Abstract
Introduction
In 2016, SB4 (Benepali®) became the first etanercept (ETN) biosimilar to obtain marketing authorisation in Europe. Despite robust analytical and clinical comparisons, outstanding questions remain on SB4 use in routine practice.
Methods
A systematic search for publications on real-world evidence of SB4 effectiveness, safety and drug survival was undertaken using search terms (SB4 OR Benepali OR biosimilar etanercept OR innovator etanercept) in the BIOSIS® Toxicology, BIOSIS Previews®, Embase® and MEDLINE® databases up to 17 January 2019.
Results
Of 959 articles identified, eight journal articles, two journal letters and 23 congress abstracts were selected on criteria of original real-world evidence with a clinical focus. As expected with real-world evidence, quality scoring showed that the evidence had high external validity but lower internal validity. A total of 13,552 patients were described across nine European countries and all approved SB4 indications: 2499 were ETN-naïve and 11,053 switched from reference ETN to SB4 (switchers). Switch acceptance rates (a combination of clinicians offering and patients accepting initiation on SB4) ranged between 51.6% and 99.0%; patient support programmes positively contributed to acceptance. Disease activity was generally similar pre- and post-switch (typically 3-month timeframe). Retention rates across studies were at least 75% (up to 12 months follow-up). No new safety signals were identified. Differences in discontinuation rates versus historic controls reported in some studies may have been influenced by differences in treatment practices, lack of clinician confidence and nocebo effects.
Conclusion
Nearly 2500 ETN-naïve patients have been initiated on SB4 and outcomes are similar to those patients receiving reference ETN. Overall this systematic review of real-world evidence provides additional reassurance that SB4 is as effective and safe as reference ETN in both switched and naïve patients.
Funding
Biogen International GmbH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A biosimilar is required by the European Medicines Agency and the Food and Drug Administration to demonstrate similarity to the reference (originator) product in terms of quality characteristics and biological activity. Furthermore, it must demonstrate bioequivalence to the reference drug and show comparable safety and efficacy in comparative clinical studies [1, 2]. Real-world evidence to supplement that from randomised clinical trials (RCTs) is important to inform and educate prescribers, patients and payers on the actual value of a medical intervention in routine clinical practice [3, 4]. Real-world evidence can address important questions remaining after approval of a biosimilar, including those on its long-term safety, immunogenicity, outcomes of switching from the reference to the biosimilar and the effectiveness and safety of the biosimilar in a wider range of clinical indications than tested pre-approval. It can also provide insights into nocebo effects, which are the apparent emergence or worsening of symptoms as a result of negative perceptions and attitudes towards an intervention, and which have the potential to adversely affect outcomes [5, 6]. Regulators encourage marketing authorisation holders of biosimilars to collect and report real-world evidence as part of post-approval risk management plans [7].
Etanercept (ETN) was the first anti-tumour necrosis factor inhibitor to receive marketing authorisation for the treatment of rheumatoid arthritis (RA), initially from the Food and Drug Administration [8, 9] and subsequently from the European Medicines Agency [10]. ETN (Enbrel®, Pfizer Europe) is now also licensed for the treatment of juvenile idiopathic arthritis, psoriatic arthritis (PsA), axial spondyloarthritis (AxSpA), ankylosing spondylitis (AS) and plaque psoriasis (PsO). ETN heralded a new age of biologic disease-modifying antirheumatic drugs (bDMARDs); however, the increased efficacy of treatment came with higher direct drug costs compared with conventional DMARDs (cDMARDs) [11]. This has produced inequalities in access to bDMARDs; in Europe, of those patients with RA who are eligible for bDMARDs according to the clinical recommendations of the European League against Rheumatism (EULAR), only 13–86% (dependent on country) can expect to receive these biologics on the basis of national reimbursement criteria [12]. Patent protection for ETN in Europe has expired, creating the opportunity for the introduction of biosimilars, which may enter the market at more affordable prices compared to the reference, potentially reducing healthcare costs for all approved indications through increased market competition, and improving patient access.
SB4 is an ETN biosimilar, which was compared to ETN in an RCT in patients with moderate to severe RA despite methotrexate treatment [13]. Therapeutic equivalence was demonstrated in terms of the American College of Rheumatology 20% response (ACR20) rate at week 24, with 78.1% of patients in the SB4 group demonstrating ACR20 vs. 80.3% of patients in the reference ETN group; the 95% confidence interval (CI) for the adjusted between-treatment difference was − 9.41% to 4.98%, thereby satisfying the predefined equivalence margin of − 15% to 15%. The safety profiles were similar, but injection site reactions occurred in fewer patients treated with SB4 compared with reference ETN—3.7% vs. 17.2% (p < 0.001) up to week 24, potentially due to differences in the formulation constituents (l-arginine) or the presence of latex in the needle shield of the reference product. SB4 had a lower incidence of antidrug antibody (ADA) development than reference ETN with an overall rate of 0.7% vs. 13.1% (p < 0.001) by week 24. These ADAs were transient and did not affect efficacy and their clinical significance was considered to be minimal by the European Medicines Agency. In an open-label extension of this trial, in which a subset of patients either continued to receive SB4 or were switched from reference ETN to SB4, SB4 continued to be effective and well tolerated with comparable performance to reference ETN up to the end of the study (100 weeks) [14, 15]. In January 2016, SB4 (Benepali®, Biogen) became the first ETN biosimilar to gain marketing authorisation in Europe and has the same indications as Enbrel® [16, 17].
The objective of this review is to provide a systematic review of published real-world evidence on the use of SB4, including effectiveness, safety, acceptance rates of the option to switch from the reference product to the biosimilar, and retention rates after switching. It includes data from prospective and retrospective observational studies (non-interventional studies), as well as analyses from European disease registries.
