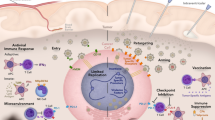
Abstract
Despite current progress in treatment, glioblastoma (GBM) remains a lethal primary malignant tumor of the central nervous system. Although immunotherapy has recently achieved remarkable survival effectiveness in multiple malignancies, none of the immune checkpoint inhibitors (ICIs) for GBM have shown anti-tumor efficacy in clinical trials. GBM has a characteristic immunosuppressive tumor microenvironment (TME) that results in the failure of ICIs. Oncolytic herpes simplex virotherapy (oHSV) is the most advanced United States Food and Drug Administration-approved virotherapy for advanced metastatic melanoma patients. Recently, another oHSV, Delytact®, was granted conditional approval in Japan against GBM, highlighting it as a promising treatment. Since oncolytic virotherapy can recruit abundant immune cells and modify the immune TME, oncolytic virotherapy for immunologically cold GBM will be an attractive therapeutic option for GBM. However, as these immune cells have roles in both anti-tumor and anti-viral immunity, fine-tuning of the TME using oncolytic virotherapy will be important to maximize the therapeutic efficacy. In this review, we discuss the current knowledge of oHSV, with a focus on the role of immune cells as friend or foe in oncolytic virotherapy.

Similar content being viewed by others
References
Ostrom QT, Cioffi G, Gittleman H et al (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 21(Supplement 5):v1–v100
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251
Otani Y, Ichikawa T, Kurozumi K et al (2019) Dynamic reorganization of microtubule and glioma invasion. Acta Med Okayama 73(4):285–297
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Stupp R, Taillibert S, Kanner A et al (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318(23):2306–2316
Reardon DA, Brandes AA, Omuro A et al (2020) Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol 6(7):1003–1010
Friebel E, Kapolou K, Unger S et al (2020) Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell 181(7):1626-1642.e1620
Goswami S, Walle T, Cornish AE et al (2020) Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat Med 26(1):39–46
Hilf N, Kuttruff-Coqui S, Frenzel K et al (2019) Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 565(7738):240–245
Keskin DB, Anandappa AJ, Sun J et al (2019) Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565(7738):234–239
Maggs L, Cattaneo G, Dal AE et al (2021) CAR T cell-based immunotherapy for the treatment of glioblastoma. Front Neurosci 15:662064
Quattrocchi KB, Miller CH, Cush S et al (1999) Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol 45(2):141–157
Desjardins A, Gromeier M, Herndon JE 2nd et al (2018) Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med 379(2):150–161
Fares J, Ahmed AU, Ulasov IV et al (2021) Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol 22(8):1103–1114
Kurozumi K, Fujii K, Shimazu Y et al (2020) Study protocol of a phase I/IIa clinical trial of Ad-SGE-REIC for treatment of recurrent malignant glioma. Future Oncol 16(6):151–159
Kurozumi K, Koizumi S, Otani Y (2021) Gene therapy and viral therapy for malignant glioma. No Shinkei Geka 49(3):608–616
Lang FF, Conrad C, Gomez-Manzano C et al (2018) Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol 36(14):1419–1427
Russell L, Swanner J, Jaime-Ramirez AC et al (2018) PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance. Nat Commun 9(1):5006
Martuza RL, Malick A, Markert JM et al (1991) Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 252(5007):854–856
Hong B, Sahu U, Mullarkey MP et al (2022) Replication and spread of oncolytic herpes simplex virus in solid tumors. Viruses 14(1):118
Mineta T, Rabkin SD, Yazaki T et al (1995) Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med 1(9):938–943
Zhou G, Roizman B (2006) Construction and properties of a herpes simplex virus 1 designed to enter cells solely via the IL-13alpha2 receptor. Proc Natl Acad Sci USA 103(14):5508–5513
Uchida H, Marzulli M, Nakano K et al (2013) Effective treatment of an orthotopic xenograft model of human glioblastoma using an EGFR-retargeted oncolytic herpes simplex virus. Mol Ther 21(3):561–569
Shibata T, Uchida H, Shiroyama T et al (2016) Development of an oncolytic HSV vector fully retargeted specifically to cellular EpCAM for virus entry and cell-to-cell spread. Gene Ther 23(6):479–488
He B, Gross M, Roizman B (1997) The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA 94(3):843–848
Farassati F, Yang AD, Lee PW (2001) Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol 3(8):745–750
