc.) were calculated for the demographic variables and baseline values. The primary endpoint was the cumulative improvement rate by doses, calculated as Kaplan–Meier estimates and 95 % confidence intervals (CI). Among the secondary endpoints were summary statistics for HR calculated at each time point (means and standard deviation); changes relative to the baseline values and the differences between values before and after administration at each dose were subjected to a paired t test. Summary statistics at each time point (mean and standard deviation) were also calculated for blood pressures (SBP and DBP) and ECG parameters (RR interval, PQ interval, QRS duration, QT interval, and QTc); changes relative to these baseline values were also calculated, and the differences between values before and after administration were compared at each dose, using a paired t test. Values of P < 0.05 were considered statistically significant (two-sided).
3 Results
3.1 Patient Characteristics
In this study, 108 patients from 60 study hospitals were eligible. Among them, 106 underwent landiolol infusion: two patients did not continue to exhibit tachycardia during the run-in period, and were consequently withdrawn from the study. Of the 106 patients treated with landiolol, an additional infusion of up to 6 h was begun in 16 patients, and eight of these patients underwent a complete 6-h infusion. The indications, exclusion criteria, and administration method were inappropriate for one patient, and another patient received another study drug during administration of landiolol; consequently, these two patients were excluded from analyses, including the effectiveness and safety evaluations. Thus, data from 104 patients were used for analysis. Of those patients, 16 were excluded from the effectiveness analysis due to violations of the entry criteria, exclusion criteria, or other criteria. Hence, data from 88 and 104 patients were used for the efficacy and safety analyses, respectively.
The demographic and other standard characteristics of the patients used for the efficacy analysis are shown in Table 1.
3.2 Efficacy
3.2.1 Bradycardic Effects
The criterion for an acceptable bradycardic effect was set at ≥20 % reduction in HR from the baseline value. At the LL dose, the relative improvement rate was 11.4 % (10 of 88 patients). In 78 patients in whom the bradycardic target was not achieved at the LL dose, an 11-min infusion at the L dose was initiated. Among those 78 patients, two were withdrawn from the study during the infusion at the L dose due to ADRs. The remaining 76 patients completed the 11-min infusion at the L dose, and the relative improvement rate was 23.7 % (18 of 76 patients). The M dose was completed in 55 of 58 patients, and the rate was 45.5 % (25 of 55 patients). The H dose was completed in 29 of 30 patients, and the rate was 65.5 % (19 of 29 patients). The cumulative improvement rate, which was the primary endpoint for effectiveness, was 11.4 % (95 % CI 4.7–18.0) at the LL dose, 32.4 % (95 % CI 22.5–42.2) at the L dose, 63.1 % (95 % CI 52.7–73.5) at the M dose, and 87.3 % (95 % CI 80.0–94.6) at the H dose, demonstrating the dose-dependent effectiveness of landiolol. The relative improvement rate by dose and the cumulative improvement rate are shown in Fig. 2a.
Dose-dependent effects of landiolol on the improvement rate (a) and the incidence of ADRs (b). ‘Cumulative’ rate or incidence is a percentage calculated from the number of responders at a given dosage or a lower dosage, divided by the number of patients analyzed. ‘Relative’ rate or incidence is a percentage calculated from the number of responders at a given dosage, divided by the number of patients analyzed and treated at that dosage. ADRs adverse drug reactions. Refer to fig. 1 for dose and infusion rates
3.2.2 Heart Rate and Blood Pressure Changes by Dose
Changes in HR (% reduction from the baseline HR) and blood pressure (SBP and DBP) are shown by dose in Table 2. Among the 88 patients whose data was used in the efficacy analysis, HR data were available for 82 patients, and blood pressure data were available for 80 patients; these data were used in the analysis. HR was reduced by 10.0 % (LL dose) to 20.9 % (H dose) in a dose-dependent manner from a mean baseline HR of 128.4 beats/min, and a significant bradycardic effect relative to the baseline HR was observed at all doses (P = 0.0001 at all doses). The mean differences in SBP were −8.0 mmHg (LL dose) to −12.2 mmHg (H dose), and a significant decrease in SBP relative to the baseline was observed at all doses (P = 0.0001 at all doses). The mean differences in DBP were −2.8 mmHg (LL dose) to −6.6 mmHg (H dose), and a significant decrease in DBP relative to the baseline was observed at all doses (LL dose, P = 0.0008; L dose, P = 0.0002; M dose, P = 0.0001; H dose, P = 0.0024).
