Abstract
Background and Objective
While biosimilars are less expensive than their originator biologics, various factors are known to impede their uptake in clinical practice including concerns regarding their interchangeability, efficacy, and safety. Pharmacists are well positioned to promote the adoption of biosimilars, thus, the aim of the review was to assess pharmacists’ knowledge and perceptions of biosimilars to identify the need for pharmacist-directed biosimilar education.
Methods
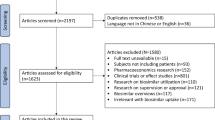
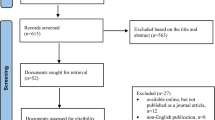
We conducted a systematic literature search for published articles indexed in MEDLINE via EBSCOHOST, Web of Science, Scopus, Cochrane Library, Dimensions, and Google Scholar databases. We included studies written in English from their earliest publication dates until December 2021. Only studies concerning pharmacists’ perspectives on biosimilars were included. Two reviewers extracted data from the studies that included pharmacists’ knowledge, perceptions, and opinions about interchangeability and automatic substitution of biosimilars. We also assessed the methodological quality of the included studies using the Joanna Briggs Institute Analytical Cross-Sectional Studies Assessment (JBI-ACSSA) for quantitative studies and the Critical Appraisal Skills Programme (CASP) for qualitative studies.
Results
Out of the 22 studies included in the review, 19 were cross-sectional quantitative studies, and the other three were qualitative studies. The sample size of the included studies ranged from 19 to 1500 participants. The level of knowledge of biosimilars graded as good, considerable, above average, or excellent among pharmacists varied from study to study, with a range of 47–86%. Only 22–51% of pharmacists were comfortable if biosimilars were prescribed for all of the indications previously used for the originator products. Pharmacists’ acceptability of switching from the originator to a biosimilar also varied, with a range of 26–84%. However, most pharmacists viewed the substitution of the originator with a biosimilar without physicians’ permission as unacceptable. Data from three studies reported that 22–74% of pharmacists had attended biosimilar training. They obtained information about biosimilars from scientific publications, pharmaceutical companies, and continuing education. Based on the criteria of JBI-ACSSA and CASP, the overall methodological quality of the studies ranged from moderate to high. The majority of the studies did not describe the sampling methods used and the strategies to deal with confounding factors.
Conclusions
Pharmacists’ knowledge and perception about biosimilars varied and were limited, especially about interchangeability and substitution, efficacy, safety, and indication extrapolation. A better understanding of biosimilars amongst pharmacists could help them to encourage prescribers’ acceptance of biosimilars.

Similar content being viewed by others
References
Tinsley SM, Grande C, Olson K, Plato L, Jacobs I. Potential of biosimilars to increase access to biologics: considerations for advanced practice providers in oncology. J Adv Pract Oncol. 2018;9(7):699–716. https://doi.org/10.6004/jadpro.2018.9.7.2.
Karateev D, Belokoneva N. Evaluation of physicians’ knowledge and attitudes towards biosimilars in Russia and issues associated with their prescribing. Biomolecules. 2019;9:1–16. https://doi.org/10.3390/biom9020057.
Ilham M, Hafidz A, Jayaraman T, Affendi R, Ali R, Lee YY. Are we ready for biosimilars in gastroenterology? EMJ Gastroenterol. 2017;6(1):83–9.
World Health Organization. Report on the expert consultation on improving access to and use of similar biotherapeutic products. 2017;1–25. https://www.who.int/medicines/access/biotherapeutics/FINAL_Report-improving-access-to-and-use-of-biotherapeutics_October2017.pdf. Accessed 20 Aug 2021.
Isaacs JD, Cutolo M, Keystone EC, Park W, Braun J. Biosimilars in immune-mediated inflammatory diseases: initial lessons from the first approved biosimilar anti-tumour necrosis factor monoclonal antibody. J Intern Med. 2016;279:41–59. https://doi.org/10.1111/joim.12432.
Kabir ER, Moreino SS, Siam MKS. The breakthrough of biosimilars: a twist in the narrative of biological therapy. Biomolecules. 2019;9:1–34. https://doi.org/10.3390/biom9090410.
European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1) (CHMP/BMWP/42832/2005 Rev 1). 2014. p. 1–13. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-0.pdf. Accessed 22 Aug 2021.
