Abstract
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infection (LRTI) in children, causing approximately 3.6 million hospitalizations per year, and has been associated with long-term pulmonary sequelae for up to 30 years after infection, yet preventative strategies and active treatment options remain elusive. The associated morbidity and healthcare related costs could be decreased substantially with the development of these much-needed medications. After an initial false start in the development of an RSV vaccine, gradual progress is now being made with the development of multiple vaccine candidates using numerous different mechanisms of action. Furthermore, nirsevimab, a new monoclonal antibody for the prevention of RSV, has recently been registered in the European Union. New novel treatments for RSV infection are also in the pipeline, which would provide the clinician with much needed ammunition in the management of the acute disease. The next few years have the potential to change the landscape of LRTI forever through the prevention and management of RSV LRTI and thereby decrease the mortality and morbidity associated with it. In this review, we discuss these new approaches, current research, and clinical trials in monoclonal antibody and vaccine development against RSV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Respiratory syncytial virus (RSV) is the most common cause of respiratory tract infections in children. |
There are currently no licensed vaccinations for preventing RSV infection, and only one licensed medication for the prevention of disease. |
Steady progress is being made in developing new therapeutics and further treatments should be available in the near future. |
1 Introduction
Lower respiratory tract infections (LRTIs) are the leading cause of death in children in the 1- to 59-month age group, accounting for approximately 653,000 (12.1% of total) annual childhood deaths globally [1, 2]. Viruses are the most common cause of LRTIs in young children, accounting for approximately 60% of cases in US and Australian children younger than 18 years with radiographic evidence of community acquired pneumonia (CAP) [3, 4]. The importance of viral pathogens, and particularly respiratory syncytial virus (RSV), in the pathogenesis of CAP was also highlighted in the Pneumonia Etiology Research for Child Health (PERCH) study, a multicenter case-control study reporting on the etiology of severe and very severe pneumonia in children (1–59 months of age) in seven low-resourced settings [5]. Viral pathogens (61.4%) were more commonly attributed as the cause of LRTIs than bacterial pathogens (27.3%) in pneumonia cases, with RSV being the most common cause of LRTIs (31.1%). Notably, only a small percentage of pneumonia cases were attributed to bacterial pathogens that were major causes of fatal pneumonia prior to the routine immunization of children against these organisms. The success of reducing the burden of pneumonia mortality and morbidity in children through vaccination against Haemophilus influenzae type b, Streptococcus pneumoniae, and Bordetella pertussis highlight the potential of the development of vaccines in further reducing childhood morbidity and mortality due to LRTI.
Furthermore, LRTI causes a substantial burden of disease in adults, especially in the elderly, causing approximately 1.2 million deaths in 2015 [6]. The contribution of RSV to LRTI and to LRTI hospitalization increases with increasing age, and accounts for 2.5–5.0 admissions/10,000 persons per year in adults over 65 years of age, making them an attractive target for further RSV vaccine development [7, 8].
2 Epidemiology of Respiratory Syncytial Virus (RSV)
RSV is transmitted through airborne droplet spread or direct contact with fomites from contaminated surfaces. Inoculation is usually through the nasopharyngeal mucosa or the conjunctival membranes [9]. The mean incubation in the nasopharynx is 5 days, after which the virus spreads via intracellular transmission, cilial motion, or aspiration of nasopharyngeal secretions to the rest of the airways [9,10,11,12]. RSV displays a direct cytopathic effect on the host’s airway epithelial cells characterized by epithelial destruction and loss of ciliary motion, as well as a multitude of indirect effects mitigated by the host’s own immune response [13].
RSV causes a wide range of respiratory tract infections ranging from asymptomatic upper respiratory tract infection to severe LRTI requiring hospitalization, and death [14, 15]. The clinical syndrome of bronchiolitis is the most common serious disease manifestation; the majority occurring in healthy full-term infants during the first year of life [14].
Both the magnitude and intensity of infection, as well as the host response to RSV infection, determine the severity of the disease [16]. Risk factors for more severe RSV disease can be divided into host, environmental, and viral factors. Host factors include the presence of co-existing medical conditions such as prematurity, congenital cardiac disease with increased pulmonary blood flow, chronic lung diseases, including bronchopulmonary dysplasia (BPD), and primary or secondary immunological suppression, including HIV infection, as well as male sex and age < 6 months at the time of RSV infection [17,18,19,20,21,22]. Demographic and environmental risk factors include low socioeconomic status, household crowding, presence of school-age siblings, crèche attendance, duration of initial breastfeeding for < 2 months, and indoor tobacco smoke exposure [17, 18, 23, 24].
Most children are infected with RSV during the first year of life, and virtually all by two years of age [25, 26]. Re-infection occurs frequently during the first few years of life, and then every 3–10 years throughout life, but these infections tend to diminish in severity [27, 28].
In 2019, it was estimated that there were 33.0 million RSV-associated acute LRTIs, 3.6 million RSV-associated acute LRTI hospital admissions, and approximately 66,000–190,000 RSV-attributable deaths in children aged < 60 months. Whereas the rate of RSV hospitalization is similar in children living in high-income countries (1.4/1000) compared with those living in low- and middle-income countries (LMICs; 0.4–2.2/1000), more than 95% of deaths transpire in LMICs, including 45% occurring outside of health facilities [29, 30]. Furthermore, RSV LRTI during infancy may also predispose to long-term pulmonary sequelae. A number of studies report increased odds of recurrent wheezing episodes up to 10 years of age after RSV LRTI. A systematic review of the pulmonary function sequelae after RSV LRTI during the first 3 years of life concluded that obstructive lung disease without a bronchodilator response is the most common pulmonary function abnormality, with effects lasting until 30 years of age, albeit heterogeneity in findings across studies [31,32,33,34,35,36,37,38,39,40,41].
A further high-risk group that warrants discussion is the elderly (> 65 years of age), accounting for the majority of RSV-associated deaths in high-income countries, and even though the disease is often milder in adults than in childhood, increased underlying comorbidities and frailty in this group leads to an increase in severe disease [7, 42].