Methods
Search Strategy and Selection Criteria
The ProQuest® platform (Ann Arbor, USA) was used to search for journal articles and conference abstracts in the databases BIOSIS® Toxicology, BIOSIS Previews®, Embase® and MEDLINE® covering the period to 17 January 2019. The search string was (SB4 OR benepali OR biosimilar ETN OR innovator ETN) AND (rtype.exact(“Journal Article” OR “Conference Abstract”). Duplicates were automatically removed and screening was undertaken by one reviewer, initially on the basis of titles and subsequently using abstracts, in order to exclude publications that were reviews rather than original data, reported interventional clinical trials, had no clinical focus (e.g. publications containing only pharmacokinetic, pharmacodynamic or cost modelling data) or lacked identifiable data on SB4. The full publications for the remaining reports were reviewed and additional criteria were applied to obtain the final list of publications to be appraised in this review. This was undertaken by one reviewer and validated by a second. The inclusion criterion was real-world evidence on the use of SB4, which was defined as observational/non-interventional retrospective and prospective studies, patient registry data and individual case reports outside the controlled interventional trial setting. Reports of universal non-medical switches from ETN reference to SB4 as a result of changes in healthcare policies were permitted, including studies which incorporated randomised processes to support patients in making a switch e.g. switch ‘visits’. The following were excluded: reviews and meta-analyses, interventional clinical trials, open-label extensions of RCTs, studies based on economic modelling (as distinct from reports on actual economic outcomes) reports from which data specific to SB4 could not be extracted from pooled data, encore reports of the same data at different congresses, congress abstracts superseded by full journal publications and analyses of data from ongoing registries superseded by analyses at a later data cut-off date, with the exception of those that reported different outcome measures.
Quality Assessment
All eligible publications were scored independently by two individuals for bias and quality using the validated Downs and Black methods [18] modified to remove questions 14 and 15 (relating to blinding of subjects and investigators), questions 23 and 24 (relating to randomisation) and question 27 (relating to power calculations), because these were not relevant to observational studies. Score differences between the two scorers were discussed and resolved.
For each publication, the scores are presented as the grand total from the 22 questions (maximum total score 23), and as the totals for each aspect of quality assessed i.e. reporting (maximum total score 11 from 10 questions), external validity (maximum total score 3 from three questions) and internal validity (maximum total score 9 from eight questions).
Data Extraction
The following data, where available, were summarized from each eligible publication: study objective or focus, study design, main inclusion and exclusion criteria, indications, patient numbers (by indication), duration of study, outcomes relating to effectiveness and safety/tolerability of SB4 and any comparators, rates of switches to or from SB4, notable characteristics of switchers vs. non-switchers (e.g. disease activity), retention rates of patients switching to SB4, reasons for discontinuation of SB4, outcomes of communication plans to support patients switching to SB4, economic outcomes associated with switching to SB4. The extraction of data was started on 1 February 2019.
Data Synthesis
Summary tables were constructed of outcomes. All congress abstracts were categorised as such and not as journal publications even if the congress proceedings had been published in a journal. Total patient numbers for each indication and by patient SB4 category were calculated across studies. Where different analyses from the same registry had overlapping dates, patient numbers were counted from the most recent report of the full registry. Patients were categorised as ETN-naïve patients initiating SB4, patients switching from reference ETN to SB4 and patients who initially switched from reference ETN to SB4 and later back-switched from SB4 to reference ETN. Back-switching patients were categorised as both switchers and back-switchers. The indications were described as reported. In cases where several indications were included in the study but the distribution of patients was not given, these patients were counted as ‘rheumatology patients not specified’.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Overall Evidence Base
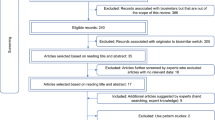
Of 951 articles retrieved by the searches there were six full journal publications [19,20,21,22,23,24], two journal letters [8, 25] and 23 congress abstracts [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] that were eligible for inclusion in this review (Fig. 1). The quality score of the full publications ranged from 16 to 19 (median 16.5) out of a maximum of 23 using the adapted Downs and Black methodology (Supplementary Fig. S1) [18]. All had the maximum score for external validity; internal validity scores were proportionately lower, with a median (range) score of 6 (5–7) out of a maximum of 9. Most congress abstracts and journal letters had lower scores than the journal publications, primarily because abstract reporting has restrictive word count limits (median [range] scores were 13 [9–17] total, 3 [1–3] for external validity and 5 [3–6] for internal validity) (Supplementary Fig. S2).
Most studies were based upon data from a single country, including the UK, Germany, Italy, France, Portugal, Sweden, Norway, Denmark and the Netherlands (Table 1 and Table S1 in the supplementary material). The studies included analyses from the following disease registries: BSRBR-RA (British Society for Rheumatology Biologics Register—Rheumatoid Arthritis) [26, 27], the German RABBIT (Rheumatoid Arthritis Observation of Biologic Therapy) cohort [28], the Denmark-wide DANBIO registry [21], the Dutch BIO-SPAN study (BIOsimilar switch, Study on Persistence and role of Attribution and Nocebo) [24], the SRQ Registry (Swedish Rheumatology Quality Register) [8, 19] and the NorArthritis Registry (Norwegian National Arthritis Registry) [31].