Inotai A, Csanadi M, Petrova G, Dimitrova M, Bochenek T, Tesar T, et al. Patient access, unmet medical need, expected benefits, and concerns related to the utilisation of biosimilars in Eastern European countries: a survey of experts. Biomed Res Int. 2018;2018:9597362. https://doi.org/10.1155/2018/9597362.
Nabhan C, Valley A, Feinberg BA. Barriers to oncology biosimilars uptake in the United States. Oncologist. 2018;23:1261–5. https://doi.org/10.1634/theoncologist.2018-0066.
Yang J, Blinzler K, Lankin J, Vijayakumar S, Maculaitis MC, Shelbaya A. Evolving perceptions, utilization, and real-world implementation experiences of oncology monoclonal antibody biosimilars in the USA: perspectives from both payers and physicians. BioDrugs. 2022;36:71–83. https://doi.org/10.1007/s40259-021-00509-3.
Aitken M, Kleinrock M. Biosimilars in the United States, 2020-2024. Biotechnol Law Rep. 2020. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/iqvia-institute-biosimilars-in-the-united-states.pdf?_=1632381810556. Accessed 1 Apr 2022.
Kolbe AR, Kearsley A, Merchant L, Temkin E, Patel A, Xu J, et al. Physician understanding and willingness to prescribe biosimilars: findings from a US national survey. BioDrugs. 2021;35:363–72. https://doi.org/10.1007/s40259-021-00479-6.
Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, Keuerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PLoS ONE. 2017;12:1–17. https://doi.org/10.1371/journal.pone.0190147.
Oskouei ST, Kusmierczyk AR. Biosimilar uptake: the importance of healthcare provider education. Pharmaceut Med. 2021;35:215–24. https://doi.org/10.1007/s40290-021-00396-7.
Inotai A, Prins CPJ, Csanádi M, Vitezic D, Codreanu C, Kaló Z. Is there a reason for concern or is it just hype? A systematic literature review of the clinical consequences of switching from originator biologics to biosimilars. Expert Opin Biol Ther. 2017;17(8):915–26. https://doi.org/10.1080/14712598.2017.1341486.
Tieu C, Lucas EJ, DePaola M, Rosman L, Alexander GC. Efficacy and safety of biosimilar insulins compared to their reference products: a systematic review. PLoS ONE. 2018;13:1–14. https://doi.org/10.1371/journal.pone.0195012.
Ebbers HC, Pieper B, Issa A, Addison J, Freudensprung U, Rezk MF. Real-world evidence on etanercept biosimilar SB4 in etanercept-naïve or switching patients: a systematic review. Rheumatol Ther. 2019;6(3):317–38. https://doi.org/10.1007/s40744-019-00169-4.
Barbier L, Ebbers HC, Declerck P, Simoens S, Vulto AG, Huys I. The efficacy, safety, and immunogenicity of switching between reference biopharmaceuticals and biosimilars: a systematic review. Clin Pharmacol Ther. 2020;108(4):734–55. https://doi.org/10.1002/cpt.1836.
Sarnola K, Merikoski M, Jyrkkä J, Hämeen-Anttila K. Physicians’ perceptions of the uptake of biosimilars: a systematic review. BMJ Open. 2020;10(5): e034183. https://doi.org/10.1136/bmjopen-2019-034183.
Giuliani R, Tabernero J, Cardoso F, McGregor KH, Vyas M, De Vries EGE. Knowledge and use of biosimilars in oncology: a survey by the European Society for Medical Oncology. ESMO Open. 2019;4(2):1–9. https://doi.org/10.1136/esmoopen-2018-000460.
Leonard E, Wascovich M, Oskouei S, Gurz P, Carpenter D. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019;25(1):102–12. https://doi.org/10.18553/jmcp.2019.25.1.102.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700. https://doi.org/10.1136/bmj.b2700.
Rehman K, Bukhari NI, Babar ZUD. Equitable access to high-cost pharmaceuticals. 1st ed. New York: Academic Press; 2018.
Ruiz S. Biosimilars in the EU. Biosimilar Drug Prod Dev. 2017;395–411. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Accessed 15 Aug 2021.
