Abstract
Introduction
Published data on the safety of biologics other than tumor necrosis factor (TNF) inhibitors during pregnancy are limited.
Objective
The aim was to detect pharmacovigilance signals for fetal and neonatal adverse drug reactions (ADRs) to biologics taken by pregnant women with autoimmune diseases.
Methods
We performed a disproportionality analysis of the World Health Organization’s VigiBase® pharmacovigilance database from 1968 to June 1, 2021. Data were collected in June 2021. By using terms for different hierarchical levels of the Medical Dictionary for Regulatory Activities, we selected the following fetal or neonatal ADRs: stillbirth, premature birth, low birth weight, small for gestational age, and congenital malformations. The frequency of all identified ADRs for biologics of interest (adalimumab, infliximab, golimumab, certolizumab, etanercept, anakinra, canakinumab, tocilizumab, sarilumab, ustekinumab, guselkumab, secukinumab, ixekizumab, belimumab, abatacept, and rituximab) was compared with that of all other reports for all other drugs and quoted as the reporting odds ratio (ROR) [95% confidence interval]. Reports with known concomitant use of teratogenic drugs were excluded from the main analysis. Other analyses included ROR stratifications by therapeutic indication in the periods 1968–2021 and 2001–2021, and an analysis after excluding reports with steroids.
Results
In the main analysis, the RORs were particularly high for musculoskeletal malformations with anakinra (7.18 [3.50–14.73]), canakinumab (19.54 [12.82–29.79]), and abatacept (5.09 [2.77–9.33]), and for immune system disorders with canakinumab (347.88 [217.9–555.50]) and rituximab (9.27 [2.95–29.15]). After the exclusion of reports with steroids, the ROR was significant for neonatal infections with belimumab (28.49 [5.75–141.25]).
Conclusion
We identified possible associations with some adverse fetal and neonatal outcomes, suggesting that vigilance is required when prescribing certain biologics during pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In comparison to tumor necrosis factor inhibitors, safety outcomes of other biologics taken in pregnancy by women with autoimmune diseases are limited, and identification of fetal and neonatal adverse drug reactions is still primarily dependent on post-marketing surveillance. |
Based on a disproportionality analysis of VigiBase® (the world's largest pharmacovigilance database) and after the exclusion of reports with known concomitant use of teratogenic drugs, strong significant signals were identified for musculoskeletal and connective tissue disorders with anakinra, canakinumab, and abatacept, and for immune system disorders with canakinumab and rituximab. |
After the exclusion of reports with steroids, there was a strong significant disproportionality signal for neonatal infections with belimumab. |
1 Introduction
In recent years, greater knowledge of the immunologic basis of human autoimmune diseases (AIDs) has prompted the development and approval of targeted biologic drugs. Biologics have greatly improved the management of these conditions by allowing more effective disease control, lowering the incidence of short- and long-term complications, and improving the patients' quality of life [1]. Given that women are more affected by immune dysfunction diseases than men, the question then arises as to whether these biologics are compatible with pregnancy [2]. During pregnancy, women with AIDs are at risk of moderate-to-severe flares with potentially serious obstetric consequences. Therefore, pregnancy must be planned during periods of disease inactivity or stability, which can sometimes be achieved by taking biologics [3, 4]. Discontinuing these drugs might expose the mother-to-be to a risk of relapse in the following months, i.e., perhaps during pregnancy [5, 6].
Immunoglobulin (Ig) G and (to a lesser extent) IgA are the only antibody (Ab) classes that pass from the mother to fetus through an active process involving the neonatal Fc receptor in the syncytiotrophoblast [7]. This process starts after 14 weeks of gestation (after organogenesis), increases in intensity during the second trimester, and continues until term [8]. Most immune-modulating biologics are monoclonal IgGs or active protein fractions bound to the Fc fragment of an Ig. The placental passage of and in utero exposure to biologics depends on the drug's molecular structure and in vivo half-life [9, 10].
Pregnant women are usually excluded from clinical trials, and the identification of teratogenic adverse effects remains dependent on post-marketing surveillance [11, 12]. Most of the available literature and registry data refer to the first class of biologics to be approved; i.e., tumor necrosis factor (TNF) inhibitors (TNFis) [13,14,15].
TNFis are prescribed for the treatment of various rheumatologic diseases (i.e., rheumatoid arthritis [RA], ankylosing spondylitis [AS], and psoriatic arthritis [PsA]), but also inflammatory bowel diseases (IBDs) (i.e., Crohn’s disease [CD] and ulcerative colitis), and plaque psoriasis (Pso). Certolizumab pegol (CZP) is a humanized polyethylene glycol (PEG)ylated Fab fragment of an anti-TNFα monoclonal Ab that does not contain an Fc fragment [10]. Thus, in vitro and ex vivo studies have shown that the structure of CZP limits its transfer through the placenta to the fetus [10]. A prospective, multicenter, pharmacokinetic study of 16 CZP-treated pregnant women then confirmed that the placental transfer of CZP was minimal [9]. Similarly, the fusion protein etanercept (ETA) crosses the placenta to a much lower extent than other monoclonal Abs like infliximab (IFX) and adalimumab (ADA) [10]. ADA and IFX were detected up to the age of 12 months in infants born to mothers exposed to these agents during pregnancy, and the drug concentration was inversely correlated with (1) time since last exposure during pregnancy and (2) maternal blood levels at delivery [16,17,18]. Nevertheless, large, comparative cohort studies and registry analyses have not highlighted higher rates of congenital malformations [19,20,21,22,23]. In 2016, the European League Against Rheumatism (EULAR) did not rule out the use of CZP prior to and during pregnancy but recommended that the maintenance or discontinuation of other anti-TNF agents during pregnancy should be considered on a case-by-case basis (depending on the woman’s disease activity) [13]. If required, treatment with ETA can be continued until 30–32 weeks of gestation, and treatment with ADA or IFX can be continued until 20 weeks of gestation [13]. The last American College of Rheumatology (ACR) guidelines are in line with those issued by the EULAR [14]. It was subsequently confirmed that discontinuing TNFi treatment at the recommended time points resulted in undetectable or low cord blood levels of TNFi [24]. Furthermore, no signal for adverse pregnancy outcomes or congenital malformations was observed in CZP-exposed pregnancies documented in a large post-marketing pharmacovigilance database [25]. Recent large cohort studies have shown an elevated risk of relapse in cases of TNFi treatment cessation in mid-pregnancy in women with IBD, and there was no evidence of severe adverse neonatal outcomes or an elevated risk of severe infections in children even when treatment was continued throughout the third trimester [26, 27]. Hence, the American Gastroenterological Association (AGA) IBD parenthood working group 2019 recommends continuation of CZP, ADA, IFX, and golimumab throughout pregnancy, without interruption in the third trimester, adjusting only the timing of the last dose to achieve the lowest possible trough levels during delivery [15].