A total of 13,552 patients were described, including 11,053 patients switching from reference ETN to SB4, of whom 3535 were reported in full journal publications and 768 back-switchers (also counted as switchers) from SB4 to reference ETN, of whom 140 were reported in full journal publications (Fig. 2). Switches for most studies were non-medical i.e. economically driven as part of a clinic-wide policy to transfer patients to a lower priced ETN biosimilar. Of the 11,053 patients who initiated SB4 without having previously received ETN, 3535 were reported in full journal publications. Most patients were described as having RA, PsA or AxSpA, but other indications were also represented, including 279 patients with PsO, 12 patients with juvenile idiopathic arthritis and 4 patients with enteropathic arthritis. Most congress abstracts did not give separate results for different indications, and consequently many patients have been summarized here as ‘rheumatology patients not otherwise specified’.
Number of patients receiving SB4 pooled from all eligible publications. Patients who switched from etanercept reference to SB4 and subsequently back-switched to etanercept reference are counted both as switchers and back-switchers. Where it was possible to determine that the same patients in a database were reported in different publications they have been counted once [7, 8]. AS ankylosing spondylitis, AxSpA axial spondyloarthritis, ETN etanercept, JIA juvenile idiopathic arthritis, PsA psoriatic arthritis, RA rheumatoid arthritis, SpA spondyloarthritis
Outcomes covered included the effectiveness of SB4, with a comparison of pre-switch and post-switch disease activity, retention rates (drug survival) for SB4, reasons for discontinuations or back-switches to reference ETN, patients’ acceptability of switching and characteristics of those patients switching (Tables 1 and S1). Several studies presented data on actual cost savings of switching and Shah et al. focused exclusively on the economics of switching [32]. There was generally little detail provided on safety outcomes; in most of the congress abstracts these were incomplete owing to the reporting of adverse events (AEs) only in those patients discontinuing SB4.
Switching Acceptance and the Characterization of Switching Patients and Clinicians and the Process
Switches from reference ETN to SB4 that were mandatory did not necessarily have higher acceptance rates than non-mandatory switches e.g. in the BIO-SPAN study, 99% of patients offered a switch agreed to this [24] compared with 79% of patients recorded as switching in the Danish, country-wide DANBIO registry following the adoption of a national guideline mandating the switch [21]. In the latter study, a reason for at least some patients not switching was because their dose strength of 25 mg ETN was not yet commercially available for SB4. That study also reported a higher switch rate for patients with PsA (86%) than patients with RA (77%) or AxSpA (77%) (Table 1) [21]. Characteristics more common in switchers were concomitant methotrexate (MTX) use (RA and PsA), longer duration of reference ETN treatment and exposure to fewer previous bDMARDs. Switchers with RA also had lower disease activity at the time of switch than non-switchers at the time of the mandated switch. In the Swedish setting of non-mandatory switching no significant differences were apparent between switching and non-switching patients except that the former had a higher concomitant use of methotrexate (58.4% vs. 52.8%) and a shorter duration of reference ETN exposure [mean (standard deviation) 5.0 (4.3) years vs. 5.6 (4.4) years] (Table 1) [19].
Some authors highlighted the importance of supportive communication with patients and patient education to achieve high rates of switching [23, 32,33,34]. The only comparison of patient support programmes was reported in a congress abstract by Shah et al., who compared the switching rate for patients provided with education on switching and a specialist switching clinic (n = 151) with the rate for patients offered a switch at a routine clinic visit [32]. Full patient support was associated with a higher switch rate over 12 months than less intensive support (95% vs. 75%). Scherlinger et al. reported 92% (48/52) initial acceptance of a non-mandatory switch in patients in a single hospital in France who were given oral and written information on biosimilars [23]. Positive clinician opinions on SB4 were reported to be the main reason for acceptance of switch in 70% of patients. The characteristics of patients who refused a switch were similar to those who accepted, with the exception of those holding a negative opinion of generic drugs (100% vs. 11%, p < 0.001) and a statistical trend towards older age and longer disease duration. A study done in a single hospital in France with a relatively low switch acceptance rate (51.6% of eligible patients) found that physicians’ behaviour rather than patients’ characteristics were associated with switching. Older clinicians and those with full-time academic posts were more likely to switch patients, but there were no independent patient characteristics associated with switching [35].
There were some data on patient attitudes to switching. In a French clinic, the experience of switching (n = 44) was considered good by 86% of patients, although 15% felt pressure to accept the switch [23]. In a UK study of non-medical switching managed with education and a dedicated biosimilar switching clinic (n = 115), 43% of patients were pleased with the switch and 23% were not pleased, with a further 33% either being indifferent, unsure or failing to answer the question [36].
Effectiveness of SB4
Three full journal publications reported effectiveness outcomes for patients switching to SB4 (Table 1) [21, 22, 24]. In the relatively large population from the DANBIO registry that was analysed by Glintborg et al., pre- and post-switch changes over 3 months were not clinically different for a range of disease activity measures in patients with RA, PsA or AxSpA (Table 1) [21]. In the single-hospital BIO-SPAN study of non-mandatory switches in the Netherlands, Tweehuysen et al. did not find any statistically significant difference (adjusted for baseline characteristics) between the 6-month change in disease activity in a historic reference ETN-treated cohort of patients with RA, PsA and AS (n = 600) and that for switchers to SB4 (n = 625), although the switchers had numerically small changes in c-reactive protein (CRP) and Disease Activity Score 28 (DAS28; Table 1) [24]. There was no clinically meaningful change in disease activity between SB4-treated patients pre- and 6 months’ post-switch: median (interquartile range [IQR]) CRP level 1 (0–5) mg/l vs. 1 (0–6) mg/l, p = 0.13; median (IQR) DAS28-CRP 1.9 (1.5–2.6) vs.1.9 (1.4–2.6), p = 0.99; and median (IQR) Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score 3.1 (1.8–5.4) vs. 3.3 (1.9–5.3), p = 0.25. A study exclusively on patients with moderate to severe PsO and/or PsA (n = 32) observed no difference in rates of clinical remission (Psoriasis Scalp Severity Index and/or DAS28 increase < 10%) before and after switch to SB4—92% and 64% for PsO and PsA patients, respectively, p < 0.001 [22].