3 Microbiology and Structure of RSV
Human RSV, then called Chimpanzee Coryza Agent, was first described after being isolated from the upper respiratory tract of a chimpanzee in 1955 [43]. Subsequently, in 1956, it was isolated from humans and identified as a virus associated with bronchiolitis in children [44]. RSV has recently (2016) been reclassified as an orthopneumovirus, in the Pneumoviridae family, within the Mononegavirales order [45].
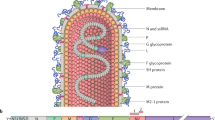
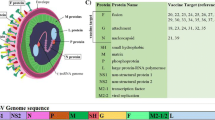
RSV is a single-stranded, negative-sense ribonucleic acid (RNA) virus that is enveloped by a host plasma membrane-derived lipid bilayer. The 15.2 kilo-base pair non-segmented and tightly encapsidated genome contains 10 genes that encode for 11 proteins, three of which are transmembrane glycoproteins: attachment protein (G protein), fusion protein (F protein), and small hydrophobic (SH) protein [46, 47]. Other virus proteins are the ribonucleocapsid and regulatory (large polymerase protein [L], matrix [M2.1 + M2.2], nucleoprotein [N] and phosphoprotein [P]), inner envelope (matrix [M]), and nonstructural proteins (NS1 + NS2) [48].
The G protein was first described as an attachment protein in 1987 [49]. It is a carbohydrate-rich (60%), heavy glycosylated protein structure that is present in a membrane form that mediates the binding of RSV to the respiratory epithelial cell, as well as a secreted form [50,51,52]. The full-length transmembrane form is a type II integral membrane protein and is the most variable structural protein, but does contain a 26 amino acid central conserved domain (CCD) that is not glycosylated and has a central role in the pathogenesis of the RSV infection [53,54,55,56]. The G protein is a less efficient neutralization antigen than the F protein [56]. The F protein is a type I integral membrane glycoprotein that mediates viral penetration into the cell and mitigates fusion between viral and cell membranes and infected neighboring cells [53]. It is highly conserved. The F protein has two unique conformations, a stable pre-fusion structure, and after binding to its host cell, a highly stable post-fusion structure [53, 57]. The F protein has six main antigenic epitopes on its surface (Ø and I–V). Antigenic epitope sites Ø, III and V are only exposed during the pre-fusion F protein conformation, while I, II and IV are exposed on both the pre- and post-fusion F protein conformations [53, 57, 58]. The number of exposed epitopes, and that the F protein is required for cell penetration and is highly genetically conserved, makes the F protein the main target for interventions such as vaccines and monoclonal antibodies targeting RSV. Epitopes II and IV are the main neutralizing epitopes, and while they do not prevent viral attachment into the affected cells, they effectively block fusion of the viral and host cellular membranes [58]. Epitope site II is the target antigen for the monoclonal antibodies palivizumab and motavizumab, while suptavumab and clesrovimab target epitope site IV.
RSV is classified into two antigenic subtypes, RSV-A and RSV-B, based on the reactivity of monoclonal antibodies directed at the G protein antigenic epitopes [55, 59]. There is a shift in the dominant circulating subtype (RSV-A or RSV-B) over cycles of one to two seasons [60, 61]. Numerous genotypes, which can co-circulate during the same RSV season, have been identified within each subtype, with dominant genotype changes in successive years [60, 62].
4 Immunology of RSV
Human T cells are essential for the resolution of acute RSV infection, as well as for the acquisition of specific immunological memory, resulting in the production of RSV-specific antibodies and production of RSV-specific T cells; this results in future infection being clinically attenuated with decreasing likelihood of severe disease [63]. CD4+ T cells stimulate B-cell antibody production, and CD8+ T cells are cytopathic to RSV-infected cells, as well as regulate the inflammatory response secondary to the virus through inhibition of the cytokine response [64]. Dysregulation of this process can lead to an unopposed inflammatory response to RSV and more severe disease, as well as enhanced disease post-vaccination, as observed after the administration of a formalin inactivated RSV vaccine to infants in 1966 [65]. Serum neutralizing antibodies against RSV are associated with a reduced risk of RSV infection progressing to LRTI, as manifested by passive immunization with monoclonal antibody (palivizumab) directed at the F-protein, which confers protection against RSV LRTI. Furthermore, maternally acquired RSV antibody has also variably been associated with a lower risk of RSV during early infancy [66]. Nevertheless, infection-induced protective antibodies against RSV are transient [67, 68]
RSV prevents an effective host immune response by a multitude of mechanisms, including inhibition of interferon responses by NS1+NS2 (non-structural proteins) [69], the binding of protein F to toll-like receptor 4 (TLR4) with interference in the TLR signaling pathway [70], and secretory protein G binding to CX3CR1 (fractalkine) and altering chemotaxis, as well as acting as a decoy for antibody binding [50, 71].
The RSV G and F proteins harbor epitopes that can elicit neutralizing antibody following RSV infection or through vaccines. Protection against RSV LRTI is conferred mainly by neutralizing antibodies, with a positive correlation between high titers of serum neutralizing antibodies and protection against RSV LRTI, and an inverse correlation with risk of progression from infection to LRTI in children [25, 28].
Maternal antibodies, including RSV-specific antibodies, are transferred to the fetus during the latter stages of pregnancy and to their offspring via breast milk [72, 73]. Lower efficiency of transplacental antibody transfer in earlier gestation could contribute to the higher risk of severe RSV LRTI in infants born preterm. Transplacental-acquired antibodies wane over the course of 3–5 months in the infant. Furthermore, immaturity of the immune system during early infancy, is further accentuated in infants born prematurely [74, 75].
5 Treatment of RSV Infection
Although multiple treatment modalities have been attempted to manage acute RSV LRTIs, including nebulized hypertonic saline, inhaled, nebulized, or intravenous β2-agonists, nebulized adrenalin, nebulized ipratropium bromide, montelukast, and inhaled, oral, intramuscular or intravenous corticosteroids, none have been shown to be effective in treating RSV [76,77,78,79,80,81,82,83,84,85,86,87]. Consequently, the management of RSV LRTIs is mainly supportive, with oxygen therapy and nutritional support.