The chimeric anti-CD20-Ab rituximab (RTX) is indicated in a large number of conditions, including hematologic malignancies, autoimmune cytopenia, systemic lupus erythematosus (SLE), RA, pemphigus vulgaris, and multiple sclerosis. Given the seriousness of some of these diseases, the EULAR and the ACR support the use of RTX in cases of severe and life-threatening maternal illness, and the literature data on pregnancies exposed to RTX are reassuring [13, 14, 28, 29].
However, data on the safety of other classes of biologics (namely interleukin [IL]-1 inhibitors [anakinra and canakinumab], IL-6 inhibitors [tocilizumab], IL-12/23 inhibitors [ustekinumab], IL-17A inhibitors [secukinumab], an anti-B cell activating factor [anti-BAFF] agent [belimumab], and cytotoxic T-lymphocyte-associated protein 4-Ig [CTLA4-Ig] [abatacept]) during pregnancy are scarce, and many experts recommend the discontinuation of these drugs once a pregnancy is confirmed [13, 14].
The objective of the present study of an international pharmacovigilance database was to evaluate outcomes in fetuses and neonates exposed in utero to biologics taken during pregnancy by women with AIDs.
2 Methods
2.1 Data Source
Individual case safety reports (ICSRs) were collected from VigiBase®—the world’s largest pharmacovigilance database, curated by the World Health Organization (WHO) [30]. Each ICSR includes anonymous administrative data (the country, and the reporter’s qualification), patient information (age and sex), drug information (the international non-proprietary name or the trade name, Anatomical Therapeutic Chemical [ATC] Classification System code, indication, start date, stop date, dosage, and administration route), and information on the suspected adverse drug reaction (ADR, coded according to the Medical Dictionary for Regulatory Activities [MedDRA]) [31]. There are five levels in the MedDRA hierarchy, ranging from very general to very specific: system organ class, high level group term, high level term, preferred term, and lowest level term. MedDRA also includes standardized MedDRA queries (SMQs), which are collections of MedDRA terms consistent with a description of a clinical syndrome associated with an ADR and drug exposure [32]. As such, SMQs are useful for wide-ranging searches. If a drug is considered to be at least probably responsible for the ADR, it is defined as “suspect” or “interacting” in the ICSR; if not, it is defined as “concomitant.” Full information on ICSRs is given on the Uppsala Monitoring Centre’s website [33].
2.2 Study Design
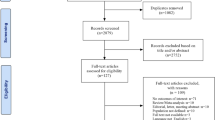
In June 2021, we searched for ICSRs on individuals of known age and sex recorded in VigiBase® between January 1, 1968, and June 1, 2021, using the “Pregnancy and neonatal topics” SMQ, which includes preferred terms related to “Congenital, familial and genetic disorders,” “Foetal disorders,” “Lactation-related topics,” “Neonatal disorders,” “Normal pregnancy conditions and outcomes,” “Pregnancy, labour and delivery complications and risk factors,” and “Termination of pregnancy and risk of abortion” (summarized in Supplementary Table S1; see the electronic supplementary material). ICSRs related to paternal exposure and to exposure through breastfeeding were excluded. We also excluded ICSRs in which the indication for treatment with a biologic drug was coronavirus disease 2019. Lastly, we identified the subset of newborns (children under the age of 1 month).
Fetal and neonatal ADRs of interest that belonged to the “Pregnancy and neonatal topics” SMQ (stillbirth, premature birth, low birth weight [LBW], small for gestational age [SGA], infection, cytopenia, chromosomal abnormalities, gene alterations, and congenital malformations [including cardiac and vascular disorders, ear and labyrinthine disorders, endocrine disorders, eye disorders, gastrointestinal tract disorders, hepatobiliary abnormalities, musculoskeletal and connective tissue disorders, neurologic disorders, renal and urinary tract disorders, reproductive tract and breast disorders, respiratory disorders, and immune system disorders]) were identified using the MedDRA hierarchy levels summarized in Supplementary Table S2. Drugs of interest considered to be suspect (monoclonal TNFis [ADA, INF, golimumab, and CZP], ETA, IL-1 inhibitors [anakinra and canakinumab], IL-6 inhibitors [tocilizumab and sarilumab], an IL-12/23 inhibitor [ustekinumab], a selective IL-23 inhibitor [guselkumab], IL-17A inhibitors [secukinumab and ixekizumab], an anti-BAFF agent [belimumab], an CTLA4-Ig [abatacept], and an anti-CD20 agent [RTX]) were identified using the ATC code (Supplementary Table S3).