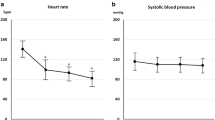
3.2.3 Time Course of Changes in Heart Rate and Blood Pressure
Time courses of changes in measured values of HR and blood pressure are shown in Fig. 3. HR decreased to a mean of 98.0 beats/min at the time of completion of administration, with the decrease occurring in both dose-dependent and time-dependent manners. HR then increased with time after completion of administration, reaching 115.2 beats/min 30 min after completion of administration. Both SBP and DBP also increased with time after completion of administration: 30 min after completion, the values were comparable to the respective baseline values.
3.2.4 ECG Parameters
The RR interval, PQ interval, QRS duration, and QT interval were significantly prolonged (P = 0.0001, 0.0002, 0.0181, and 0.0001, respectively), compared with the baseline parameters. However, no significant prolongation of QTc was observed.
3.2.5 Cardiac Function
The cardiac output (mean ± SD) significantly decreased from 6.2 ± 1.9 L/min before administration to 5.4 ± 1.6 L/min during administration (paired t test: P = 0.0116), and returned to 5.8 ± 1.5 L/min after completion of administration. The cardiac index significantly decreased from 3.7 ± 1.0 L/min/m2 before administration to 3.1 ± 0.6 L/min/m2 during administration (paired t test: P = 0.0063), and recovered to a mean of 3.4 ± 0.7 L/min/m2 after completion of administration. No significant changes were observed in pulmonary capillary pressure or central venous pressure.
3.2.6 Changes in Heart Rate and Blood Pressure during the Maintenance Period of Up to 6 h
Changes in HR and blood pressure during the maintenance period are shown in Fig. 4. Of the 104 patients analyzed, 16 underwent an additional infusion of landiolol (beyond the four-dose escalation period), and the duration (mean ± SD) of administration for this maintenance period was 259.8 ± 131.5 min. HR remained at about 100 beats/min during this period, and increased to a mean of 110.8 beats/min 30 min after completion of administration. The mean dosages used during the maintenance period were 0.0117–0.0184 mg/kg/min; this range corresponds to the L and M doses during the dose-escalation period. SBP was in the range of 112.1–130.0 mmHg, and DBP varied between 54.1 and 70.4 mmHg, during the maintenance period.
Changes in HR (a), BP (b), and landiolol dose (c) during and after the maintenance period. Data are mean ± SD. C the time of completion of administration, DBP diastolic blood pressure, HR heart rate, Pre before administration (baseline), SBP systolic blood pressure, SD standard deviation. Refer to fig. 1 for dose and infusion rates
3.3 Safety
3.3.1 Incidence of Adverse Drug Reactions
The relative incidence of ADRs as determined by subjective symptoms and objective findings was 5.8 % (6 of 103 patients) at the LL dose, 7.8 % (7 of 90 patients) at the L dose, 8.6 % (6 of 70 patients) at the M dose, and 12.5 % (5 of 40 patients) at the H dose. The cumulative incidence of ADRs increased in a dose-dependent manner: 5.8 % at the LL dose, 13.1 % at the L dose, 20.6 % at the M dose, and 30.5 % at the H dose (Fig. 2b).
3.3.2 Details of Adverse Drug Reactions
Subjective symptoms and objective findings were defined as ADRs, and a total of 27 ADR events were observed: six, eight, eight, and five events at the LL, L, M, and H doses, respectively. Among these events, the most frequently reported ADR was hypotension (blood pressure ≤90/60 mmHg), which occurred in 23 cases (four at the LL dose, seven at the L dose, seven at the M dose, and five at the H dose). The remaining four ADRs were bradycardia and right-bundle branch block at the LL dose, respiratory distress at the L dose, and hypoxemia at the M dose. In terms of severity, 15 of the ADRs were judged to be mild, ten were moderate, and two were severe, with no apparent dose-dependent increase in severity. In the case of bradycardia that developed at the LL dose, an approximately 5-s cardiac arrest occurred in a patient with SSS; this was considered to be a serious adverse event.
4 Discussion
The importance of β-blockers for treatment of various cardiac diseases has been established. In particular, ultra-short-acting β1-selective blockers are very effective for acute-stage coronary vascular disorders and for postoperative SVT [13], in which involvement of catecholamines through sympathetic nerve excitation has been suggested [1–4]. Esmolol is the first ultra-short-acting β1-selective blocker to be developed, and it is now established as an SVT-treatment drug that is easily regulated, due to its short half-life (about 9 min) [23]. Landiolol, the drug used in this study, is a new β1-blocker with an elimination half-life of 3–4 min in human blood [18, 19]. Landiolol exhibits approximately ninefold more β1-blocking effect in vivo than esmolol, and about eightfold more heart selectivity in vitro [24]. Consequently, landiolol is expected to be of considerable clinical utility.