MacLean AR, ul-Fareed M, Robertson L et al (1991) Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the “a” sequence. J Gen Virol 72(Pt 3):631–639
Dufour F, Sasseville AM, Chabaud S et al (2011) The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFα- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis 16(3):256–271
Todo T, Martuza RL, Rabkin SD et al (2001) Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA 98(11):6396–6401
Goldsmith K, Chen W, Johnson DC et al (1998) Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J Exp Med 187(3):341–348
Peters C, Paget M, Tshilenge KT et al (2018) Restriction of replication of oncolytic herpes simplex virus with a deletion of γ34.5 in glioblastoma stem-like cells. J Virol 92(15)
Kambara H, Okano H, Chiocca EA et al (2005) An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res 65(7):2832–2839
Hu JC, Coffin RS, Davis CJ et al (2006) A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 12(22):6737–6747
Kaufman HL, Ruby CE, Hughes T et al (2014) Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer 2:11
Cheema TA, Wakimoto H, Fecci PE et al (2013) Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc Natl Acad Sci USA 110(29):12006–12011
Bolyard C, Meisen WH, Banasavadi-Siddegowda Y et al (2017) BAI1 orchestrates macrophage inflammatory response to HSV infection—implications for oncolytic viral therapy. Clin Cancer Res 23(7):1809–1819
Fujii K, Kurozumi K, Ichikawa T et al (2013) The integrin inhibitor cilengitide enhances the anti-glioma efficacy of vasculostatin-expressing oncolytic virus. Cancer Gene Ther 20(8):437–444
Hardcastle J, Kurozumi K, Dmitrieva N et al (2010) Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol Ther 18(2):285–294
Nair M, Khosla M, Otani Y et al (2020) Enhancing antitumor efficacy of heavily vascularized tumors by RAMBO virus through decreased tumor endothelial cell activation. Cancers (Basel). 12(4):1040
Tomita Y, Kurozumi K, Yoo JY et al (2019) Oncolytic herpes virus armed with vasculostatin in combination with bevacizumab abrogates glioma invasion via the CCN1 and AKT signaling pathways. Mol Cancer Ther 18(8):1418–1429
Brennan CW, Verhaak RG, McKenna A et al (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477
Rutledge WC, Kong J, Gao J et al (2013) Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res 19(18):4951–4960
Markert JM, Medlock MD, Rabkin SD et al (2000) Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 7(10):867–874
Markert JM, Razdan SN, Kuo HC et al (2014) A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther 22(5):1048–1055
Friedman GK, Johnston JM, Bag AK et al (2021) Oncolytic HSV-1 G207 immunovirotherapy for pediatric high-grade gliomas. N Engl J Med 384(17):1613–1622
Waters AM, Johnston JM, Reddy AT et al (2017) Rationale and design of a phase 1 clinical trial to evaluate HSV G207 alone or with a single radiation dose in children with progressive or recurrent malignant supratentorial brain tumors. Hum Gene Ther Clin Dev 28(1):7–16
Bernstock JD, Bag AK, Fiveash J et al (2020) Design and rationale for first-in-human phase 1 immunovirotherapy clinical trial of oncolytic HSV G207 to treat malignant pediatric cerebellar brain tumors. Hum Gene Ther 31(19–20):1132–1139
Friedman GK, Bernstock JD, Chen D et al (2018) Enhanced sensitivity of patient-derived pediatric high-grade brain tumor xenografts to oncolytic HSV-1 virotherapy correlates with nectin-1 expression. Sci Rep 8(1):13930
Rampling R, Cruickshank G, Papanastassiou V et al (2000) Toxicity evaluation of replication-competent herpes simplex virus (ICP 345 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther 7(10):859–866
Papanastassiou V, Rampling R, Fraser M et al (2002) The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther 9(6):398–406
Harrow S, Papanastassiou V, Harland J et al (2004) HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther 11(22):1648–1658
Chiocca EA, Solomon I, Nakashima H et al (2021) First-in-human CAN-3110 (ICP-34.5 expressing HSV-1 oncolytic virus) in patients with recurrent high-grade glioma. J Clin Oncol 39(15_suppl):2009
Kurozumi K, Hardcastle J, Thakur R et al (2007) Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst 99(23):1768–1781
Hong B, Muili K, Bolyard C et al (2019) Suppression of HMGB1 released in the glioblastoma tumor microenvironment reduces tumoral edema. Mol Ther Oncolytics 12:93–102
Saha D, Martuza RL, Rabkin SD (2017) Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell 32(2):253-267.e255