2.3 Statistical Analysis
In our descriptive analysis, categorical variables (number of ICSRs, number of ICSRs with a single suspect drug, age groups of pregnant women, the biologics’ therapeutic indication, the geographical source [the continent] of ICSRs, the reporter’s qualification of the ICSRs, seriousness criteria, and the concomitant use of steroids and teratogenic drugs) were expressed as the number (percentage), and continuous variables (pregnant women’s age, and the vigiGrade™ completeness score [a measure of the amount of clinically relevant information in an ICSR as it appears in VigiBase®]) were expressed as the median (range).
Several types of disproportionality analysis have been described in the literature. Here, we chose to calculate the reporting odds ratio (ROR) and its 95% confidence interval (CI) as a guide to the strength of an association between a suspect drug and fetal or neonatal ADRs in the ICSRs filtered over the study period. This case/non-case approach is the best way to deal with the limitations of an ICSR database and to interpret the results reliably [34]. Calculation of the ROR has been described in detail elsewhere [35]. Briefly, ROR = (a/c)/(b/d), where a is the number of ADRs of interest with the drug of interest, b is the number of ADRs of interest with all other drugs in the study population, c is the number of ADRs other than those of interest but with the drug of interest, and d is the number of ADRs other than those of interest with all other drugs in the study population. If the ROR and the lower boundary of its 95% CI are above 1, the ADR of interest is reported more frequently with the drug of interest than with all other drugs. It has been suggested that an ROR above 4 corresponds to a “large” effect size [35]. The ROR is only interpretable if a drug is reported in at least three ICSRs [36].
Considering that infection and cytopenia can be reported in an ICSR on a mother and an ICSR on her child, RORs for each drug–ADR pair were calculated in the subgroup of newborns.
In the main analysis and given the ADRs of interest, we excluded ICSRs in which known teratogenic drugs were reported (namely valproic acid, acitretin, diethylstilbestrol, alitretinoin, isotretinoin, misoprostol, mycophenolic acid, testosterone, danazol, methotrexate, cyclophosphamide, lithium, carbimazole, warfarin, acenocoumarol, fluindione, carbamazepine, phenobarbital, phenytoin, and topiramate) [37]. Given the potential fetal and neonatal ADRs to steroids (i.e., a risk of premature birth, SGA, LBW, and infections but not congenital malformations [38, 39]), we excluded ICSRs in which this drug class was reported when analyzing stillbirth, premature birth, LBW, SGA, and infection. In another analysis of all the ICSRs that met the selection criteria (including those with known teratogenic drugs and steroids), we stratified by indication when the RORs were statistically significant. Since RTX was the drug of interest that had been on the market for longest (since 1998), we calculated RORs from January 1, 2001, to June 1, 2021. To assess the stability of our results in the sensitivity analyses, we selected valproate as a positive control (since this drug is known to be associated with congenital malformations [40]) and paracetamol as a negative control.
All analyses were performed using R software (version 3.6.3, R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Study Population
After the exclusion of ICSRs related to paternal exposure and exposure through breastfeeding, 190,023 ICSRs on individuals of known age and sex reported to VigiBase® between 1968 and June 1, 2021, matched the “Pregnancy and neonatal topics” SMQ, and 9636 of these ICSRs featured at least one drug of interest. The characteristics of the study population are summarized in Table 1 and (for each monoclonal TNFi [mTNFi] specifically) in Supplementary Table S4 (see the electronic supplementary material). In 5704 (59.2%) of the ICSRs, only one suspect drug was involved. Most of the selected ADRs occurred in patients taking a TNFi. The main therapeutic indications for RTX documented in VigiBase® were hematologic diseases; detailed results are not shown here. Among the individuals with serious ADRs, 266 (2.8%) displayed a congenital anomaly or other birth defect, and 133 (1.4) died.
3.2 Disproportionality Analyses
3.2.1 Main Analysis: The Exclusion of Known Teratogenic Drugs
The significant RORs for neonatal ADRs of interest after the exclusion of ICSRs involving teratogenic drugs (n = 18,804) are represented graphically in Fig. 1.
The RORs [95% CI] were particularly high (above 4) for (1) musculoskeletal disorders with anakinra (7.18 [3.50–14.73]), canakinumab (19.54 [12.82–29.79]), and abatacept (5.09 [2.77–9.33]), and (2) immune system disorders (detailed in Supplementary Table S5; see the electronic supplementary material) with canakinumab (347.88 [217.90–555.50]) and RTX (9.27 [2.95–29.15]).
3.2.2 Analysis After the Exclusion of ICSRs with Steroids
After the exclusion of ICSRs with suspected or concomitant use of steroids (n = 8164), the ROR [95% CI] was particularly high for infection with belimumab (28.49 [5.75–141.25], Fig. 2).
3.2.3 Stratified Analyses by Indication
RORs were stratified by indication for cases with available data on this variable (Fig. 3). In patients with other AIDs (including familial Mediterranean fever, TNF receptor-associated periodic syndrome, hyperimmunoglobulinemia D with periodic fever syndrome, and cryopyrin-associated periodic syndromes), the RORs were particularly high for musculoskeletal malformations with anakinra (7.74 [2.51–32.90]) and canakinumab (31.22 [12.91–75.49]) and for immune system disorders with canakinumab (31.25 [6.63–147.41]). In patients with Pso/PsA, the RORs were particularly high for stillbirth with mTNFis (5.73 [2.98–10.99]) and for neurologic disorders with ETA (4.38 [1.88–10.17]). In patients with RA, the ROR was particularly high for LBW with mTNFis (9.10 [2.03–40.73]). In patients with AS, the ROR was particularly high for eye disorders with ETA (6.30 [1.94–34.72]). In patients with SLE, the ROR was particularly high for infection with belimumab (16.12 [2.75–94.60]). In patients with IBD, the ROR was particularly high for infection with ustekinumab (11.50 [1.84–71.95]).