Antiviral drugs against RSV include ribavirin, a broad-spectrum antiviral guanosine analog [88]. In a recent systematic review, analysis of the pooled data concluded that there was no difference in mortality between individuals treated with ribavirin compared with those receiving supportive care; however in subgroup analysis in subjects with hematological disease, ribavirin significantly decreased mortality [89]. The use of ribavirin is further complicated by the cost, complicated delivery, and adverse effect profile and therefore is not regularly administered [88].
Novel drug and treatment therapies are constantly being sought, including a trivalent nanobody that binds antigenic epitope site II of the F protein (ALX-0171), fusion inhibitors (GS-5806, JNJ-53718678, BTA-C585 +AK-0529), a non-fusion N-protein inhibitor (EDP-938), and RSV polymerase inhibitors (ALS-008176 + PC768) [90,91,92,93,94].
Based on the limited therapeutic options available for the management of RSV LRTIs, other than supportive care for symptoms, the focus has been prevention of RSV LRTIs either by way of passive or active immunization.
6 Passive Immunization
Passive immunization involves administration of antibodies targeted against a pathogen, which is used in instances of individuals requiring immediate protection, or where they are unable to timeously produce antibodies, as in newborn babies with an immature immune system, or in individuals with underlying immunodeficiencies. Prevention of RSV through passive immunization involves administration of polyclonal or monoclonal RSV-neutralizing antibodies (Table 1).
RSV immune globulin intravenously (RSV-IGIV) was the first commercially available preparation (1992) and consisted of purified polyclonal antibodies sourced from donors with high-titer RSV-neutralizing activity [100]. Monthly high-dose RSV-IGIV administration in children (n = 81) < 2 years of age (mean age 8 months) at high-risk for developing severe RSV disease, i.e. with either prematurity, BPD or congenital heart disease, was associated with 62%, 72%, 63% and 92% risk reduction of RSV LRTI, severe RSV LRTI, RSV hospitalization, and duration of RSV-associated intensive care unit (ICU) admission, respectively [101, 102]. A large follow-up, randomized, placebo-controlled study of RSV-IGIV in 54 centers across America (PREVENT trial) in children with a history of prematurity and/or BPD reported a 41%, 54%, 53% and 60% reduction in RSV-associated hospitalization, moderate or severe LRTI hospitalization, duration of RSV hospital stay and days requiring supplemental oxygen, respectively. However, RSV-IVIG failed to reach its primary efficacy endpoint in a randomized controlled trial in children with congenital heart disease (CARDIAC trial) and resulted in an increased number of cyanotic spells and poor post-surgery outcomes in cases [103]. Therefore, RSV-IVIG was only indicated in premature children or those with chronic lung disease, and was eventually withdrawn with the subsequent development of palivizumab [104].
Palivizumab is a humanized monoclonal antibody directed at RSV Protein F site II epitope [105]. The IMPact-RSV trial, a randomized, double-blind, placebo-controlled trial in 139 centers across America, Canada and the UK, randomized 1502 children with prematurity or bronchopulmonary dysplasia to either palivizumab or placebo monthly for 5 months (Table 1) [95]. Palivizumab was efficacious against RSV hospitalization (78% risk reduction in children with prematurity and no BPD, and 39% risk reduction in children with prematurity and BPD). Furthermore, when compared with placebo, palivizumab was associated with a reduced number of days for RSV admission, fewer days on supplemental oxygen, and fewer admissions to the ICU.
Palivizumab is licensed for use in children born at < 36 weeks gestation and younger than six months of age at the beginning of the RSV season, or children < 2 years of age requiring treatment for BPD within the past six months or with a hemodynamically significant congenital heart disease, although regional guidelines vary significantly [107]. The high cost of palivizumab, approximately $1700–$12,500 per patient per RSV season in the US, depending on patient birth month and dosing regimen, has resulted in more restrictive use even in high-income countries (HICs), and it being largely inaccessible in LMICs [106]. In 2014, the American Academy of Pediatrics updated their recommendations for the administration of palivizumab, limiting its recommended use only to those in the high-risk groups for severe RSV LRTI, defined as infants born < 29 weeks gestation and younger than one year at the start of the RSV season, infants < 32 weeks gestation with chronic lung disease of infancy during the first year of life, and infants younger than one year with a hemodynamically significant congenital heart lesion [107]. Furthermore, palivizumab may also be considered in severely immunocompromised children during the first two years of life and in children unable to clear pulmonary secretions, such as those with neuromuscular diseases or abnormal pulmonary anatomy, during the first year of life. Palivizumab has not been approved for prophylaxis against RSV illness in elderly patients, especially in those > 65 years of age, a group that is prone to more severe infection, with prolonged hospitalization and increased mortality [108].
The development of the next-generation monoclonal antibodies was facilitated through the introduction of a triple YTE mutation (M252Y/S254T/T256E) into the Fc portion of the antibody (IgG), thereby enhancing the binding to the Fc receptor, resulting in a fourfold increase in the half-life of the molecule and possibly only necessitating a single dose that could confer protection through the average RSV epidemic period, which usually lasts 5–6 months [109, 110].
Palivizumab was modified by in vitro affinity maturation to create motavizumab, a monoclonal antibody, which, compared with palivizumab, has 70 times the affinity for the RSV F protein and 20 times higher in vitro neutralization activity [111]. In a phase III, randomized, double-blind study comparing motavizumab (n = 3329) with palivizumab (n = 3306), in children born before 36 weeks gestation and either < 6 months of age or < 2 years of age having received treatment for chronic lung disease in the past six months, motavizumab was non-inferior to palivizumab. Compared with palivizumab, motavizumab was associated with a 26% and 50% relative risk reduction of RSV hospitalization and medically attended acute LRTI, respectively [96]. However, motavizumab was associated with an increase in cutaneous hypersensitivity reactions in recipients, subsequently leading to it not being licensed [112].
Suptavumab, a monoclonal antibody that targets the F protein prefusion site IV epitope, was discontinued following the results of a phase III efficacy trial in healthy preterm infants < 6 months of age, which failed to show protection against RSV-associated hospitalization or outpatient illness, despite being effective against select RSV-A isolates [98]. This was attributed to a genetic strain of RSV-B that harbored mutations of the epitope to which suptavumab was directed.