In this study, administration of landiolol was initiated as a 1-min loading infusion at 0.015 mg/kg/min, followed by a 10-min infusion at 0.005 mg/kg/min (the LL dose). An open-label study was performed using a four-dose titration regimen, in which stepwise increases of the LL dose to the L, M, and H doses were performed until ≥20 % reduction of HR from the baseline value was achieved, at which time the dose escalation was stopped. The cumulative improvement rate, which is the percentage of patients obtaining the bradycardic target (≥20 % reduction of HR) was 11.4 % at the LL dose and increased to 87.3 % at the H dose, demonstrating a dose-dependent effect. In terms of the mean slowing of HR, a significant bradycardic effect relative to the baseline HR was observed at all doses, and HR increased with time after completion of administration. SBP and DBP also significantly decreased at all doses compared with their respective values before administration. HR decreased with an increase in dose, and increased with time after completion of administration. Blood pressure slightly decreased at all doses, but increased with time after completion of administration.
The cumulative incidence of ADRs increased in a dose-dependent manner from 5.8 % at the LL dose to 30.5 % at the H dose, but the severity of the ADRs did not increase in a dose-dependent manner. Furthermore, the most frequently reported ADR was hypotension (≥20 % reduction in blood pressure from the baseline value and a value ≤90/60 mmHg), and all cases were rapidly resolved or remitted with or without intervention. Together, these observations confirm that landiolol has no associated safety problems at doses up to the H dose. One serious ADR, bradycardia with cardiac arrest, was reported at the LL dose in a patient with SSS. Because the cardiac conduction system is suppressed by a β-blocker, and symptoms may be aggravated, any treatment with a β-blocker is contraindicated in patients with bradyarrhythmia, including atrioventricular block (II or severe) and SSS. Although the exclusion criteria were established such that patients with SSS would not be enrolled in this study, two patients with SSS were nonetheless enrolled and received landiolol. Hence, pre-treatment examinations and medical interviews are important in order to confirm a patient’s diagnosis prior to clinical use of landiolol. No ADRs occurred in the second patient with SSS.
Because landiolol can be optionally administered continuously for a maximum of 6 h, a maintenance period for up to 6 h was used to evaluate maintenance of the target HR; 16 patients underwent this maintenance period. In eight patients, the drug was continuously administered at a dose between those of the LL and H doses for 6 h, and it was possible to maintain the target HR by changing the dose during the period of continuous administration. HR increased in a time-dependent manner after completion of drug administration, reflecting the pharmacological profile of landiolol as an ultra-short-acting β1-selective blocker.
Because we did not include a placebo group, this study design could not reveal the natural progress of HR and other parameters measured in this study. Therefore, it would be difficult to obtain a definitive evaluation of this drug from the results of this study. However, because the study was performed in accordance with real clinical practice, these results should be considered as preliminary data for the next round of placebo-controlled studies.
5 Conclusion
Landiolol exhibited a rapid bradycardic effect in patients with postoperative SVT. This effect was dose-dependent, and HR returned to normal levels within 30 min after completion of administration. Our data suggest that landiolol can be used safely at doses of up to 0.04 mg/kg/min for 10 min, with a loading infusion at 0.125 mg/kg/min for 1 min.
References
Halter JB, Pflug AE, Porte D. Mechanism of plasma catecholamine increases during surgical stress in man. J Clin Endocrinol Metab. 1977;45:936–44.
Kalman JM, Munawar M, Howes LG, Louis WJ, Buxton BF, Gutteridge G, et al. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann Thorac Surg. 1995;60:1709–15.
Breslow MJ, Jordan DA, Christopherson R, Rosenfeld B, Miller CF, Hanley DF, et al. Epidural morphine decreases postoperative hypertension by attenuating sympathetic nervous system hyperactivity. JAMA. 1989;261:3577–81.
Reves JG, Karp RB, Buttner EE, Tosone S, Smith LR, Samuelson PN, et al. Neuronal and adrenomedullary catecholamine release in response to cardiopulmonary bypass in man. Circulation. 1982;66(1):49–55.
Leitch JW, Thomson D, Baird DK, Harris PJ. The importance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1990;100:338–42.
Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–49.
Hashimoto K, Ilstrup DM, Schaff HV. Influence of clinical and hemodynamic variables on risk of supraventricular tachycardia after coronary artery bypass. J Thorac Cardiovasc Surg. 1991;101:56–65.
Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVilet M, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–7.
Olshansky B. Management of atrial fibrillation after coronary artery bypass graft. Am J Cardiol. 1996;78(8A):27–34.