RORs calculated in the period 1968–2021 for fetal and neonatal ADRs, with stratification by indication. ADR adverse drug reaction, AID autoimmune disease, AS ankylosing spondylitis, CI confidence interval, IBD inflammatory bowel disease, ICSR individual case safety report, N number of drug–ADR pairs, PsA psoriatic arthritis, RA rheumatoid arthritis, ROR reporting odds ratio, SLE systemic lupus erythematosus, TNF tumor necrosis factor. * Including familial Mediterranean fever, TNF receptor-associated periodic syndrome, hyperimmunoglobulinemia D with periodic fever syndrome, and cryopyrin-associated periodic syndromes
3.2.4 Sensitivity Analyses
When we repeated the analyses for the period 2001–2021, the results were generally consistent with those of the main analysis (Fig. 4).
RORs calculated in the period 2001–2021 for fetal and neonatal ADRs, with stratification by indication. ADR adverse drug reaction, AID autoimmune disease, AS ankylosing spondylitis, CI confidence interval, IBD inflammatory bowel disease, ICSR individual case safety report, N number of drug–ADR pairs, PsA psoriatic arthritis, RA rheumatoid arthritis, ROR reporting odds ratio, SLE systemic lupus erythematosus, TNF tumor necrosis factor. * Including familial Mediterranean fever, TNF receptor-associated periodic syndrome, hyperimmunoglobulinemia D with periodic fever syndrome, and cryopyrin-associated periodic syndromes
The RORs with valproate were significant for many malformations (i.e., cardiac, vascular, ear, eye, gastrointestinal tract, musculoskeletal, neurologic, renal and reproductive tract malformations) and for SGA (Supplementary Figure S1; see the electronic supplementary material). None of the RORs were significant with paracetamol, except for infections (Supplementary Figure S2).
4 Discussion
Potential fetal and neonatal ADRs related to in utero exposure of biologics are difficult to identify and document because pregnant women are very often excluded from clinical trials [41,42,43], and few biologics are authorized in pregnant women. Hence, post-marketing pharmacoepidemiologic studies are important for detecting these ADRs. Disproportionality analysis lies at the interface between pharmacovigilance and pharmacoepidemiology. It can detect early disproportionality signals for specific ADRs, which must then be confirmed in more specific pharmacoepidemiologic studies [35]. To the best of our knowledge, the present study is the first to have examined reports on these potential ADRs in VigiBase®—the world’s largest pharmacovigilance database.
After the exclusion of reports with known teratogenic drugs, one of our main findings was a set of strong disproportionality signals for musculoskeletal malformations with the IL-1 inhibitors anakinra and canakinumab and the CTLA-4-Ig abatacept. We do not have a clear mechanistic, pharmacologic explanation for these associations. In fact, the biologics of interest in the present study do not readily cross the placenta until after the critical organogenesis stage because they are all high-molecular-weight macromolecules (17.3 kilodaltons [kDa] for anakinra, 92 kDa for abatacept, and more than 100 kDa for the others) [44, 45]. Thus, a direct teratogenic effect of these molecules is unlikely.
Regarding anakinra and canakinumab, it was shown that the pro-inflammatory IL-1 pathway has a major role in pregnancy (i.e., embryo implantation, placenta development, and protection against infections) and is involved in several disorders of pregnancy (such as pre-eclampsia). Blockade of the IL-1 pathway appears to reduce the incidence of these complications and protect the placenta and fetal/neonatal development [46]. However, there are no data on whether inhibition of this signaling pathway is teratogenic. Indication bias is a possible explanation for the association between IL-1 inhibitors and musculoskeletal malformations, since cryopyrin-associated autoinflammatory syndrome—an indication for IL-1 inhibitors—corresponds to a range of often inherited genetic diseases with skeletal abnormalities [47]. However, in the present study, the disproportionality signal remained significant after stratification for this indication. It should be noted that studies in monkeys have prompted concerns about a relationship between rilonacept (another IL-1-blocking agent) and fetal skeletal abnormalities [48]. Furthermore, the authors of a recent systematic review (including 88 pregnancies exposed to IL-1 inhibitors from 22 studies) did not reveal any musculoskeletal malformation, and found only two cases of renal agenesis [46].
Regarding abatacept, the mechanism underlying fetal malformations has not been well characterized. This molecule downregulates activated T cells via selective modulation of their co-stimulatory signal. Activated T cells are closely involved in bone formation by promoting the differentiation of mesenchymal stem cells into osteoblasts, and then increasing osteoblast proliferation and differentiation [49]. In Kumar et al.’s clinical study (including clinical trials and post-marketing data), seven congenital anomalies were observed among 86 live births (from 151 pregnant women exposed to abatacept): cardiovascular disorders (n = 2), cleft lip (n = 1), meningocele (n = 1), pyloric stenosis (n = 1), skull malformation (n = 1), and Down’s syndrome (n = 1). However, an effect of concomitantly administered methotrexate or other teratogenic drugs could not be ruled out [50]. No fetal disorders were described in two other case series [29, 51].
In the present study, the other main findings were strong disproportionality signals for immune system disorders with canakinumab and RTX, after the exclusion of concomitant known teratogenic drugs. As mentioned above, there is no clear pharmacologic mechanism for the putative effect of canakinumab. Again, confounding bias (i.e., an indication bias) might explain these results because many of the AIDs for which IL-1 receptor antagonists are prescribed are hereditary genetic pathologies with innate immune system disorders and often severe immunodeficiencies [47]. Regarding RTX, this chimeric monoclonal Ab induces B cell depletion (often associated with hypogammaglobulinemia) by directly targeting CD20 on the surface of B cells. Since RTX crosses the placental barrier, hypogammaglobulinemia can be observed in fetuses exposed in utero to RTX and can lead to transient lymphopenia and a decrease in IgG levels in the first days of life [29]. However, no major infectious complication was observed [28, 29].