Nirsevimab is a recombinant human IgG1 monoclonal antibody with affinity for the highly conserved site Ø of the prefusion RSV F protein, and can be administered intramuscularly as a single dose before the RSV season [113]. In a phase IIb randomized, placebo-controlled trial of nirsevimab in premature infants (29 weeks to < 37 weeks gestation) without other underlying comorbidities, a single dose of nirsevimab, administered before the start of the RSV season, resulted in 70.1% (95% confidence interval [CI] 52.3–81.2) vaccine efficacy against medically attended RSV-LRTI, and a 78.4% (95% CI 51.9–90.3) lower rate of RSV LRTI hospitalization [114]. In a further randomized, placebo-controlled trial of nirsevimab in late–preterm (> 34 weeks gestation) and term infants (MELODY trial), the efficacy of nirsevimab was 76.4% (95% CI 62.3–85.2) against medically attended RSV LRTI and 76.8% (95% CI 49.4 to 89.4) against hospitalization for RSV LRTI through to 150 days post-enrolment [99, 115]. Furthermore, nirsevimab may confer protection beyond 180 days, including through the second RSV season up to 510 days post-enrolment, as indicated by a 43% lower risk of medically attended RSV LRTI in the nirsevimab group compared with the placebo group between 361 and 511 days [116]. In a pooled analysis of the phase IIb and phase III trials, nirsevimab efficacy through to 180 days post-enrolment was 79.5% (95% CI 65.9–87.7) against the primary endpoint of medically attended RSV LTRI, 77.3% (95% CI 50.3–98.7) for any medically attended RSV LRTI with hospitalization, and 86.0% (95% CI 62.5–94.8) against very severe RSV LRTI [117]. Nirsevimab has recently (October/November 2022) been registered for use in the European Union and the UK.
Clesrovimab, another long-acting monoclonal antibody that binds to epitope site IV of the RSV F protein, is currently being evaluated in a multicenter, randomized, partially blinded, phase III trial. Clesrovimab safety and efficacy data will be compared with placebo and the active comparator palivizumab in infants and children at increased risk of severe RSV disease, with an estimated completion date of April 2026 (NCT04938830).
7 Active Immunization
The pathway to an RSV vaccine has been impeded following initial failure of an inactivated whole virus RSV vaccine in 1966, which culminated in vaccinated children developing more severe disease following RSV infection, particularly if they were seronegative prior to vaccination [65]. The investigational, inactivated RSV vaccine provided no protection against subsequent RSV infection in 100 children administered either a single or three doses, compared with the control arm that received a parainfluenza vaccine. Furthermore, 80% of the RSV vaccine recipients required hospitalization during the subsequent RSV infections, compared with 5% of the controls. There were also two deaths due to RSV illness in the vaccine recipients, and none in the control group.
The mechanism for vaccine-associated enhanced disease (VAED) and the unfavorable outcome in vaccine recipients has been attributed to the formation of an abundance of non-neutralizing antibodies, as opposed to neutralizing antibodies, which is the desired effect of vaccines against viral pathogens [118]. Neutralizing antibodies bind to receptor-binding domains of viruses; surface proteins are recognized and bound by the neutralizing antibodies and subsequently inhibit attachment, entry and fusion with the host cell. Furthermore, antibody-mediated antigen-binding fragments (Fab) binding to the antigenic proteins on the virus surface induce immune complexes, which through binding of immune effector cells via the Fc receptor of the antibody complex (FcR) triggers further enhancement of the immune response. An accumulation or deposition of these immune complexes could lead to overstimulation of the FcR-mediated immune response, with exaggerated immune cell recruitment, antibody production, and T-helper cell 2 type response, with the resultant increase in inflammation and VAED [118, 119]. Future vaccines targeting RSV, or any pathogen, need to be assessed and studied vigorously, to avoid a repeat of VAED experienced after vaccination against RSV in the 1960s.
There are currently multiple RSV vaccine candidates in various stages of development and testing, targeting either infants, pregnant women or the elderly (Electronic Supplementary Table 1). Vaccination of pregnant women aims to prevent RSV LRTI in their young infants, through maternal–fetal antibody transfer (Table 2). Prevention of RSV LRTI in early infancy is important, since the median age of RSV LRTI hospitalization is 3–4 months, with approximately 50% of RSV LRTI hospitalizations and RSV-associated deaths in children occurring in the first six months of life [120, 122, 122]. Illustrative of the potential of vaccination during pregnancy in preventing infections due to respiratory pathogens in young infants, is the effectiveness of vaccinating pregnant women with the acellular pertussis vaccine and inactivated influenza virus vaccine (IIV) [123, 124]. In the case of maternal pertussis vaccination, it protects infants in the first two months of life, which is the age period of greatest susceptibility for fatal pertussis in children, and when infants are too young to be protected through direct active immunization. In keeping with the kinetics of maternally derived hemagglutination inhibition antibody in infants, there is waning of protection against influenza illness beyond three months of age in babies born to women vaccinated with IIV [125]. Furthermore, the infants born to women vaccinated with IIV had a 43% lower risk of all-cause severe pneumonia, or hospitalization for pneumonia, during the three months of life, indicating that maternal IIV vaccination conferred protection beyond only directly preventing influenza illness [126].
There are multiple phase I, II and III trials currently underway examining the different RSV vaccine candidates [120]. PATH provides an updated snapshot of RSV vaccines and immunoprophylactic options currently under investigation or in production (https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/). Different vaccine types under investigation for prevention of RSV include live attenuated virus vaccines, chimeric vaccines, protein-based vaccines, including nanoparticles, nucleic acid vaccines and recombinant vector based vaccines (Electronic Supplementary Table 1).