Most of the congress reports with data on SB4 effectiveness found no clinically meaningful differences between pre- and post-switch disease activity or changes over time in historic and switching cohorts of patients (Table S1) [8, 31, 35,36,37,38,39,40,41,42]. In one study of 120 switchers, 39.1% (47/120) of patients had increases in DAS28 resulting in 22.5% (27/120) of patients moving to a higher disease state [43]. Of patients with worsening DAS28, 38.3% had disease flares, mainly in the first 4 months’ post-switch. The authors concluded that these observations may have reflected ‘natural’ fluctuations as increases in disease activity were more frequent in patients not in disease remission at the time of switching.
Safety
In patients switching from reference ETN to SB4 in the DANBIO registry, no major safety signals were observed, and AEs were mainly non-specific [21]. The overall rate of AEs observed in patients with PsO in the DERMBIO registry did not differ for SB4 vs. reference ETN treatment; the incidence rate (95% CI) per 100 treatment years was 34.2 (17.1–68.3) vs. 32.5 (29.7–35.6) [20]. In SB4 patients, the incidence rate of infections (95% CI) per 100 treatment years was 17.1 (6.4–45.5), compared with 16.4 (14.4–18.6) for reference ETN-treated patients. Safety data appertaining to discontinuations is included in the switching outcomes section above. Additional safety data for switching patients reported in congress abstracts are summarised in Table S1.
In bionaïve patients in the BSRBR-RA registry the risk of serious AEs was similar between patients initiating SB4 (n = 310) and those initiating ETN (n = 192), as assessed at a 6-month follow-up visit, (HR = 0.5, 95% CI 0.3–1.1, p = 0.1) [26]. In the Italian Psobiosimilars registry, the authors concluded that there was no significant difference in numbers of AEs between switch patients and ETN-naïve patients initiating SB4, with AEs reported in 3/158 (1 worsening PsA, 1 upper respiratory tract infection,1 melanoma) and 3/39 patients (2 worsening PsO and 1 herpes simplex virus infection), respectively [25]. In the RABBIT registry, the AE profile of bionaïve patients initiating reference ETN differed from that of patients initiating SB4 due to a higher rate of injection site reactions—7.3% (23/313 patients) vs. 2.6% (7/266 patients).
Immunogenicity as assessed by incidence of anti-ETN antibodies was generally not reported.
Persistence Following a Switch to SB4
Retention rates (i.e. drug survival) and discontinuations were reported as outcomes of switching in the majority of studies [8, 20,21,22,23,24, 28, 31, 33, 35,36,37, 39, 41, 42, 44,45,46,47,48]. Glintborg et al. and Tweehuysen et al. distinguished between all discontinuations of SB4, from which the retention rate could be derived, and a sub-category of discontinuations that led to a back-switch to reference ETN [21, 24]. They adjusted for potential baseline confounders in comparisons of rates with historic reference ETN controls, although this did not include time-varying confounders such as methotrexate usage. In contrast, most of the congress abstracts did not indicate whether discontinuations included back-switches and did not appear to adjust for clinically relevant confounders [reflected in the Downs and Black quality scoring (Fig. S1)].
In the BIO-SPAN study, 10% of patients switching from reference ETN to SB4 discontinued vs. 8% in an historic cohort of reference ETN-treated patients. The relative risk (adjusted hazard ratio, HR) of discontinuing treatment in the 6 months following the switch was 1.57 (95% CI 1.05–2.36), which implied an adjusted discontinuation rate of 12.5% in switchers [24]. The main reasons for discontinuation of SB4 and reference ETN were lack of effect (43% vs. 61%) and AEs (47% vs. 28%). These AEs were more frequently subjective for switch patients: 84% (46/55 AEs in 28 patient) vs. 40% (6/15 in 13 patients) for the historical reference ETN cohort. Patient questionnaires prior to switching did not show an association between patients’ beliefs about medications or treatment expectations and discontinuation. SB4 discontinuation was associated with lower reported self-efficacy with regard to coping with pain and other symptoms related to arthritis and a shorter duration of reference ETN treatment. Analysis of the DANBIO registry also found a lower adjusted retention rate over 12 months for switchers compared with historic ETN controls, who had similar baseline characteristics, but differences between switchers and contemporary non-switchers were not significantly different [21]. In both switchers and non-switchers retention rates were lower for patients not in remission at switch or baseline (crude HR [95% CI] 1.7 [1.3–2.2] and 2.4 [1.7–3.6], respectively), leading the authors to conclude that patient-related rather than drug-related factors were more important in the decision to discontinue. The possibility of a nocebo effect contributing to discontinuation was suggested by Scherlinger et al., who noted variability in the pain indexes between patients after switches, which might be linked to fluctuation of painful periods in the natural process of rheumatic disease.
In patients with PsO in the DERMBIO registry discontinuation rate did not differ significantly between SB4 and reference ETN: adjusted HR adjusted for sex, methotrexate use and presence of PsA was 0.50 (95% CI 0.11–2.02, p = 0.317) [20].
