Abstract
Teserpaturev/G47Δ (Delytact®) is a third-generation (triple-mutated) recombinant oncolytic herpes simplex virus type 1 being developed by Daiichi Sankyo Co., Ltd. for the treatment of certain solid cancers. Teserpaturev/G47Δ has been approved for the treatment of malignant glioma in Japan and is currently in clinical development for the treatment of prostate cancer (phase II), malignant pleural mesothelioma (phase I) and recurrent olfactory neuroblastoma (phase I). This article summarizes the milestones in the development of teserpaturev/G47Δ leading to this first approval for the treatment of malignant glioma.
Similar content being viewed by others
References
Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373–9.
Taguchi S, Fukuhara H, Todo T. Oncolytic virus therapy in Japan: progress in clinical trials and future perspectives. Jpn J Clin Oncol. 2019;49(3):201–9.
Bommareddy PK, Shettigar M. Kaufman HL Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18:498–513.
Fukuhara H, Takeshima Y, Todo T. Triple-mutated oncolytic herpes virus for treating both fast- and slow-growing tumors. Cancer Sci. 2021;112(8):3293–301.
Zeng J, Li X, Sander M, et al. Oncolytic viro-immunotherapy: an emerging option in the treatment of gliomas. Front Immunol. 2021;12: 721830.
Kaufman HL, Bommareddy PK. Two roads for oncolytic immunotherapy development. J Immunother Cancer. 2019;7:26.
Mondal M, Guo J, He P, et al. Recent advances of oncolytic virus in cancer therapy. Hum Vaccines Immunother. 2020;16(10):2389–402.
Ghouse JN, Martuza SM, Rabkin SD. In situ cancer vaccination and immunovirotherapy using oncolytic HSV. Viruses. 2021;13:1740.
Mineta T, Rabkin SD, Yazaki T, et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1(9):938–43.
Otani Y, Yoo JY, Shimizu T, et al. Implications of immune cells in oncolytic herpes simplex virotherapy for glioma. Brain Tumor Pathol. 2022;39(2):57–64.
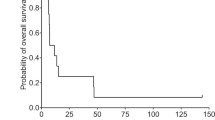
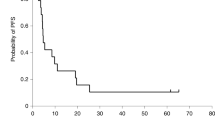
Todo T, Ito H, Ino Y, et al. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat Med. 2022;28:1630-9.
UMIN Clinical Trials Registry. Unique UMIN ID UMIN000015995. http://upload.umin.ac.jp. Accessed 29 July 2022.
Daiichi Sankyo Co. Ltd. DELYTACT® oncolytic virus G47∆ approved in Japan for treatment of patients with malignant glioma [media release]. 11 June 2021. https://www.daiichisankyo.com.
Daiichi-Sankyo Co. Ltd. DELYTACT: Japanese package insert. 2021.
Daiichi Sankyo Co. Ltd. Daiichi Sankyo launches DELYTACT(R) oncolytic virus G47 delta in Japan [media release]. 1 Nov 2021. https://www.daiichisankyo.com.
Daiichi Sankyo Co. Ltd. Oncolytic virus G47delta (DS-1647) designated as orphan drug under Orphan Drug/Medical Device designation system [media release]. http://www.daiichisankyo.com. Accessed 19 July 2021.
Daiichi Sankyo Co. Ltd. Daiichi Sankyo submits application for oncolytic virus teserpaturev (G47Δ) for treatment of patients with malignant glioma in Japan [media release]. http://www.daiichisankyo.com. Accessed 19 July 2021.
Daiichi Sankyo Co. Ltd. Reference data (consolidated financial results for Q3 FY2020). http://www.daiichisankyo.com. Accessed 29 July 2022.
Todo T, Martuza RL, Rabkin SD, et al. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–401.
Zhang W, Fulci G, Wakimoto H, et al. Combination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma models. Neoplasia. 2013;15(6):591–9.
Barnard Z, Wakimoto H, Zaupa C, et al. Expression of FMS-like tyrosine kinase 3 ligand by oncolytic herpes simplex virus type I prolongs survival in mice bearing established syngeneic intracranial malignant glioma. Neurosurgery. 2012;71(3):741–8.
Hoffmann D, Wildner O. Comparison of herpes simplex virus- and conditionally replicative adenovirus-based vectors for glioblastoma treatment. Cancer Gene Ther. 2007;14(7):627–39.
Wakimoto H, Kesari S, Farrell CJ, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69(8):3472–81.
Cheema TA, Wakimoto H, Fecci PE, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc Natl Acad Sci USA. 2013;110(29):12006–11.
Cheema TA, Kanai R, Kim GW, et al. Enhanced antitumor efficacy of low-dose etoposide with oncolytic herpes simplex virus in human glioblastoma stem cell xenografts. Clin Cancer Res. 2011;17(23):7383–93.