Motohara T, Mayumi T, Kuroshima S, Takahashi T, Okushiba S, Kato H. Perioperative management for patients having cardiac disease—esophageal disease. ICU & CCU. 1995;19(7):575–81 (Japanese).
Matloff JM, Wolfson S, Gorlin R, Harken DE. Control of postcardiac surgical tachycardias with propranolol. Circulation. 1968;37(4 Suppl):II-133–8.
Balser JR, Martinez EA, Winters BD, Perdue PW, Clarke AW, Huang W, et al. β-Adrenergic blockade accelerates conversion of postoperative supraventricular tachyarrhythmias. Anesthesiology. 1998;89:1052–9.
Gray RJ, Bateman TM, Czer LSC, Conklin CM, Matloff JM. Esmolol: a new ultrashort-acting beta-adrenergic blocking agent for rapid control of HR in postoperative supraventricular tachyarrhythmias. J Am Coll Cardiol. 1985;5:1451–6.
Mooss AN, Wurdeman RL, Mohiuddin SM, Reyes AP, Sugimoto JT, Scott W, et al. Esmolol versus diltiazem in the treatment of postoperative atrial fibrillation/atrial flutter after open heart surgery. Am Heart J. 2000;140:176–80.
Daoud EG. Management of atrial fibrillation in the post-cardiac surgery setting. Cardiol Clin. 2004;22:159–66.
Andrews TC, Reimold SC, Berlin JA, Antman EM. Prevention of supraventricular arrhythmias after coronary artery bypass surgery: a meta-analysis of randomized control trials. Circulation. 1991;84(3 Suppl):III-236–44.
Kowey PR, Taylor JE, Rials SJ, Marinchak RA. Meta-analysis of the effectiveness of prophylactic drug therapy in preventing supraventricular arrhythmia early after coronary artery bypass grafting. Am J Cardiol. 1992;69:963–5.
Nakashima M, Kanamaru M. Phase I study of ONO-1101, a new ultra short acting β1-blocking agent in healthy volunteers. Rinshoiyaku. 2000;16(10):1531–56 (Japanese).
Shiroya T, Ichioka Y, Yosida K, Nishijima K, Oomawari N, Naka M, et al. Pharmacological studies of ONO-1101 as a β blocking agent with high β1 selectivity and ultra-short duration of action. Kiso to Rinsho. 1997;31(9):2913–23 (Japanese).
Yoshiya I, Ogawa R, Okumura F, Shimada Y, Hanaoka K. Clinical evaluation of landiolol hydrochloride (ONO-1101) on perioperative supraventricular tachyarrhythmia—a phase III, double-blind study in comparison with placebo. Rinshoiyaku. 1997;13(19):4949–78 (Japanese).
Yoshiya I, Ogawa R, Okumura F, Shimada Y, Kuro M. Clinical evaluation of landiolol hydrochloride (ONO-1101) on perioperative supraventricular tachyarrhythmia in patients with hypertension or cardiac ischemia—double-blind study in comparison with placebo. Rinshoiyaku. 2002;18(9):1049–76 (Japanese).
Atarashi H, Kuruma A, Yashima M, Saitoh H, Ino T, Endoh Y, et al. Pharmacokinetics of landiolol hydrochloride, a new ultra-short-acting β-blocker, in patients with cardiac arrhythmias. Clin Pharmacol Ther. 2000;68:143–50.
Sum CY, Yacobi A, Kartzinel R, Stampfli H, Davis CS, Lai CM. Kinetics of esmolol, an ultra-short-acting beta blocker, and of its major metabolite. Clin Pharmacol Ther. 1983;34:427–34.
Iguchi S, Iwamura H, Nishizaki M, Hayashi A, Senokuchi K, Kobayashi K, et al. Development of a highly cardioselective ultra short-acting β-blocker, ONO-1101. Chem Pharm Bull (Tokyo). 1992;40:1462–9.
Acknowledgments
The authors thank all trial members, investigators, and study sites for their participation in this study. This study was supported by Ono Pharmaceutical Co., Ltd, Osaka, Japan.
Conflicts of interest
N.T. has no conflicts of interest to declare. S.K. is an employee of Ono Pharma USA, Inc. The authors thank all trial members, investigators, and study sites for their participation in this study.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Trial members, Investigators, and Study Sites are as follows.
Trial members: Jun Takezawa, Shu Matsukawa, Kimitaka Tajimi, Hisashi Sugimoto, Tsuyoshi Maekawa, Hideki Origasa, Keisuke Amaha.