O’Callaghan J, Barry SP, Bermingham M, Morris JM, Griffin BT. Regulation of biosimilar medicines and current perspectives on interchangeability and policy. Eur J Clin Pharmacol. 2019;75:1–11. https://doi.org/10.1007/s00228-018-2542-1.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Checklist for analytical cross sectional studies. Joanna Briggs Inst Rev Man. 2017;1–7. http://joannabriggs.org/research/critical-appraisal-tools. Accessed 25 Aug 2021.
Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):1–11. https://doi.org/10.1186/s40779-020-00238-8.
Makhlouf SM, Pini S, Ahmed S, Bennett MI. Managing pain in people with cancer: a systematic review of the attitudes and knowledge of professionals, patients, caregivers and public. J Cancer Educ. 2020;35(2):214–40. https://doi.org/10.1007/s13187-019-01548-9.
Critical Appraisal Skills Programme. CASP qualitative studies checklist. 2018. https://casp-uk.b-cdn.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist-2018.pdf. Accessed 27 Aug 2021.
Ricoy-Cano AJ, Obrero-Gaitán E, Caravaca-Sánchez F, La F-R. Factors conditioning sexual behavior in older adults: a systematic review of qualitative studies. J Clin Med. 2020;9(6):1716. https://doi.org/10.3390/jcm9061716.
Tanabe K, Sugimoto N, Fujimoto Y. A web-based survey to investigate the extent of awareness and understanding for biosimilar among Japanese physicians and pharmacists. Value Health. 2015;18(7):A658. https://doi.org/10.1016/j.jval.2015.09.2381.
Almalki ZS, Iqbal MS, Alossaimi MA, Alsaber Z, Alanazi RA, Alruwaili YM, et al. Pharmacists’ awareness and perceptions of biosimilars in Saudi Arabia. 2020;14(3):3–6. https://doi.org/10.22377/ajp.v14i03.3757.
Alahmari AK, Almalki ZS, Shahid Iqbal M, Alossaimi MA, Mohammed Zaid Alsaber M, Alanazi RA, et al. Knowledge and attitude of pharmacists about biosimilar medications in Saudi Arabia. Int J Pharm Investig. 2021;11(1):123–6. https://doi.org/10.5530/ijpi.2021.1.23.
Foreman E, Patel H, Siderov J, Harchowal J, Bubalo J, Chan A. A survey of global biosimilar implementation practice conducted by the International Society of Oncology pharmacy practitioners. J Oncol Pharm Pract. 2020;26:22–32. https://doi.org/10.1177/1078155220913098.
Marín-Jiménez I, Carrascosa J, Guigini M, Monte-Boquet E. Knowledge, perceptions, attitude, barriers and facilitators of biosimilars use across specialty physicians and hospital pharmacists: a national survey. Farm Hosp. 2021;45:240–6. https://doi.org/10.7399/fh.11662.
Mhiri A, Khemakhem M, Kalboussi N, Kacem B. Knowledge and perceptions of biosimilar medicines by health professionals in Tunisia. Ann Pharm Françaises. 2021. https://doi.org/10.1016/j.pharma.2021.08.001.
Tomaszewski D. Biosimilar naming conventions: pharmacist perceptions and impact on confidence in dispensing biologics. J Manag Care Spec Pharm. 2016;22:919–26. https://doi.org/10.18553/jmcp.2016.22.8.919.
Aladul MI, Fitzpatrick RW, Chapman SR. Healthcare professionals’ perceptions and perspectives on biosimilar medicines and the barriers and facilitators to their prescribing in UK: a qualitative study. BMJ Open. 2018;8(11):1–8. https://doi.org/10.1136/bmjopen-2018-023603.
Olave M, Lavery C, Leonard CE, Lo Re III V, Shea JA, Kay J, et al. Knowledge of biosimilars and perceptions of the naming conventions for biosimilar products in clinical practice in the United States. Drugs Ther Perspect. 2021;37:338–46. https://doi.org/10.1007/s40267-021-00844-z.
Chan A, Patel H, Siderov J, Bubalo J, Foreman E. Assessing biosimilar education needs among oncology pharmacy practitioners worldwide: an ISOPP membership survey. J Oncol Pharm Pract. 2020;26(3_Suppl.):11–21. https://doi.org/10.1177/1078155219898510.
Łukasik ZM, Nowicki M. Knowledge and attitude of community pharmacy employees towards an automatic drug substitution of generics and biosimilars. Acta Pol Pharm Drug Res. 2018;75(5):1247–54. https://doi.org/10.32383/appdr/85315.