Kim Y, Yoo JY, Lee TJ et al (2018) Complex role of NK cells in regulation of oncolytic virus-bortezomib therapy. Proc Natl Acad Sci USA 115(19):4927–4932
Yoo JY, Jaime-Ramirez AC, Bolyard C et al (2016) Bortezomib treatment sensitizes oncolytic HSV-1-treated tumors to NK cell immunotherapy. Clin Cancer Res 22(21):5265–5276
Otani Y, Yoo JY, Lewis CT et al (2022) NOTCH-induced MDSC recruitment after oHSV virotherapy in CNS cancer models modulates antitumor immunotherapy. Clin Cancer Res 28(7):1460–1473
Bernstock JD, Vicario N, Rong L et al (2019) A novel in situ multiplex immunofluorescence panel for the assessment of tumor immunopathology and response to virotherapy in pediatric glioblastoma reveals a role for checkpoint protein inhibition. Oncoimmunology 8(12):e1678921
Kaufman HL, Kohlhapp FJ, Zloza A (2015) Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 14(9):642–662
Serrano-Del Valle A, Anel A, Naval J et al (2019) Immunogenic cell death and immunotherapy of multiple myeloma. Front Cell Dev Biol 7:50
Alvarez-Breckenridge CA, Yu J, Price R et al (2012) NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat Med 18(12):1827–1834
Han J, Chen X, Chu J et al (2015) TGFβ treatment enhances glioblastoma virotherapy by inhibiting the innate immune response. Cancer Res 75(24):5273–5282
Hambardzumyan D, Gutmann DH, Kettenmann H (2016) The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 19(1):20–27
Van Hove H, Martens L, Scheyltjens I et al (2019) A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci 22(6):1021–1035
Wang Q, Hu B, Hu X et al (2017) Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32(1):42-56.e46
Uneda A, Kurozumi K, Fujimura A et al (2021) Differentiated glioblastoma cells accelerate tumor progression by shaping the tumor microenvironment via CCN1-mediated macrophage infiltration. Acta Neuropathol Commun 9(1):29
Denton NL, Chen CY, Scott TR et al (2016) Tumor-associated macrophages in oncolytic virotherapy: friend or foe? Biomedicines 4(3):13
Meisen WH, Wohleb ES, Jaime-Ramirez AC et al (2015) The impact of macrophage- and microglia-secreted TNFalpha on oncolytic HSV-1 therapy in the glioblastoma tumor microenvironment. Clin Cancer Res 21(14):3274–3285
Delwar ZM, Kuo Y, Wen YH et al (2018) Oncolytic virotherapy blockade by microglia and macrophages requires STAT1/3. Cancer Res 78(3):718–730
Fortin C, Yang Y, Huang X (2017) Monocytic myeloid-derived suppressor cells regulate T-cell responses against vaccinia virus. Eur J Immunol 47(6):1022–1031
Tan Z, Liu L, Chiu MS et al (2019) Virotherapy-recruited PMN-MDSC infiltration of mesothelioma blocks antitumor CTL by IL-10-mediated dendritic cell suppression. Oncoimmunology. 8(1):e1518672
Clements DR, Sterea AM, Kim Y et al (2015) Newly recruited CD11b+, GR-1+, Ly6C(high) myeloid cells augment tumor-associated immunosuppression immediately following the therapeutic administration of oncolytic reovirus. J Immunol 194(9):4397–4412
Otani Y, Yoo JY, Chao S et al (2020) Oncolytic HSV-infected glioma cells activate NOTCH in adjacent tumor cells sensitizing tumors to gamma secretase inhibition. Clin Cancer Res 26(10):2381–2392
Chang AL, Miska J, Wainwright DA et al (2016) CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res 76(19):5671–5682
Alayo QA, Ito H, Passaro C et al (2020) Glioblastoma infiltration of both tumor- and virus-antigen specific cytotoxic T cells correlates with experimental virotherapy responses. Sci Rep 10(1):5095
Ramelyte E, Tastanova A, Balázs Z et al (2021) Oncolytic virotherapy-mediated anti-tumor response: a single-cell perspective. Cancer Cell 39(3):394-406.e394
Markert JM, Liechty PG, Wang W et al (2009) Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther 17(1):199–207
Todo T (2019) ATIM-14. Results of phase ii clinical trial of oncolytic herpes virus G47δ in patients with glioblastomA. Neuro Oncol 21(Suppl 6):vi4
Acknowledgements
This study was supported by grants-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (No. 21K20803, YO) and research grants from Teraoka Scholarship Foundation to YO.
Funding
This study was supported by grants-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (No. 21K20803, YO) and research grants from Teraoka Scholarship Foundation to YO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflicts of interest exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Otani, Y., Yoo, J.Y., Shimizu, T. et al. Implications of immune cells in oncolytic herpes simplex virotherapy for glioma. Brain Tumor Pathol 39, 57–64 (2022). https://doi.org/10.1007/s10014-022-00431-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-022-00431-8