Two factors prompted us to perform an analysis after the exclusion of reports with steroid use. First, given that the most frequently reported indications for the investigated biologics were RA, Pso/PsA, AS, and IBD, steroid use can be an indirect marker of disease activity. Thus, analyzing reports without steroids indirectly avoided a confounding bias—namely, the activity of these diseases. In fact, several studies have shown that women with these conditions are at high risk of obstetrical complications (including premature birth) even when they are not being treated with biologics and especially when the disease is not controlled [3, 4, 52,53,54]. Nevertheless, steroid use was a very indirect estimate of disease activity in our study. Second, steroid use in pregnant women with AIDs is independently associated with premature birth, SGA, LBW, and infections (but not congenital malformations) [38, 39] in a dose-dependent manner. In a cohort of 528 pregnant women with RA and after adjustment for a large number of confounding factors (including maternal age, comorbidities, disease activity, and other RA-related medications), the relative risk of premature birth was higher in both high-use and medium-use steroid groups than in a non-steroid-use group before 20 weeks of gestation. The mean total cumulative prednisone equivalent doses in the high use and medium use groups were 2208.6 mg and 883.0 mg (adjusted relative risk 4.77 [95% CI 2.76–8.26] and 1.81 [95% CI 1.10–2.97], respectively) [39]. Prednisone equivalent doses ≥ 10 mg later in pregnancy were also associated with a higher premature birth rate (adjusted hazard ratio 2.45 [95% CI 1.32–4.56]) [39]. Desai et al. showed that high-dose steroid use (average daily doses > 10 mg) was an independent risk factor of serious maternal infections during pregnancy in women with AIDs; these infections might contribute to premature deliveries and poor neonatal outcomes [55]. Moreover, according to a recent pregnancy registry study of 1490 mothers with IBD, steroid use was associated with an elevated risk of premature birth, SGA, LBW, and intrauterine growth restriction. In a multivariate analysis adjusted for biologics and other immunomodulator drugs, steroid use was also associated with preterm birth (odds ratio [OR] 1.79 [95% CI 1.18–2.73]) and LBW (OR 1.76 [95% CI 1.07–2.88]) [26]. Furthermore, among live births, late corticosteroid use (second and third trimesters) is associated with a high risk of serious infections during the first year after birth in children exposed in utero [38]. In the present study and after the exclusion of reports with steroid use, we still observed a strong disproportionality signal for infection with belimumab. This association was somewhat unexpected because (1) the risk of infections with belimumab is moderate in large cohorts of men and non-pregnant women with SLE, and (2) no infectious events were reported among the newborns born to 13 women with SLE exposed to belimumab during pregnancy [56,57,58]. Neonatal infections were not mentioned in another analysis of 13 pregnancies in patients with SLE exposed to belimumab [59]. In a recent prospective cohort study of 55 pregnancies exposed to belimumab (performed by the manufacturer), six out of 46 infants (13%) had at least one infection or episode of fever of "unknown origin" within the first 3 months of life [60]. The investigators emphasized the scarcity of data on the use of belimumab in pregnancy, and noted that they had reported several major birth defects [60].
Araujo et al. also performed a disproportionality analysis of ADRs to biologics (TNFis, abatacept, anakinra, RTX, and tocilizumab) used by patients with AIDs, albeit not specifically in pregnant women [61]. The researchers identified 75 congenital anomalies (out of the 411,063 reports analyzed) but did not report any RORs. Araujo et al.’s study population [61] differed from ours because (1) the pharmacovigilance data came from the US Food and Drug Administration’s Adverse Event Reporting System (which contains fewer reports than VigiBase®), (2) patients under 18 were excluded, (3) the study period was 2003–2016 (prior to the EULAR recommendations about biologics during pregnancy [13]), and (4) several biologics (such as canakinumab or belimumab) were not included.
It is important to bear in mind that disproportionality studies do not prove the existence of an association between drug exposure and an effect; in fact, they generate pharmacovigilance signals that must then be confirmed in pharmacoepidemiologic studies like the VALORE project. The potential of this Italian project (designed to optimize post-marketing surveillance of biologics, including biosimilars) has recently been emphasized [12]. Many biologics (both originators and biosimilars: TNFis, anakinra, tocilizumab, secukinumab, ustekinumab, ixekizumab, brodalumab, sarilumab, guselkumab, tildrakizumab, risankizumab, abatacept and vedolizumab) were studied during the period 2010–2019. During this time, 794 women with at least one delivery were exposed to biologics during pregnancy. Post-marketing surveillance for this project includes ADRs to biologics in newborns exposed in utero (such as congenital anomalies, preterm delivery, and stillbirth). Many confounding factors (such as disease activity and the trimester of pregnancy) will be documented.