Paczkowska A, Kuś K, Kopciuch D, Zaprutko T, Stachowiak Z, Ratajczak P, et al. Safety of biodrugs: pharmacistsí knowledge and practice with regard to polish and European legal aspects and substitution of biotechnology drugs: multicenter study in Poland. Acta Pol Pharm Drug Res. 2020;77(4):657–64. https://doi.org/10.32383/appdr/127257.
Chapman SR, Fitzpatrick RW, Aladul MI. Knowledge, attitude and practice of healthcare professionals towards infliximab and insulin glargine biosimilars: result of a UK web-based survey. BMJ Open. 2017;7(6):1–8. https://doi.org/10.1136/bmjopen-2017-016730.
O’Callaghan J, Bermingham M, Leonard M, Hallinan F, Morris JM, Moore U, et al. Assessing awareness and attitudes of healthcare professionals on the use of biosimilar medicines: a survey of physicians and pharmacists in Ireland. Regul Toxicol Pharmacol. 2017;88:252–61. https://doi.org/10.1016/j.yrtph.2017.06.013.
Barbier L, Vandenplas Y, Simoens S, Declerck P, Vulto AG, Huys I. Knowledge and perception of biosimilars in ambulatory care: a survey among Belgian community pharmacists and physicians. J Pharm Policy Pract. 2021;14(1):1–16. https://doi.org/10.1186/s40545-021-00330-x.
Dylst P, Vulto A, Simoens S. Barriers to the uptake of biosimilars and possible solutions: a Belgian case study. Pharmacoeconomics. 2014;32(7):681–91. https://doi.org/10.1007/s40273-014-0163-9.
Poon SYK, Hsu JC, Ko Y, Chiang SC. Assessing knowledge and attitude of healthcare professionals on biosimilars: a national survey for pharmacists and physicians in Taiwan. Healthcare (Basel). 2021;9(11):1600. https://doi.org/10.3390/healthcare9111600.
Barbier L, Simoens S, Vulto AG, Huys I. European stakeholder learnings regarding biosimilars. Part I: improving biosimilar understanding and adoption. BioDrugs. 2020;34(6):783–96. https://doi.org/10.1007/s40259-020-00452-9.
Barbier L, Simoens S, Vulto AG, Huys I. European stakeholder learnings regarding biosimilars. Part II: improving biosimilar use in clinical practice. BioDrugs. 2020;34(6):797–808. https://doi.org/10.1007/s40259-020-00440-z.
Reilly MS, Schneider P. Naming and labelling of biologicals: the perspective of hospital and retail pharmacists. GaBI J. 2016;5(4):151–5. https://doi.org/10.5639/gabij.2016.0504.040.
Greene L, Singh RM, Carden MJ, Pardo CO, Lichtenstein GR, Care JM, et al. Strategies for overcoming barriers to adopting biosimilars. J Manag Care Spec Pharm. 2019;25(8):904–12. https://doi.org/10.18553/jmcp.2019.18412.
Adé A, Bourdon O, Bussières J-F. A survey of pharmacists’ knowledge and views of biosimilars in Quebec and France. Ann Pharm Françaises. 2017;75(4):267–75. https://doi.org/10.1016/j.pharm.a.2017.01.003.
Beck M, Michel B, Rybarczyk-Vigouret MC, Levêque D, Sordet C, Sibilia J, et al. Knowledge, behaviors and practices of community and hospital pharmacists towards biosimilar medicines: results of a French web-based survey. MAbs. 2017;9(2):383–90. https://doi.org/10.1080/19420862.2016.1267087.
Fernandez-Lopez S, Kazzaz D, Bashir M, McLaughlin T. Assessment of pharmacists’ views on biosimilar naming conventions. J Manag Care Pharm. 2015;21(3):188–95. https://doi.org/10.18553/jmcp.2015.21.3.188.
Mohammed JA, Kadhim DJ. Knowledge and perception of Iraqi pharmacists towards biosimilar medicines. Iraqi J Pharm Sci. 2021;30(1):226–32. https://doi.org/10.31351/vol30iss1pp226-232.