Live attenuated vaccines (LAVs) contain a live replicating pathogen, thereby eliciting a potent immune response, both humoral and cellular, with the virulence of the pathogen having been reduced [127]. Earlier attempts at developing LAVs were unsuccessful in inducing immunity to RSV or were too reactogenic [128]. Current RSV LAVs include attenuation through reverse genetic engineering that delete proteins that regulate viral synthesis or responses [129]. A potential advantage of the LAV is the intranasal route of administration, which could induce mucosal immunity better than when vaccines are administered systemically. There are currently multiple LAV candidates undergoing phase I and phase II trials [130]. MV-012-968 (Meissa Vaccines, Inc.), in which there is codon deoptimization of NS1/NS2/G, as well as SH deletion and secreted G ablation, has just completed a randomized, double-blind, placebo-controlled phase IIa trial to evaluate the safety and efficacy against RSV infection in a human challenge model in adults. VAD00001 (SP0125) [Sanofi Pasteur] is undergoing a randomized, placebo-controlled trial evaluating safety, immunogenicity and dosing in 300 children aged 6–18 months, with completion expected in April 2023.
Chimeric vaccines comprise selected attenuated viruses that contain genetic material from the organisms of interest, forming a hybrid organism. There are currently three chimeric vaccines in phase I trials and none in phase II trials [130]. These include parainfluenza 5 virus/RSV chimera, Bacillus Calmette–Guerin expressing the RSV N gene, and Sendai virus/RSV protein F.
Protein-based vaccines are either particle or subunit based. These contain nanoscopic particles that mimic selected antigens on the virus surface and can be manufactured with or without an adjuvant. Furthermore, they elicit a robust humoral and cellular immune response. The F protein has been the main protein epitope utilized, with both the pre-F and post-F conformation of the protein explored. Other antigenic particles that are being investigated include epitopes of the G protein, SH protein, matrix protein, and nucleocapsid (N) protein. There are two maternal F-protein vaccines currently in phase III trials (Table 2). The F-protein vaccine is also being evaluated in adults older than 65 years of age and in the pediatric population.
A phase III, randomized, observer-blind, placebo-controlled trial to determine the immunogenicity and safety of a prefusion RSV F protein nanoparticle vaccine with aluminum adjuvant (Novavax) administered to healthy third-trimester pregnant women did not meet predetermined efficacy targets [122]. In the trial that enrolled healthy pregnant women between 28 and 36 weeks gestational age, a single dose of vaccine was associated with a 39.4% (95% CI 5.3–61.2) reduction in the primary endpoint of RSV-associated medically significant LRTIs in the first 90 days of life in the infants. However, the vaccine efficacy for medically significant RSV LRTI was higher in LMICs (40.5%, 95% CI − 3.1 to 65.7) than in HICs (37.7%, 95% CI − 33.8 to 71.0), which had increased even more by 180 days (33.1, 95% CI − 8.6 to 58.7 versus 14.6, 95% CI − 61.6 to 54.8). These differences were even more marked for RSV LRTI with hospitalization, with vaccine efficacy of 54.2% (95% CI 29.5–70.2) and 50.4% (95% CI 26.2–66.7) at 90 and 180 days in LMICs versus 7.7% (95% CI − 92.4 to 55.8) and 2.1% (95% CI − 96.3 to 51.2), respectively.
A further phase III, randomized, double-blind, placebo-controlled study evaluated a single dose of unadjuvanted prefusion F protein RSV vaccine administered intramuscularly. This study, led by GlaxoSmithKline™, evaluated vaccine efficacy in pregnant women 18–49 years of age in protecting against RSV-associated LRTIs in their infants through to 180 days of age (NCT04605159). Enrolment into the GSK maternal RSV vaccine program was terminated following a recommendation from the independent data monitoring committee following an excess of serious adverse events in the vaccine arm [131].
A phase IIb proof-of-concept study evaluating the efficacy of a bivalent RSV-A and RSV-B stabilized prefusion F protein vaccine (Pfizer) administered to pregnant women between 24 and 36 weeks gestational age induced robust neutralizing antibody responses in the women and high efficiency of transplacental antibody transfer to the newborn, with geometric mean transplacental ratios for neutralizing antibodies ranging from 1.41 to 1.67 in those who received RSVpreF with aluminum adjuvant and 1.68 to 2.10 in those who received RSVpreF without aluminum adjuvant (NCT04032093). Furthermore, neutralizing antibodies in the infant persisted through to 180 days of age above the putative threshold associated with protection against RSV LRTI. An exploratory analysis of the phase II trial reported vaccine efficacy of 85% (95% CI 21.5–97.6%) for medically attended LRTI and 91.5% (95% CI − 5.6 to 99.8%) for medically attended severe LRTI through to 180 days of age in the infants. The subsequent phase III trial is currently being evaluated in pregnant women. Enrolment of pregnant women into the phase III study was completed in late 2022 and analysis for vaccine efficacy is expected early in 2023 (NCT04424316). Preliminary data released by Pfizer reported a vaccine efficacy of 81.8% (99% CI 40.6–96.3) against severe medically attended LRTI due to RSV from birth to 90 days of life and 69.4% (99% CI 44.3–84.1) up to 6 months [132]. GSK recently reported data from a phase II observer-blind, placebo-controlled RCT (NCT04126213) [133]. An RSVPreF3 maternal vaccine administered to 213 pregnant women aged 18–40 years during the second or third trimester was well tolerated. Maternal neutralizing antibodies against both RSV-A and RSV-B were induced with successful transfer to the newborn.
A limitation of the maternal RSV vaccine trials in determining the durability of protection is that enrolment into the studies are generally planned for women to be vaccinated so that the birth of their babies coincides with the onset of the predicted RSV season. Consequently, exposure to RSV is enriched in children < 3 months of age in such studies, and vaccine efficacy beyond three months of age remains uncertain.
A phase II, randomized, double-blinded, placebo-controlled trial investigating the safety and tolerability of BARS13, a recombinant G protein plus cyclosporine A (which induces regulatory T lymphocytes and suppresses interleukin-2) vaccine, is currently underway (NCT04681833), and a further five phase I trials are currently ongoing, either targeting adult or elderly participants (Table 2).