Our study had several limitations, most of which are inherent to pharmacovigilance database studies and case/non-case designs [35]. Firstly, underreporting prevented us from determining the absolute frequency of drugs associated with each maternal and neonatal ADR of interest. Nevertheless, widespread underreporting would not affect the results of a disproportionality analysis [35]. Secondly, data on some variables were missing or incomplete. For example, the indications for treatment were rarely stated. As a result, the vigiGrade™ completeness score in the present study was not very high (median [interquartile range {IQR}] 0.44 [0.34–0.63], Table 1). This completeness score—measuring the amount of clinically relevant information in an ICSR as it appears in VigiBase®, and ranging from 0.07 to 1 [62]—does not imply or reflect causality between a drug and an adverse event but focuses on information that is important when assessing causality. To the best of our knowledge, a well-documented threshold has not been defined, but a vigiGrade™ score > 0.8 has already been suggested [63]. However, in Bergvall et al.’s study of report completeness and predictors of well-documented reports in VigiBase®, the median (IQR) completeness score was 0.41 (0.26–0.63) over the period 2007–2012 (including all VigiBase® ICSRs), which is consistent with the values observed in the present study [63]. Thirdly, it is important to note that the VigiBase® pharmacovigilance database was not specifically designed to record ADRs during pregnancy. Consequently, several data items are not collected, including the patient’s medical history, the trimester of pregnancy, the gestational age (conditioning several ADRs studied here) at live and preterm birth, and the duration of drug exposure. Moreover, we did not consider the age of the mother or other potential underlying conditions in the mother (e.g., obesity, diabetes mellitus, cardiovascular disease, and nephropathy) that can impact the course of the pregnancy [64]. Fourthly, RORs that were below 4 but were statistically significant and had a lower boundary of the 95% CI above 1 should be interpreted with caution because a significant size effect is not usually considered below this threshold [35]. Fifthly, due to the large number of countries involved in VigiBase® and the heterogeneity of each country's methods for assessing causality, the database does not include a causality assessment. The likelihood that the reported event was caused by the medicine varies from one report to another; some countries collect only ADRs with at least a possible causal relationship between the drug and the adverse event, while other countries collect all adverse events observed in patients using the drug in question [65].
Conversely, our case/non-case study has several important strengths [35]. Firstly, we studied the world’s largest pharmacovigilance database, which reflects routine medication use. Secondly, the case/non-case design is a validated method of investigating disproportionality between reports and drugs [35]. Thirdly, we used the “Pregnancy and neonatal topics” SMQ to identify the population of interest. SMQs are validated, pre-determined sets of MedDRA terms grouped together after extensive reviewing, testing, analysis, and expert discussion [32]. The specific “Pregnancy and neonatal topics” SMQ was built according to the European Medicines Agency's guideline on exposure to medicinal products during pregnancy [66, 67].
5 Conclusion
Using data from the world's largest pharmacovigilance database, we found high disproportionality signals for certain fetal or neonatal ADRs with some biologics: musculoskeletal malformations with anakinra, canakinumab, and abatacept, and immune system disorders with canakinumab and RTX. Until more robust, post-marketing, pharmacoepidemiologic studies are conducted, we recommend particular vigilance and close monitoring in pregnancies for which these drugs are needed to control the woman’s AID.
References
Burmester GR, Bijlsma JWJ, Cutolo M, McInnes IB. Managing rheumatic and musculoskeletal diseases—past, present and future. Nat Rev Rheumatol. 2017;13:443–8.
Beltagy A, Aghamajidi A, Trespidi L, Ossola W, Meroni PL. Biologics During Pregnancy and Breastfeeding Among Women With Rheumatic Diseases: Safety Clinical Evidence on the Road. Front Pharmacol [Internet]. Frontiers; 2021 [cited 2021 Jul 19];0. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fphar.2021.621247/full
Bandoli G, Singh N, Strouse J, Baer RJ, Donovan BM, Feuer SK, et al. Mediation of adverse pregnancy outcomes in autoimmune conditions by pregnancy complications: a mediation analysis of autoimmune conditions and adverse pregnancy outcomes. Arthritis Care Res. 2020;72:256–64.
Somers EC. Pregnancy and autoimmune diseases. Best Pract Res Clin Obstet Gynaecol. 2020;64:3–10.
Masson Regnault M, Shourick J, Jendoubi F, Tauber M, Paul C. Time to Relapse After Discontinuing Systemic Treatment for Psoriasis: A Systematic Review. Am J Clin Dermatol [Internet]. 2022 [cited 2022 May 3]; Available from: https://doi.org/10.1007/s40257-022-00679-y
Kennedy NA, Warner B, Johnston EL, Flanders L, Hendy P, Ding NS, et al. Relapse after withdrawal from anti-TNF therapy for inflammatory bowel disease: an observational study, plus systematic review and meta-analysis. Aliment Pharmacol Ther. 2016;43:910–23.
Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–31.
Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248–55.
Mariette X, Förger F, Abraham B, Flynn AD, Moltó A, Flipo R-M, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228–33.
Porter C, Armstrong-Fisher S, Kopotsha T, Smith B, Baker T, Kevorkian L, et al. Certolizumab pegol does not bind the neonatal Fc receptor (FcRn): consequences for FcRn-mediated in vitro transcytosis and ex vivo human placental transfer. J Reprod Immunol. 2016;116:7–12.
Cavadino A, Sandberg L, Öhman I, Bergvall T, Star K, Dolk H, et al. Signal detection in EUROmediCAT: identification and evaluation of medication-congenital anomaly associations and use of vigibase as a complementary source of reference. Drug Saf. 2021;44:765–85.
Trifirò G, Isgrò V, Ingrasciotta Y, Ientile V, L’Abbate L, Foti SS, et al. Large-scale postmarketing surveillance of biological drugs for immune-mediated inflammatory diseases through an italian distributed multi-database healthcare network: the VALORE project. BioDrugs. 2021;35:749–64.
Skorpen CG, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795–810.
Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res. 2020;72:461–88.
Mahadevan U, Robinson C, Bernasko N, Boland B, Chambers C, Dubinsky M, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American gastroenterological association IBD parenthood project working group. Gastroenterology. 2019;156:1508–24.
Mahadevan U, Wolf DC, Dubinsky M, Cortot A, Lee SD, Siegel CA, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286–92.
Julsgaard M, Christensen LA, Gibson PR, Gearry RB, Fallingborg J, Hvas CL, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110–9.