Retention rates over 6 months post-switch in analyses presented in six congress abstracts (as reported or calculated from the number of discontinuations reported) ranged from 82% to 91% (Table S1) [33, 35, 37, 39, 41, 44]. Two studies found lower retention rates (Table S1). In a small study in the Netherlands (n = 69 switchers), the retention rate for SB4 was 75% [42]. In a UK study (n = 72 switchers) retention rate was 73.6%; the main reasons for SB4 discontinuation were loss of effect (58%) and AEs (32%); withdrawal in patients with RA was associated with duration on reference ETN (odds ratio 1.43 [95% CI 1.02–2.00]) [46]. The authors highlighted the fact that there was no significant change in disease activity for SpA and PsA, and the loss of effect in RA was due to subjective measures except for CRP, suggesting that there were reasons for withdrawal not detected by the study. An analysis of non-medical switchers in a single rheumatology centre in Sweden (reported in a journal letter) showed retention rate declining from 90% at 6 months to 78% at 12 months and 69% at 18 months, which was significantly lower compared with a historical cohort (p = 0.0015) [8]. This was explained by patient preference rather than objective worsening of disease.
Back-Switching
A proportion of patients who switched from reference ETN to SB4 discontinued and back-switched to reference ETN. Some of the smaller studies highlighted disease flares as a reason for back-switching [23, 30, 43]. This was the case for 3 out of 44 patients back-switching in a single-centre French study [23]. In a national audit of Wales, 18 of 120 switching patients experienced flares and nine of these back-switched to reference ETN [43].
Evidence from the large DANBIO registry study suggests the importance of subjective reasons for back-switching. Glintborg et al. found that 7% (120/1641) of switchers back-switched after a median duration of SB4 treatment of 120 days [IQR (interquartile range) 73–193], mainly because of lack of effect of SB4 (48–65% depending upon indication) and AEs (30–42% depending upon indication) [21]. The baseline characteristics of back-switchers were not significantly different from switchers. However, the authors highlighted that changes in disease activity in patients restarting ETN compared to while receiving SB4 were mainly subjective. Changes in disease activity at the time of ETN restart were mainly confined to Patient Global Scores, with little or no changes in CRP and swollen joint counts. Median (IQR) changes for patients with RA, PsA and AxSpA were as follows: Patient Global Score 30 (12–52) mm, 15 (7–77) mm and 25 (19–35) mm, respectively; swollen joint count 1 (0–4), 0 (0–0) and not applicable (AxSpA), respectively; and CRP 0 (− 1 to 5) mg/l, 1 (0–2) mg/l and 0 (0–4), respectively. Similarly, subjective reasons in terms of patient preference were the primary reason for back-switching in a Dutch single-centre study. At time of censoring in the DANBIO study, 104/120 (87%) of patients who back-switched were still being treated with ETN with a median treatment duration of 236 (155–302) days.
Three small studies reported in congress abstracts found AEs to be the most frequent reason for back-switching, accounting for 58% (7/12) [42], 60% (6/10) [48] and 62.5% (6/8) [36] of back-switching, respectively (Table S1). Four congress abstracts reported ineffectiveness rather than AEs as the main reason for back-switching, accounting for 50% (7/14) [37], 80% (4/5) [39], 61% (11/18) [47] and 58% (11/19) [46] of back-switching, respectively (Table S1). In the last of these studies, the observed lack of effect was mainly subjective (see switching outcomes section above).
One study investigated the rate of back-switching in patients switching to SB4 in different time periods [44]. Over time, there was a constant increase in the use of SB4 but the proportion of patients back-switching to reference ETN significantly increased from 7% (53/707) of patients starting SB4 between February 2016 and September 2016 (p < 0.05) to 10% (153/1607) of those starting between February 2016 and March 2017 to 14% (320/2229) of patients starting SB4 between February 2016 and August 2017 (p < 0.05). No reasons for back-switching were reported in this study.
SB4 Effectiveness and Retention Rates in ETN-Naïve Patients
Data from the BSRBR-RA registry found that the effectiveness of SB4 in bionaïve patients was not significantly different from reference ETN after adjusting for baseline characteristics [27]. SB4 was also found to be effective in two studies of ETN-naïve patients with PsO. In a study of 39 patients from the Psobiosimilars registry, mean (SD) Psoriasis Area Severity Index (PASI) reduced from 12.5 (6.2) at baseline to 6.7 (2.2) at month 6 [24]. In an Italian study of 12 ETN naïve patients, PASI score improved by more than 50% in eight patients at week 12 of SB4 treatment, and by week 2, by which time two patients had discontinued because of PsA reactivation and psoriasis worsening, a 75% reduction in PASI score was achieved by 9 of 10 patients [22]. Baganz et al. concluded from their study of the German RABBIT registry that reference ETN and SB4 were equally effective in bionaïve patients initiating these treatments (n = 313 and n = 266, respectively) [28]. Retention was greater for SB4, with 17.3% discontinuations up to 180 days after drug commencement compared with 29.4% discontinuations in the reference ETN cohort; adjusting for disease duration and co-morbidities made no significant difference. AEs were the most frequent cause of discontinuations in both SB4- (48%) and ETN-treated patients (54%). The most frequent AEs resulting in discontinuations were disease flares in SB4 patients (12% of AEs) and skin reactions at the injection site in reference ETN patients (26% of AEs).
Health Economics
Six congress abstracts reported economic outcomes of switching from reference ETN to SB4 and these were all cost savings (Table S1) [32, 34, 36, 37, 39, 41]. For example, switching 151 patients to SB4 in a UK hospital was estimated to result in a total saving of approximately £500,000 per annum [36]. The investment in a high intensity programme of patient support for switching saved approximately £9000 per biologic-treated patient over a year. In another group of hospitals in the UK potential yearly savings were estimated at £660,000 and enabled the employment of an additional clinical nurse and secretary.