Nucleic acid vaccines, such as messenger RNA (mRNA), is a relatively new approach to vaccine development that was used in the development of vaccines against coronavirus disease 2019 (COVID-19) [134]. mRNA vaccines use laboratory-based prefabricated mRNA to encode for the production of a protein, or part thereof, in the recipient’s cellular nucleus [135]. The protein stimulates the recipient’s immune system to deliver an immune response, with subsequent antibody production. There are currently three RSV nucleic acid vaccines in phase I human trials, including a randomized, observer-blinded, placebo-controlled, dose-escalation trial to evaluate the safety, reactogenicity and immunogenicity of the mRNA-1345 vaccine, which encodes stabilized RSV pre-F protein, in healthy adults aged 18–49 years, women of childbearing age 18–40 years of age, healthy older adults aged 65–79 years, and RSV-seropositive children aged 12–59 months, with results expected by September 2023 (NCT04528719). The phase II + III trial of mRNA-1345 (Moderna) is currently underway (NCT05127434). There is also the future possibility of combining RSV mRNA with targets against other respiratory viruses (human metapneumovirus [hMPV] and parainfluenza), such as is being undertaken in preclinical studies of a monovalent RSV and bivalent RSV and hMPV vaccine.
Recombinant vector vaccines use replicating or non-replicating viruses that have been engineered to contain extra genetic material from a pathogen of interest. This genetic material is then delivered to the recipient and an immune response is elicited. There is currently one non-replicating RSV vaccine undergoing a phase III trial, and two in phase II trials. These include a phase III randomized, double-blinded, placebo-controlled study to evaluate the safety and immunogenicity of an Ad26.RSV.pre-F-based vaccine in adults aged 18–59 years (NCT05070546). Ad26.RSV.pre-F delivers pre-F protein via an adenoviral vector. Completion is predicted during 2022. A phase II, randomized, double-blinded, placebo-controlled trial evaluating the safety, tolerability and immunogenicity of the same vaccine in RSV seropositive toddlers aged 12–24 months has finished recruitment and is awaiting results. A further phase III, randomized, double-blind trial assessing the clinical efficacy, safety and reactogenicity of a recombinant modified vaccinia virus (Ankara-BN) containing five different RSV-specific antigens encoding the RSV F protein, G protein, nucleoprotein, and transcription elongation factor (M2-1) derived from RSV subtype A, as well as another G protein of RSV subtype B, in adults older than 60 years of age is currently taking place, with a completion date set for December 2024.
8 Conclusion
RSV is the most common cause of LRTIs in children, causing approximately 3.6 million hospitalizations per year, and has been associated with long-term pulmonary sequelae for up to 30 years after infection, yet preventative strategies and active treatment options remain elusive. After an initial false start in the development of an RSV vaccine, for the first time in decades gradual progress is being made with the development of new-generation monoclonal antibodies with extended half-lives and multiple vaccine candidates. Building on these successes, and with the recent registration of the monoclonal antibody nirsevimab, as well as promising data from a recent phase III maternal vaccination trial, the future of RSV morbidity and mortality looks to change substantially. We have the potential to change the landscape of RSV LRTI, and therefore all-cause LRTI, through continuing along this path and by expanding on the work that is currently being performed.
References
Levels and Trends in Child Mortality: Report 2020, Estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation. New York: United Nations Children's Fund; 2020.
World Health Statistics. Monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization; 2019. p. 2019.
Jain S, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–45.
Bhuiyan MU, et al. The contribution of viruses and bacteria to community-acquired pneumonia in vaccinated children: a case–control study. Thorax. 2019;74(3):261–9.
Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394(10200):757–79.
Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(11):1133–61.
Falsey AR, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–59.
Jain S, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–27.
Hall CB. Respiratory syncytial virus: its transmission in the hospital environment. Yale J Biol Med. 1982;55(3–4):219–23.
Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2014;35(12):519–30.
Johnson KM, et al. Respiratory syncytial virus. IV. Correlation of virus shedding, serologic response, and illness in adult volunteers. JAMA. 1961;176:663–7.
Shigeta S, et al. The cell to cell infection of respiratory syncytial virus in HEp-2 monolayer cultures. J Gen Virol. 1968;3(1):129–31.
Aherne W, et al. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23(1):7–18.
Borchers AT, et al. Respiratory syncytial virus—a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–79.
Papenburg J, Boivin G. The distinguishing features of human metapneumovirus and respiratory syncytial virus. Rev Med Virol. 2010;20(4):245–60.
Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82(5):2040–55.
Hall CB, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132(2):e341–8.
Boyce TG, et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137(6):865–70.
Madhi SA, et al. Five-year cohort study of hospitalization for respiratory syncytial virus associated lower respiratory tract infection in African children. J Clin Virol. 2006;36(3):215–21.
Stensballe LG, et al. Atopic disposition, wheezing, and subsequent respiratory syncytial virus hospitalization in Danish children younger than 18 months: a nested case-control study. Pediatrics. 2006;118(5):e1360–8.
Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96(2):179–86.
Krinzman S, et al. Respiratory syncytial virus-associated infections in adult recipients of solid organ transplants. J Heart Lung Transplant. 1998;17(2):202–10.
Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143(5 Suppl):S118–26.
Chu HY, et al. Molecular epidemiology of respiratory syncytial virus transmission in childcare. J Clin Virol. 2013;57(4):343–50.
Glezen WP, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–6.
Kutsaya A, et al. Prospective clinical and serological follow-up in early childhood reveals a high rate of subclinical RSV infection and a relatively high reinfection rate within the first 3 years of life. Epidemiol Infect. 2016;144(8):1622–33.
Henderson FW, et al. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300(10):530–4.
Hall CB, et al. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–8.
Cohen C, et al. In- and out-of-hospital mortality associated with seasonal and pandemic influenza and respiratory syncytial virus in South Africa, 2009–2013. Clin Infect Dis. 2018;66(1):95–103.
Li Y, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64.
Verwey C, et al. Pulmonary function sequelae after respiratory syncytial virus lower respiratory tract infection in children: a systematic review. Pediatr Pulmonol. 2020;55:1567–83.
Coutts J, et al. Association between respiratory syncytial virus hospitalization in infancy and childhood asthma. Pediatr Pulmonol. 2020;55(5):1104–10.
Escobar GJ, et al. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97.
Escobar GJ, et al. Recurrent wheezing in the third year of life among children born at 32 weeks’ gestation or later: relationship to laboratory-confirmed, medically attended infection with respiratory syncytial virus during the first year of life. Arch Pediatr Adolesc Med. 2010;164(10):915–22.