Bortlik M, Machkova N, Duricova D, Malickova K, Hrdlicka L, Lukas M, et al. Pregnancy and newborn outcome of mothers with inflammatory bowel diseases exposed to anti-TNF-α therapy during pregnancy: three-center study. Scand J Gastroenterol. 2013;48:951–8.
Viktil KK, Engeland A, Furu K. Outcomes after anti-rheumatic drug use before and during pregnancy: a cohort study among 150,000 pregnant women and expectant fathers. Scand J Rheumatol. 2012;41:196–201.
Weber-Schoendorfer C, Oppermann M, Wacker E, Bernard N, Beghin D, et al. Pregnancy outcome after TNF-α inhibitor therapy during the first trimester: a prospective multicentre cohort study. Br J Clin Pharmacol. 2015;80:727–39.
Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, Ornoy A. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol Elmsford N. 2014;43:78–84.
PIANO: A 1000 Patient Prospective Registry of Pregnancy Outcomes in Women With IBD Exposed to Immunomodulators and Biologic Therapy [Internet]. Epistemonikos. [cited 2022 Sep 22]. Available from: https://www.epistemonikos.org/en/documents/74ab54ddb26493ee5d6490b16f6a2a2b87532d24
Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREATTM registry. Am J Gastroenterol. 2012;107:1409–22.
Ghalandari N, Kemper E, Crijns IH, Wolbink G, Rispens T, Smeele HT, et al. Analysing cord blood levels of TNF inhibitors to validate the EULAR points to consider for TNF inhibitor use during pregnancy. Ann Rheum Dis. 2022;81:402–5.
Clowse M, Fischer-Betz R, Nelson-Piercy C, Scheuerle AE, Stephan B, Dubinsky M, et al. Pharmacovigilance pregnancy data in a large population of patients with chronic inflammatory disease exposed to certolizumab pegol. Ther Adv Musculoskelet Dis. 2022;14:1759.
Luu M, Benzenine E, Doret M, Michiels C, Barkun A, Degand T, et al. Continuous anti-TNFα use throughout pregnancy: possible complications for the mother but not for the fetus. A retrospective cohort on the French national health insurance database (EVASION). Am J Gastroenterol. 2018;113:1669–77.
Chaparro M, Verreth A, Lobaton T, Gravito-Soares E, Julsgaard M, Savarino E, et al. Long-term safety of in utero exposure to anti-TNFα drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY study. Am J Gastroenterol. 2018;113:396–403.
Perrotta K, Kiernan E, Bandoli G, Manaster R, Chambers C. Pregnancy outcomes following maternal treatment with rituximab prior to or during pregnancy: a case series. Rheumatol Adv Pract. 2021;5:74.
Ojeda-Uribe M, Afif N, Dahan E, Sparsa L, Haby C, Sibilia J, et al. Exposure to abatacept or rituximab in the first trimester of pregnancy in three women with autoimmune diseases. Clin Rheumatol. 2013;32:695–700.
Lindquist M. VigiBase, the WHO Global ICSR database system: basic facts. Drug Inf J. 2008;42:409–19.
Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20:109–17.
Standardised MedDRA Queries [Internet]. [cited 2022 Sep 25]. Available from: https://www.meddra.org/standardised-meddra-queries
Vigibase [Internet]. Available from: https://www.who-umc.org/vigibase/vigibase/
Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13:519–23.
Montastruc J-L, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011;72:905–8.
Roux E, Thiessard F, Fourrier A, Bégaud B, Tubert-Bitter P. Evaluation of statistical association measures for the automatic signal generation in pharmacovigilance. IEEE Trans Inf Technol Biomed Publ IEEE Eng Med Biol Soc. 2005;9:518–27.
Gelder MM, Jong Berg LTW, Roeleveld N. Drugs associated with teratogenic mechanisms. Part II: a literature review of the evidence on human risks. Hum Reprod Oxf Engl. 2014;29:168–83.
Odufalu F-D, Long M, Lin K, Mahadevan U. Exposure to corticosteroids in pregnancy is associated with adverse perinatal outcomes among infants of mothers with inflammatory bowel disease: results from the PIANO registry. Gut. 2022;71:1766–72.
Palmsten K, Bandoli G, Vazquez-Benitez G, Xi M, Johnson DL, Xu R, et al. Oral corticosteroid use during pregnancy and risk of preterm birth. Rheumatol Oxf Engl. 2020;59:1262–71.
Veroniki AA, Cogo E, Rios P, Straus SE, Finkelstein Y, Kealey R, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15:95.
Shields KE, Lyerly AD. Exclusion of pregnant women from industry-sponsored clinical trials. Obstet Gynecol. 2013;122:1077–81.
Wiland P, Jeka S, Dokoupilová E, Brandt-Jürgens J, Miranda Limón JM, Cantalejo Moreira M, et al. Switching to biosimilar SDZ-ADL in patients with moderate-to-severe active rheumatoid arthritis: 48-week efficacy, safety and immunogenicity results from the phase III, randomized double-blind ADMYRA study. BioDrugs. 2020;34:809–23.
Haridas VM, Katta R, Nalawade A, Kharkar S, Zhdan V, Garmish O, et al. Pharmacokinetic similarity and comparative pharmacodynamics, safety, efficacy, and immunogenicity of DRL_RI versus reference rituximab in biologics-naïve patients with moderate-to-severe rheumatoid arthritis: a double-blind, randomized, three-arm study. BioDrugs. 2020;34:183–96.
Zhao L, Ren T, Wang DD. Clinical pharmacology considerations in biologics development. Acta Pharmacol Sin. 2012;33:1339–47.
Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–9.