Investigators and Study Sites: Takahiro Ichimiya, Asahikawa City Hospital; Hidetoshi Aoki, Asahikawa City Hospital; Masao Hosokawa, Keiyukai Sapporo Hospital; Kazuhiro Myojin, National Sapporo Hospital; Akitomo Matsuki, Hirosaki University School of Medicine and Hospital; Kohei Kawazoe, Iwate Medical University Hospital; Yasuhiko Hashimoto, Tohoku University Hospital; Kunihiko Hoshi, Furukawa City Hospital; Yasunori Watanabe, Hitachi General Hospital; Toshio Mitsui, University of Tsukuba Hospital; Katsuo Fuse, Jichi Medical University Hospital; Minoru Nakano, Maebashi Red Cross Hospital; Fumio Kunimoto, Gunma University Hospital; Hideshige Shihara, Fukaya Red Cross Hospital; Takashi Yamada, Dokkyo Koshigaya Hospital; Norimasa Seo, Omiya Medical Center, Jichi Medical School; Tatsuumi Sasaki, Saitama Ohara Cardiovascular Center; Hirokazu Murayama, Chiba Prefectural Cardiopulmonary Center Tsurumai Hospital; Takeshi Kasai, Emergency and Trauma Center, Kameda General Hospital; Akihiro Konishi, New Tokyo Hospital; Nariyuki Hayashi, Nihon University Itabashi Hospital; Keiichi Katoh, Japanese Red Cross Medical Center; Hiroyuki Ohsawa, Tokyo Metropolitan Police Hospital; Miyuki Yokota, Cancer Institute Hospital; Kunihiro Mashiko, Nippon Medical School Hospital; Yasuyuki Hosoda, Juntendo University Hospital; Yoshinobu Sumiyama, Toho University, Medical Center Ohashi Hospital; Yoshihiro Yagishita, International Medical Center of Japan; Mamoru Takiguchi, Tokai University Hospital; Tadashi Aoki, St. Marianna University School of Medicine Hospital; Akito Ohmura, Teikyo University School of medicine University Hospital, Mizonokuchi; Shoji Eguchi, Niigata University Hospital; Tsutomu Kobayashi, Kanazawa University Hospital; Takahiro Takemura, National East Nagano Hospital; Kousuke Baba, Hokushin General Hospital; Shuji Dohi, Gifu University Hospital; Kazuyuki Ikeda, Hamamatsu University School of Medicine, University Hospital; Yoshito Shiraishi, Shizuoka General Hospital; Jun Takezawa, Nagoya University Hospital; Jyurou Hotta, Japanese Red Cross Nagoya Daiichi Hospital; Masashi Ueyama, Social Insurance Chukyo Hospital; Shuichiro Sugimura, Fujita Health University Hospital; Kazuo Maruyama, Mie University Hospital; Yoshifumi Tanaka, University Hospital, Kyoto Prefectural University of Medicine; Akira Asada, Osaka City University Hospital; Ikuto Yoshiya, Osaka University Hospital; Hisashi Sugimoto, Osaka University Hospital; Makoto Satani, Osaka City General Hospital; Junichiro Yokota, Osaka Prefectural Senshu Critical Care Medical Center; Hidefumi Obara, Kobe University Hospital; Chikara Tashiro, Hyogo College of Medicine Hospital; Tatsuya Sugino, Hyogo Prefectural Nishinomiya Hospital; Masahiro Shinozaki, Wakayama Medical University Hospital; Toru Sato, Tottori University Hospital; Akitomo Yonei, Kurashiki Central Hospital; Tsuyoshi Maekawa, Yamaguchi University Hospital; Yoshitoyo Miyauchi, Tokuyama Central Hospital; Yasutoshi Matayoshi, Yamaguchi Prefectural Central Hospital; Shuzo Oshita, Tokushima University Hospital; Kenji Oguli, Kagawa Medical University Hospital; Tatsuru Arai, Ehime University Hospital; Kingo Nishiyama, Kochi Red Cross Hospital; Akinori Zaitsu, Kyushu University Hospital; Hisataka Yasui, Kyushu University Hospital; Sumitaka Haseba, Nagasaki University Hospital; Kazufumi Okamoto, Kumamoto University Hospital; Takayuki Noguchi, Oita Medical University Hospital; Toru Fujigaki, Oita Prefectural Hospital; Nozomu Yoshimura, Kagoshima University Hospital.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Taenaka, N., Kikawa, S. Dose-Dependent Effect of Landiolol, a New Ultra-Short-Acting β1-Blocker, on Supraventricular Tachyarrhythmias in Postoperative Patients. Clin Drug Investig 33, 505–514 (2013). https://doi.org/10.1007/s40261-013-0093-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0093-x