Schauer U, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J. 2002;20(5):1277–83.
Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed). 1982;284(6330):1665–9.
Stein RT, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–5.
Sigurs N, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–52.
Sigurs N, et al. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161(5):1501–7.
Sigurs N, et al. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95(4):500–5.
Sigurs N, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–41.
Korsten K, Adriaenssens N, Coenen S. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J. 2021;57(4): 2002688.
Blount RE Jr, Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956;92(3):544–9.
Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg. 1957;66(3):281–90.
Afonso CL, Amarasinghe GK. Taxonomy of the order Mononegavirales: update 2016. Arch Virol. 2016;161(8):2351–60.
Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162(1–2):80–99.
Lee WJ, et al. Complete genome sequence of human respiratory syncytial virus genotype A with a 72-nucleotide duplication in the attachment protein G gene. J Virol. 2012;86(24):13810–1.
Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol. 2013;372:3–38.
Levine S, Klaiber-Franco R, Paradiso PR. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987;68(Pt 9):2521–4.
Bukreyev A, et al. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol. 2008;82(24):12191–204.
Roberts SR, et al. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J Virol. 1994;68(7):4538–46.
Hendricks DA, et al. Appearance of a soluble form of the G protein of respiratory syncytial virus in fluids of infected cells. J Gen Virol. 1987;68(Pt 6):1705–14.
McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol. 2013;372:83–104.
Fedechkin SO, et al. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci Immunol. 2018;3(21):eaar3534.
Mufson MA, et al. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985;66(Pt 10):2111–24.
Olmsted RA, et al. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci USA. 1986;83(19):7462–6.
Swanson KA, et al. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci USA. 2011;108(23):9619–24.
McLellan JS. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr Opin Virol. 2015;11:70–5.
Anderson LJ, et al. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985;151(4):626–33.
Cane PA. Molecular epidemiology of respiratory syncytial virus. Rev Med Virol. 2001;11(2):103–16.
Waris M. Pattern of respiratory syncytial virus epidemics in Finland: two-year cycles with alternating prevalence of groups A and B. J Infect Dis. 1991;163(3):464–9.
Bose ME, et al. Sequencing and analysis of globally obtained human respiratory syncytial virus A and B genomes. PLoS One. 2015;10(3): e0120098.
Openshaw PJM, et al. Protective and harmful immunity to RSV infection. Annu Rev Immunol. 2017;35:501–32.
Christiaansen AF, et al. The CD4 T cell response to respiratory syncytial virus infection. Immunol Res. 2014;59(1–3):109–17.
Kim HW, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34.
Buchwald AG, et al. Respiratory syncytial virus (RSV) neutralizing antibodies at birth predict protection from RSV illness in infants in the first 3 months of life. Clin Infect Dis. 2021;73(11):e4421–7.
Chiu C, Openshaw PJ. Antiviral B cell and T cell immunity in the lungs. Nat Immunol. 2015;16(1):18–26.
Habibi MS, et al. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med. 2015;191(9):1040–9.
Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol. 2005;79(9):5353–62.
Rallabhandi P, et al. Respiratory syncytial virus fusion protein-induced toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. MBio. 2012;3(4): e00218-12.
Jeong KI, et al. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a G protein-dependent manner. PLoS One. 2015;10(6): e0130517.
Ogilvie MM, et al. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol. 1981;7(4):263–71.
Ochola R, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One. 2009;4(12): e8088.
de Sierra TM, et al. Respiratory syncytial virus-specific immunoglobulins in preterm infants. J Pediatr. 1993;122(5 Pt 1):787–91.
Sande CJ, Cane PA, Nokes DJ. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine. 2014;32(37):4726–9.
Bisgaard H, et al. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am J Respir Crit Care Med. 2008;178(8):854–60.
Bulow SM, et al. Prednisolone treatment of respiratory syncytial virus infection: a randomized controlled trial of 147 infants. Pediatrics. 1999;104(6): e77.
Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–93.
Steiner RW. Treating acute bronchiolitis associated with RSV. Am Fam Physician. 2004;69(2):325–30.
Buckingham SC, et al. A randomized, double-blind, placebo-controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: effects on RSV quantity and clinical outcome. J Infect Dis. 2002;185(9):1222–8.
Cade A, et al. Randomised placebo controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch Dis Child. 2000;82(2):126–30.
Hammer J, Numa A, Newth CJ. Albuterol responsiveness in infants with respiratory failure caused by respiratory syncytial virus infection. J Pediatr. 1995;127(3):485–90.
Proesmans M, et al. Montelukast does not prevent reactive airway disease in young children hospitalized for RSV bronchiolitis. Acta Paediatr. 2009;98(11):1830–4.
Rodriguez WJ, et al. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics. 1997;100(6):937–42.
Roosevelt G, et al. Dexamethasone in bronchiolitis: a randomised controlled trial. Lancet. 1996;348(9023):292–5.
van Woensel JB, et al. Randomised double blind placebo controlled trial of prednisolone in children admitted to hospital with respiratory syncytial virus bronchiolitis. Thorax. 1997;52(7):634–7.
Dalziel SR, et al. Bronchiolitis. Lancet. 2022;400(10349):392–406.
DeVincenzo JP. Therapy of respiratory syncytial virus infection. Pediatr Infect Dis J. 2000;19(8):786–90 (discussion 802–4, 811–3).
Tejada S, et al. Ribavirin for treatment of subjects with respiratory syncytial virus-related infection: a systematic review and meta-analysis. Adv Ther. 2022;39(9):4037–51.
DeVincenzo J, et al. Safety and antiviral effects of nebulized PC786 in a respiratory syncytial virus challenge study. J Infect Dis. 2022;225(12):2087–96.
DeVincenzo JP, et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371(8):711–22.
DeVincenzo JP, et al. Activity of oral ALS-008176 in a respiratory syncytial virus challenge study. N Engl J Med. 2015;373(21):2048–58.
Stevens M, et al. Antiviral activity of oral JNJ-53718678 in healthy adult volunteers challenged with respiratory syncytial virus: a placebo-controlled study. J Infect Dis. 2018;218(5):748–56.