Brien M-E, Gaudreault V, Hughes K, Hayes DJL, Heazell AEP, Girard S. A systematic review of the safety of blocking the IL-1 system in human pregnancy. J Clin Med. 2021;11:225.
Georgin-Lavialle S, Ducharme-Benard S, Sarrabay G, Savey L, Grateau G, Hentgen V. Systemic autoinflammatory diseases: Clinical state of the art. Best Pract Res Clin Rheumatol. 2020;34: 101529.
Drug Approval Package: Arcalyst (Rilonacept) NDA #125249 [Internet]. [cited 2021 Aug 30]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/125249s000TOC.cfm
Pacifici R. T cells: critical bone regulators in health and disease. Bone. 2010;47:461–71.
Kumar M, Ray L, Vemuri S, Simon TA. Pregnancy outcomes following exposure to abatacept during pregnancy. Semin Arthritis Rheum. 2015;45:351–6.
Bazzani C, Scrivo R, Andreoli L, Baldissera E, Biggioggero M, Canti V, et al. Prospectively-followed pregnancies in patients with inflammatory arthritis taking biological drugs: an Italian multicentre study. Clin Exp Rheumatol. 2015;33:688–93.
Kishore S, Mittal V, Majithia V. Obstetric outcomes in women with rheumatoid arthritis: results from nationwide inpatient sample database 2003–2011✰. Semin Arthritis Rheum. 2019;49:236–40.
Xie W, Huang H, Ji L, Zhang Z. Maternal and neonatal outcomes in pregnant women with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Rheumatology [Internet]. 2021 [cited 2021 Aug 23]; Available from: https://doi.org/10.1093/rheumatology/keab357
Kim M-A, Kim Y-H, Chun J, Lee HS, Park SJ, Cheon JH, et al. The influence of disease activity on pregnancy outcomes in women with inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2021;15:719–32.
Desai RJ, Bateman BT, Huybrechts KF, Patorno E, Hernandez-Diaz S, Park Y, et al. Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohort study. BMJ. 2017;356: j895.
Kao J-H, Lan T-Y, Lu C-H, Cheng C-F, Huang Y-M, Shen C-Y, et al. Pregnancy outcomes in patients treated with belimumab: report from real-world experience. Semin Arthritis Rheum. 2021;51:963–8.
Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383:1117–28.
Kirou KA, Dall’Era M, Aranow C, Anders H-J. Belimumab or anifrolumab for systemic lupus erythematosus? A risk-benefit assessment. Front Immunol. 2022;13:980079.
Crisafulli F, Gerardi MC, Moschetti L, Fredi M, Nalli C, Urban ML, et al. Pos0702 pregnancy in sle patients treated with belimumab: experience from 3 Italian centers. Ann Rheum Dis. 2021;80:600–1.
Juliao P, Wurst K, Pimenta JM, Gemzoe K, Landy H, Moody MA, et al. Belimumab use during pregnancy: Interim results of the belimumab pregnancy registry. Birth Defects Res [Internet]. [cited 2022 Oct 3];n/a. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/bdr2.2091
Araujo AGS, Borba HHL, Tonin FS, Lenzi L, Venson R, Pontarolo R, et al. Safety of biologics approved for the treatment of rheumatoid arthritis and other autoimmune diseases: a disproportionality analysis from the FDA adverse event reporting system (FAERS). BioDrugs. 2018;32:377–90.
Technical description of vigiGradeTM Completeness score [Internet]. [cited 2022 Sep 25]. Available from: https://www.zva.gov.lv/archive/doc_upl/Technical-description-of-vigiGrade-Completeness-score.pdf
Bergvall T, Norén GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37:65–77.
Holness N. High-risk pregnancy. Nurs Clin N Am. 2018;53:241–51.
Guideline for using VigiBase data in studies [Internet]. [cited 2022 Sep 25]. Available from: https://who-umc.org/media/05kldqpj/guidelineusingvigibaseinstudies.pdf
Pregnancy and neonatal topics (SMQ) [Internet]. [cited 2022 Sep 25]. Available from: https://bioportal.bioontology.org/ontologies/MEDDRA?p=classes&conceptid=20000185
Guideline on the exposure to medicinal products during pregnancy: need for post-authorisation data [Internet]. [cited 2022 Sep 25]. Available from: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-exposure-medicinal-products-during-pregnancy-need-post-authorisation-data_en.pdf
Acknowledgements
The authors acknowledge the Uppsala Monitoring Centre (UMC), which provided and gave permission to use the data analyzed in this present study. The authors are also grateful to the National Pharmacovigilance Centers, which contributed to the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
AD contributed to the conception/design of the work, acquisition, analysis, and interpretation of data for the work, and drafting the manuscript. She approved the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SL contributed to the analysis and interpretation of data for the work, and drafting the manuscript. She approved the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. YB revised the work critically for important intellectual content. He approved the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. KM revised the work critically for important intellectual content. He approved the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SB revised the work critically for important intellectual content. She approved the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SL revised the work critically for important intellectual content. She approved the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. ASHL revised the work critically for important intellectual content. She approved the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. VGC contributed to the analysis and interpretation of data for the work, and drafting the manuscript. She approved the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. BB contributed to the conception/design of the work, acquisition, analysis, and interpretation of data for the work, and drafting the manuscript. He approved the version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research did not receive any specific funding from agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Patient consent to participate/publish
Not applicable.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dernoncourt, A., Liabeuf, S., Bennis, Y. et al. Fetal and Neonatal Adverse Drug Reactions Associated with Biologics Taken During Pregnancy by Women with Autoimmune Diseases: Insights from an Analysis of the World Health Organization Pharmacovigilance Database (VigiBase®). BioDrugs 37, 73–87 (2023). https://doi.org/10.1007/s40259-022-00564-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00564-4