Mohd Sani N, Aziz Z, Kamarulzaman A. Biosimilar medicines: a cross-sectional study on hospital pharmacists’ perceived confidence and barriers to promote prescribing among clinicians in Malaysia. J Public Heal Emerg. 2021;5:AB043. https://doi.org/10.21037/jphe-21-ab043.
Okoro RN, Hedima EW, Aguiyi-ikeanyi CN. Knowledge, attitudes, and practices of pharmacists toward biosimilar medicines in Nigeria. J Am Pharm Assoc (2003). 2021;62(1):79-85.e2. https://doi.org/10.1016/j.japh.2021.09.014.
Pawłowska I, Pawłowski L, Krzyżaniak N, Kocić I. Perspectives of hospital pharmacists towards biosimilar medicines: a survey of Polish pharmacy practice in general hospitals. BioDrugs. 2019;33(2):183–91. https://doi.org/10.1007/s40259-019-00341-w.
Shakeel S, Hassali MA, Rehman H, Iffat W, Yasmin R, Farrukh U. Explanatory findings of prescribing biosimilar medicines in oncology care settings of Pakistan. Lat Am J Pharm. 2020;39(10):2041–5.
Shakeel S, et al. Knowledge, attitude, and practice towards biosimilars and interchangeable products: a prescriptive insight by the pharmacists. Int J Gen Med. 2020;13:1075–82. https://doi.org/10.2147/IJGM.S266545.
Rémuzat C, Dorey J, Cristeau O, Ionescu D, Radière G, Toumi M. Key drivers for market penetration of biosimilars in Europe. J Mark Access Heal Policy. 2017;5:1272308. https://doi.org/10.1080/20016689.2016.1272308.
Stevenson JG, Popovian R, Jacobs I, Hurst S, Shane LG. Biosimilars: practical considerations for pharmacists. Ann Pharmacother. 2017;51(7):590–602. https://doi.org/10.1177/1060028017690743.
Rumore MM, Vogenberg FR. Biosimilars: still not quite ready for prime time. 2017;41(6). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4894513/pdf/ptj4106366.pdf. Accessed 1 Sep 2021.
Li E, Liu J, Ramchandani M. A framework for integrating biosimilars into the didactic core requirements of a doctor of pharmacy curriculum. Am J Pharm Educ. 2017. https://doi.org/10.5688/ajpe81357.
Barbier L, Vulto AG. Interchangeability of biosimilars: overcoming the final hurdles. Drugs. 2021;81:1897–903. https://doi.org/10.1007/s40265-021-01629-4.
Cohen HP, Blauvelt A, Rifkin RM, Danese S, Gokhale SB, Woollett G. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78:463–78. https://doi.org/10.1007/s40265-018-0881-y.
Larkin H, Macdonald J, Lumsden R. Pharmacy-mediated substitution of biosimilars: a global survey benchmarking country substitution policies. GaBI J. 2017;6(4):157–64. https://doi.org/10.5639/gabij.2017.0604.034.
Schneider PJ, Reilly MS. Policy recommendations for a sustainable biosimilars market: lessons from Europe. Generics Biosimilars Initiat J. 2020;9(2):76–83. https://doi.org/10.5639/gabij.2020.0902.013.
Acknowledgments
The authors thank the Director-General of Health Malaysia for his permission to publish this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no financial support for the research, authorship, or publication of this systematic review.
Conflicts of interest/competing interests
Noraisyah Mohd Sani, Zoriah Aziz, Rema Panickar, and Adeeba Kamarulzaman have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Availability of data and material
All relevant materials for this systematic review are incorporated in the article, are fully cited, or are supplied as Electronic Supplementary Material.
Code availability
Not applicable.
Author contributions
This study was conceptualized and designed by NMS and ZA. NMS carried out the search. NMS and RP independently performed the screening of studies for inclusion and quality evaluation. NMS extracted the data, which RP validated, and all authors thoroughly reviewed them. NMS prepared the manuscript’s first draft. ZA and AK conducted a critical assessment of the manuscript for significant intellectual content. ZA made critical revisions to the draft manuscript. All authors read and approved the final manuscript for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohd Sani, N., Aziz, Z., Panickar, R. et al. Pharmacists’ Perspectives of Biosimilars: A Systematic Review. BioDrugs 36, 489–508 (2022). https://doi.org/10.1007/s40259-022-00541-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00541-x