Discussion
This systematic review provides a comprehensive summary of published observational studies on patients in Europe receiving the ETN biosimilar SB4 in routine practice over the 3 years after it gained marketing authorisation. Data from more than 13,500 patients in observational studies and patient registries have been published. As to be expected in the early years after the introduction of a biosimilar into the market, most studies presented results from patients switching from reference ETN to SB4. However, large numbers of patients have also been initiated on SB4 therapy without any previous exposure to ETN.
Across Europe, different national or local policies for the introduction of SB4 have influenced its switch acceptance rate; in most of the studies there was a clinic-wide, non-medical switch of patients from reference ETN to SB4. When the acceptance rate was reported, this was in a majority of patients, ranging from 51.6% to 99% across nine studies; in eight of these, the acceptance rate was at least 79% [21, 23, 24, 33,34,35, 38, 42, 47]. The highest rate of switching reported was in the non-mandatory setting of the BIO-SPAN study (99%) [24]. A lower acceptance rate (79%) in the DANBIO Registry in Denmark, where switching was mandated [21], was thought to be influenced by patient-related factors such as co-morbidities and higher disease activity in non-switchers and the absence of a 25 mg dose of SB4 at the time of the study [21]. A higher rate of methotrexate use was also observed in switchers vs. non-switchers in both the DANBIO registry [21] and the Swedish Rheumatology Quality register [19]; in the latter this was also seen for switchers to infliximab biosimilar [19]. The studies’ findings diverged, however, with respect to duration of treatment with reference ETN, which was longer in switchers in the DANBIO registry compared with switchers in the Swedish Rheumatology Quality register.
The importance of patient education and support and clinician attitude towards switching has been highlighted with respect to acceptance rate. The lowest rate of optional acceptance rate was in a smaller French study (51.6%) and this was attributed to clinician behaviour [35]. In a real-world study on the acceptance of biosimilars in Germany, the most common concern of patients was that they did not know enough about the drug, and this was more frequent in patients prescribed a biosimilar than a reference product (36–41% vs. 25–30%) [49]. Patients in the UK have similarly reported that better communication by healthcare professionals would increase their acceptance of biosimilars [50]. Experts across Europe have cited lack of education of both clinicians and patients as the main hurdle for acceptance of biosimilars [51].
Overall the effectiveness of SB4 was comparable with reference ETN in terms of disease activity pre- and post-switch and also in ETN-naïve patients. Results from DANBIO suggest that SB4 effectiveness did not vary with indication, but most reports did not give sufficient details to evaluate this [21]. In patients from the Psobiosimilars registry, switching to SB4 did not result in any meaningful change in PASI or additional AEs, and SB4 was also effective in ETN-naïve patients [25].
Safety data were incompletely reported in most of the studies, but there was no indication from the AEs summarised that the safety profile of SB4 was different in routine practice from that demonstrated in the controlled clinical trial situation. The lack of immunogenicity data was in line with data from clinical studies that reported low rates of ADAs without clinical relevance and consequently ADAs are not commonly collected in rheumatology practice [15]. In a non-interventional study of ETN-treated patients, in which all samples were assessed in a single laboratory using the same methodology, no ADAs were detected [52].
Retention rates for SB4 were generally high. However, two comparisons between SB4 switchers and historic cohorts of ETN-treated patients showed lower retention rates in patients switching to SB4 [21, 24]. The small difference in the retention rate in the BIO-SPAN study might be attributable to calendar time bias, because clinicians were more likely to use a ‘treat-to-target’ approach in 2016 than in 2014—the time of the historic cohort [24]. SB4 retention appeared to be negatively influenced by subjective (symptoms only perceptible to the patient) [24] or non-specific AEs [21], subjective assessment of disease activity (e.g. self-reported pain, Patient Global Score) [21, 24] and patient characteristics such as remission status at baseline (assessed by DAS28 or the Ankylosing Spondylitis Disease Activity Score) [21]. A similar finding was reported by Tweehuysen et al. in separate study, where a quarter of patients switching from infliximab reference to biosimilar (CT-P13) discontinued because of subjective assessments of disease activity or subjective AEs [53]. Defined nocebo effects have been reported in 12.8% of patients switching to an infliximab biosimilar, where a nocebo response was defined as “an unexplained, unfavourable therapeutic effect subsequent to a non-medical switch from originator infliximab to biosimilar infliximab with regaining of the beneficial effects after reinitiating the originator” [54]. Tweehuysen et al. hypothesized that the higher retention rate they found for SB4 compared with that for an infliximab biosimilar in an earlier study could be due to increased confidence among clinicians after this previous biosimilar experience, and the implementation of communication strategies that may have positively influenced patients and reduced the likelihood of nocebo effects [24, 53].
SB4 was also as effective as reference ETN in bionaïve patients in the BSRBR-RA registry with respect to DAS28 and remission status [27]. These real-world data indicate that the clinical equivalence between SB4 and reference ETN that was demonstrated in bionaïve patients in the phase 3 RCT [13, 14] and in switched patients in the RCT open-label extension to this [15] is also seen in routine clinical practice. Furthermore, analysis of patients in a multi-switching cohort suggested that switching from reference ETN to SB4 and then back to reference ETN for non-medical reasons does not impact disease activity [8].
Back-switching rates across all the reports reviewed varied from 4.6% to 26.4% [8, 21, 23, 44, 46,47,48], probably reflecting the diversity in settings and patient numbers. There was also one study of a clinic-wide non-medical back-switch following a reduction in the price of reference ETN [8]. Some of the data on back-switching and discontinuations were suggestive of a nocebo effect; notably Glintborg et al. found the reasons given for back-switching were mainly subjective on the part of patients [21].