DeVincenzo J, et al. A randomized, placebo-controlled, respiratory syncytial virus human challenge study of the antiviral efficacy, safety, and pharmacokinetics of RV521, an inhibitor of the RSV-F protein. Antimicrob Agents Chemother. 2020;64(2): e01884-19.
Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–7.
Carbonell-Estrany X, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125(1):e35-51.
O’Brien KL, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15(12):1398–408.
Simões EAF, et al. Suptavumab for the prevention of medically attended respiratory syncytial virus infection in preterm infants. Clin Infect Dis. 2021;73(11):e4400–8.
Hammitt LL, Dagan R. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386(9):837–46.
Siber GR, et al. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J Infect Dis. 1992;165(3):456–63.
Groothuis JR, Simoes EA, Hemming VG. Respiratory syncytial virus (RSV) infection in preterm infants and the protective effects of RSV immune globulin (RSVIG). Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics. 1995;95(4):463–7.
Groothuis JR, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329(21):1524–30.
Simoes EA, et al. Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus disease in infants and children with congenital heart disease. The Cardiac Study Group. J Pediatr. 1998;133(4):492–9.
Respiratory syncytial virus immune globulin intravenous: indications for use. American Academy of Pediatrics Committee on Infectious Diseases, Committee on Fetus and Newborn. Pediatrics. 1997;99(4):645–50.
Johnson S, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176(5):1215–24.
Shahabi A, et al. Assessing variation in the cost of palivizumab for respiratory syncytial virus prevention in preterm infants. Pharmacoecon Open. 2018;2(1):53–61.
Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415–20.
Stephens LM, Varga SM. Considerations for a respiratory syncytial virus vaccine targeting an elderly population. Vaccines (Basel). 2021;9(6):624.
Obando-Pacheco P, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356–64.
Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem. 2006;281(33):23514–24.
Wu H, et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368(3):652–65.
FDA Panel Nixes Licensing Request for Motavizumab; 2010. https://www.medscape.com/viewarticle/722903 [cited 25 October 2022].
Zhu Q, McLellan JS. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med. 2017;9(388): eaaj1928.
Griffin MP, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415–25.
Muller WJ, Madhi SA. Nirsevimab for Prevention of RSV in Term and Late-Preterm Infants. 2023. https://doi.org/10.1056/NEJMc2214773
Dagan R, Nunez BS, Cots MB, Bosheva M, Madhi SA, Muller WJ, Zar HJ, Grenham A, Shroff M, Takas T, Mankad VS, Leach A, Villafana T. Nirsevimab for the prevention of RSV disease in healthy late-preterm and term infants: follow-up through second RSV season. In: 12th International RSV Symposium; 2022.
Simoes E, Zar HJ, Botsheva M, Muller WJ, Takas T, Griffin PM, Leach A, Yuan Y, Villafana T. Pooled efficacy of nirsevimab against RSV lower respiratory tract infection in preterm and term infants. In: European Society for Paediatric Infectious Diseases; 2022.
Bigay J, et al. Vaccine-associated enhanced disease in humans and animal models: lessons and challenges for vaccine development. Front Microbiol. 2022;13: 932408.
Polack FP, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196(6):859–65.
PATH. RSV Vaccine and mAb Snapshot; 2021. https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ [cited 2 Sep 2022].
Graham BS. Vaccines against respiratory syncytial virus: the time has finally come. Vaccine. 2016;34(30):3535–41.
Madhi SA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383(5):426–39.
Furuta M, et al. Efficacy and safety of pertussis vaccination for pregnant women—a systematic review of randomised controlled trials and observational studies. BMC Pregnancy Childbirth. 2017;17(1):390.
Benowitz I, et al. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51(12):1355–61.
Nunes MC, et al. Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin Infect Dis. 2017;65(7):1066–71.
Jarvis JR, et al. The effectiveness of influenza vaccination in pregnancy in relation to child health outcomes: systematic review and meta-analysis. Vaccine. 2020;38(7):1601–13.
Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100.
Wright PF, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–42.
Karron RA, et al. Live-attenuated vaccines prevent respiratory syncytial virus-associated illness in young children. Am J Respir Crit Care Med. 2021;203(5):594–603.
ClinicalTrials.gov; 2022. https://www.clinicaltrials.gov/ct2/home [cited 5 Sep 2022].
GSK provides further update on phase III RSV maternal vaccine candidate programme; 2022. https://www.gsk.com/en-gb/media/press-releases/gsk-provides-further-update-on-phase-iii-rsv-maternal-vaccine-candidate-programme/ [cited 16 Nov 2022].
Pfizer Announces Positive Top-Line Data of Phase 3 Global Maternal Immunization Trial for its Bivalent Respiratory Syncytial Virus (RSV) Vaccine Candidate; 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-phase-3-global [cited 4 Nov 2022].
Bebia Z, et al. Safety and immunogenicity of an investigational respiratory syncytial virus vaccine (RSVPreF3) in mothers and their infants: a phase 2 randomized trial. J Infect Dis. 2023. https://doi.org/10.1093/infdis/jiad024.
Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597(7876):318–24.
Pardi N, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by University of the Witwatersrand.
Conflict of interest
Charl Verwey has previously received personal funding from AstraZeneca, Merck, and GSK. Shabir A. Madhi has received grant support to the institution from the Bill & Melinda Gates Foundation (BMGF) and has been involved in clinical trials with Pfizer, GSK, Merck, and AstraZeneca, with funding to the institution. Charl Verwey and Shabir A. Madhi declare no conflicts of interest in relation to this manuscript.
Ethics approval
No ethics approval was required.
Patient consent to participate/publish
Not applicable.
Data availability
All data represented are available in the public domain.
Code availability
Not applicable.
Author contributions
This manuscript is the sole work of the authors. CV and SAM contributed equally to the planning, development, writing, and final approval of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Verwey, C., Madhi, S.A. Review and Update of Active and Passive Immunization Against Respiratory Syncytial Virus. BioDrugs 37, 295–309 (2023). https://doi.org/10.1007/s40259-023-00596-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-023-00596-4