Ning J, Wakimoto H, Peters C, et al. Rad51 degradation: role in oncolytic virus-poly(ADP-ribose) polymerase inhibitor combination therapy in glioblastoma. J Natl Cancer Inst. 2017;109(3):1–13.
Kanai R, Rabkin SD, Yip S, et al. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. J Natl Cancer Inst. 2012;104(1):42–55.
Nigim F, Esaki S, Hood M, et al. A new patient-derived orthotopic malignant meningioma model treated with oncolytic herpes simplex virus. Neuro Oncol. 2016;18(9):1278–87.
Saha D, Wakimoto H, Peters CW, et al. Combinatorial effects of VEGFR kinase inhibitor axitinib and oncolytic virotherapy in mouse and human glioblastoma stem-like cell models. Clin Cancer Res. 2018;24(14):3409–22.
Esaki S, Nigim F, Moon E, et al. Blockade of transforming growth factor-β signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models. Int J Cancer. 2017;141(11):2348–58.
Farrell CJ, Zaupa C, Barnard Z, et al. Combination immunotherapy for tumors via sequential intratumoral injections of oncolytic herpes simplex virus 1 and immature dendritic cells. Clin Cancer Res. 2008;14(23):7711–6.
Sugawara K, Iwai M, Ito H, et al. Oncolytic herpes virus G47Δ works synergistically with CTLA-4 inhibition through dynamic intratumoral immune modulation. Mol Ther Oncol. 2021;22:129–42.
Fan J, Jiang H, Cheng L, et al. Oncolytic herpes simplex virus and temozolomide synergistically inhibit breast cancer cell tumorigenesis in vitro and in vivo. Oncol Lett. 2021;21(2):99.
Sugawara K, Iwai M, Yajima S, et al. Efficacy of a third-generation oncolytic herpes virus G47Δ in advanced stage models of human gastric cancer. Mol Ther Oncol. 2020;17:205–15.
Fan J, Jiang H, Cheng L, et al. The oncolytic herpes simplex virus vector, G47Δ, effectively targets tamoxifen-resistant breast cancer cells. Oncol Rep. 2016;35(3):1741–9.
Wang L, Ning J, Wakimoto H, et al. Oncolytic herpes simplex virus and PI3K inhibitor BKM120 synergize to promote killing of prostate cancer stem-like cells. Mol Ther Oncol. 2019;13:58–66.
Yamada T, Tateishi R, Iwai M, et al. Neoadjuvant use of oncolytic herpes virus G47Δ enhances the antitumor efficacy of radiofrequency ablation. Mol Ther Oncol. 2020;18:535–45.
Ito H, Ino Y, Todo T. Therapeutic efficacy of third generation oncolytic HSV-1 (G47delta) for glioma cells with stem cell property [abstract]. In: 19th annual meeting of the American Society of Gene and Cell Therapy, 2016.
Saha D, Martuza RL, Rabkin SD. Glioblastoma eradication by combination oncolytic immunovirotherapy and immune checkpoint blockade [abstract]. Mol Ther. 2017;25(5 Suppl 1):361.
Uchihashi T, Nakahara H, Fukuhara H, et al. Oncolytic herpes virus G47Δ injected into tongue cancer swiftly traffics in lymphatics and suppresses metastasis. Mol Ther Oncol. 2021;22:388–98.
Yajima S, Sugawara K, Iwai M, et al. Efficacy and safety of a third-generation oncolytic herpes virus G47Δ in models of human esophageal carcinoma. Mol Ther Oncol. 2021;23:402–11.
Inoue K, Ito H, Iwai M, et al. Neoadjuvant use of oncolytic herpes virus G47delta prevents stage advancement of tongue cancer. Cancer Sci. 2022;113:1422.
Cheema TA, Fecci PE, Ning J, et al. Immunovirotherapy for the treatment of glioblastoma. OncoImmunology. 2014;3(1): e27218.
Esaki S, Rabkin SD, Martuza RL, et al. Transient fasting enhances replication of oncolytic herpes simplex virus in glioblastoma. Am J Cancer Res. 2016;6(2):300–11.
Saha D, Rabkin SD, Martuza RL. Temozolomide antagonizes oncolytic immunovirotherapy in glioblastoma. J Immunother Cancer. 2020;8(Suppl 1): e000345.
Todo T, Ino Y, Ohtsu H, et al. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat Commun. 2022;13(1):4119.
Todo T. Results of phase ii clinical trial of oncolytic herpes virus G47delta in patients with glioblastoma [abstract no. ATIM-14]. Neuro Oncol. 2019;21(Suppl 6):vi4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of teserpaturev/G47Δ was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. James E. Frampton is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frampton, J.E. Teserpaturev/G47Δ: First Approval. BioDrugs 36, 667–672 (2022). https://doi.org/10.1007/s40259-022-00553-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00553-7