Real-world data opens the window to what is actually happening in clinical practice. It benefits from external validity and can provide insights into indications not investigated in RCTs. The real-world evidence base presented here is rich in terms of overall patient numbers, geography and spread of indications; but its limitations must be equally recognised. To a degree, high external validity sacrifices internal validity, making it difficult to definitively attribute differences or similarities in outcomes to the intervention. Comparisons of retention rates and drug effectiveness for non-switchers receiving reference ETN and switchers receiving SB4 are likely to be influenced by selection bias. Some of the analyses by Glintborg et al. and Sigurdardottir and Svard extended to follow-up durations of 12 and 24 months, respectively [8, 21]. However, many of the other studies had follow-up durations of only 6 months, which is likely to have made their findings more susceptible to the effects of nocebo responses. As a result of the observational setting, controlling for bias e.g. selection bias, attrition bias is challenging. As expected, the quality scoring of publications showed the quality of evidence from congress abstracts to be generally lower and more varied than that from full journal publications. Lower quality was primarily due to incomplete reporting as a result of word count restriction, lack of clarity in the methodology and lack of statistical consideration for confounders. Nevertheless, balancing the strengths and limitations of real-world evidence on SB4, the importance of continued commitment to generation of post-approval data and to maintenance of high-quality patient registries is apparent.
Most switches from reference ETN to SB4 were made for reasons of cost-saving, and the realisation of these was demonstrated in several studies. Savings were reported only briefly in congress abstracts with insufficient detail for critical appraisal; however, they are an indication of tangible benefits from clinic-wide switching policies.
Limitations of the Current Review
Systematic reviews are a robust tool for gathering, evaluating and summarising data from existing studies on a particular topic and drawing conclusions from this larger data pool. However, the quality of the subsequent review is dependent upon the data available. In the case of the current review only 6 of the 31 selected publications were full articles, with the majority of the remainder being conference abstracts. This limited the available data for each study and the level of analysis that could be performed. Thus, while we believe the overall conclusions of the review are robust, it is possible that some of the contributing data could alter when the full articles are published.
The fact that all of the authors are employees of the company that markets the product of interest could be viewed as introducing potential bias into the review. However, one of the main purposes of a systematic review is to reduce potential bias as much as possible by introducing clear selection and scoring processes through the use of checklists as detailed in the “Methods” section. We are confident that the conclusions of the current article are a true reflection of the data published.
Conclusion
A substantial body of real-world evidence now exists on the use of SB4, the first subcutaneous anti-tumour necrosis factor biosimilar to become available in Europe. While the quality of the reported evidence is variable, the experience of switching from reference ETN to SB4 has been generally positive, without loss of effectiveness or detriment to safety and tolerability compared with the reference ETN, and with persistence rates of at least 75%. Reported acceptance has been mainly high, ranging from 51.6% to 99% across nine studies, in eight of which it was at least 79%; patient acceptance has benefited from a supportive communications programmes. ETN-naïve patients have been initiated on SB4 treatment (n = 2499 reviewed here) and no differences are reported in their clinical outcomes compared with those of patients treated with ETN. Overall, the body of real-world evidence from 13,552 patients presented here provides additional reassurance that the biosimilar approval pathway is robust and that SB4 is as effective and safe as ETN reference in both switched and naïve patients.
References
Committee for Medicinal Products for Human Use. Guideline on similar biological medicinal products. 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf. Accessed 6 Feb 2019.
Food and Drug Administration. Biosimilars and interchangeable products. 2017. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm580419.htm. Accessed 7 May 2019.
Katkade VB, Sanders KN, Zou KH. Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc. 2018;11:295–304.
Scavone C, di Mauro G, Mascolo A, Berrino L, Rossi F, Capuano A. The new paradigms in clinical research: from early access programs to the novel therapeutic approaches for unmet medical needs. Front Pharmacol. 2019. https://doi.org/10.3389/fphar.2019.00111.
Kristensen LE, Alten R, Puig L, et al. Non-pharmacological effects in switching medication: the nocebo effect in switching from originator to biosimilar agent. Biodrugs. 2018;32(5):397–404.
Rezk MF, Pieper B. Treatment outcomes with biosimilars: be aware of the nocebo effect. Rheumatol Ther. 2017;4(2):209–18.
European Medicines Agency. Summary of the risk management plan (RMP) for Benepali (etanercept). 2015. https://www.ema.europa.eu/en/documents/rmp-summary/benepali-epar-risk-management-plan-summary_en.pdf. Accessed 6 Feb 2019.
Sigurdardottir V, Svard A. Repeated switches between reference product etanercept and biosimilar do not affect disease activity or retention rate of etanercept over 24 months—a cohort study with historical controls. Joint Bone Spine. 2019;86(4):529–30.
Amgen. Enbrel Prescribing Information. Thousand Oaks: Amgen. 2019. https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/enbrel/enbrel_pi.pdf. Accessed 2 Aug 2019.
Pfizer Europe. Enbrel summary of product characteristics. Brussels: Pfizer Europe; 2018.
Gulacsi L, Brodszky V, Baji P, et al. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev Clin Immunol. 2015;11(Suppl 1):S43–52.
Kalo Z, Voko Z, Ostor A, et al. Patient access to reimbursed biological disease-modifying antirheumatic drugs in the European region. J Mark Access Health Policy. 2017;5(1):1345580.
Emery P, Vencovsky J, Sylwestrzak A, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):51–7.
Emery P, Vencovsky J, Sylwestrzak A, et al. 52-week results of the phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatol (Oxford). 2017;56(12):2093–101.
Emery P, Vencovsky J, Sylwestrzak A, et al. Long-term efficacy and safety in patients with rheumatoid arthritis continuing on SB4 or switching from reference etanercept to SB4. Ann Rheum Dis. 2017;76:1986–91.
Charles C. The evolving biosimilar landscape: approval of the first etanercept biosimilar in Europe an interview with Emilio Martin-Mola. Eur Med J. 2016;1(3):76–84.
European Public Assessment Report for Benepali, European Medicines Agency. 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/benepali. Accessed 2 Aug 2019.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84.
Di Giuseppe D, Frisell T, Ernestam S, et al. Uptake of rheumatology biosimilars in the absence of forced switching. Expert Opin Biol Ther. 2018;18(5):499–504.
Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178(2):509–19.
Glintborg B, Loft AG, Omerovic E, et al. To switch or not to switch: results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. One-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann Rheum Dis. 2019;78(2):192–200.
Pescitelli L, Lazzeri L, Di Cesare A, Tripo L, Ricceri F, Prignano F. Clinical experience with the etanercept biosimilar SB4 in psoriatic patients. Int J Clin Pharm. 2019;41(1):9–12.
Scherlinger M, Langlois E, Germain V, Schaeverbeke T. Acceptance rate and sociological factors involved in the switch from originator to biosimilar etanercept (SB4). Semin Arthritis Rheum. 2019;48(5):927–32.
Tweehuysen L, Huiskes VJB, van den Bemt BJF, et al. Open-label, non-mandatory transitioning from originator etanercept to biosimilar SB4: six-month results from a controlled cohort study. Arthritis Rheumatol. 2018;70(9):1408–18.
Gisondi P, Bianchi L, Calzavara-Pinton P, et al. Etanercept biosimilar SB4 in the treatment of chronic plaque psoriasis: data from the Psobiosimilars registry. Br J Dermatol. 2019;180(2):409–10.
De Cock D, Dyball S, Kearsley-Fleet L, Watson K, Hyrich K. Profiling rheumatoid arthritis biosimilar switchers: data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (UK). 2018;57(suppl 3):iii148.
De Cock D, Kearsley-Fleet L, Davies R, Watson K, Hyrich K. SB4 shows comparable short-term effectiveness to its etanercept originator as first-line biologic treatment for patients with rheumatoid arthritis in routine clinical care. Arthritis Rheumatol. 2018;70(suppl 10):2539.
Baganz L, Meissner Y, Herzer P, Braun J, Grassler A, Strangfeld A, Zink A. Treatment continuation on the etanercept original in comparison with a biosimilar. Arthritis Rheumatol. 2018;70(suppl 10):2512.
Rabbitts R, Jewell T, Marrow K-L, Herbison C, Laversuch C. Switching to biosimilars: an early clinical review. Rheumatology. 2017;56(suppl 2):ii132.
Steel L, Marshall T, Loke M. Real world experience of biosimilar switching at the Norfolk and Norwich University Hospital, United Kingdom. Ann Rheum Dis. 2018;77(Suppl):321.
Haugeberg G, Bakland G, Rødevand E, Fevang BT, Hansen IJW, Diamantopoulos A. Drug survival and reason for drop-out in rheumatoid arthritis patients with a non-medical switch from originator to biosimilar etanercept—preliminary data from a Norwegian multicenter study. Ann Rheum Dis. 2018;77(suppl 2):1383.
Shah K, Flora K, Penn H. Cost effectiveness of a high intensity programme (HIP) compared to a low intensity programme (LIP) for switching patients with rheumatoid arthritis to the etanercept biosimilar. Rheumatology (UK). 2018;57(suppl 3):iii68–9.
Holroyd C, Wallis D, Bennett S, Clayton P, Edwards CJ. Switching from bio-original etanercept to biosimilar etanercept SB4: patient acceptability and outcomes in the real world. Ann Rheum Dis. 2017;76(supple 2):1180.
Szlumper C, Topping K, Blackler L, et al. Switching to biosimilar etanercept in clinical practice. Rheumatology (UK). 2017;56(suppl 2):ii139.
Al Tabaa O, Etcheto A, Miceli-Richard C. Switch from innovator etanercept to biosimilar etanercept in inflammatory rheumatic diseases: the experience of Cochin University Hospital Paris-France. Ann Rheum Dis. 2018;77(suppl 2):960.
Shah K, Flora K, Penn H. Clinical outcomes of a multi-disciplinary switching programme to biosimilar etanercept for patients with rheumatoid arthritis. Rheumatol (UK). 2018;57:iii143–4.
Alkoky H, Pakozdi A, Tahir H. Benepali switches in clinical practice—a positive single centre experience. Arthritis Rheumatol. 2018;70(suppl 10):2511.
Brites L, Costa F, Freitas J, et al. Impact of block switch to biosimilar etanercept in practice, across different rheumatic diseases. Arthritis Rheumatol. 2018;70(suppl 10):2534.
Dyball S, Hoskins V, Christy-Kilner S, Haque S. Effectiveness and tolerability of benepali in rheumatoid arthritis patients switched from enbrel. Arthritis Rheumatol. 2017;69(Suppl 10):2447.
Kruger K, Selmi C, Cantagrel A, et al. Benefit study: results of interim analysis of a pan European observational study to evaluate real-world effectiveness of SB4 following transition from originator etanercept (ETN) in patients with rheumatoid arthritis or axial spondyloarthritis. Arthritis Rheumatol. 2018;69(suppl 10):2537.
Ma J, Petford S, Jones L, Douglas K, John H. Audit of the clinical efficacy and safety of etanercept biosimilar to its reference product in patients with inflammatory arthritis: experience from a district general hospital in the United Kingdom. Ann Rheum Dis. 2018;77(suppl 2):1386.
Müskens WD, Rongen-van Dartel SAA, Adang E, van Riel PL. The influence of switching from etanercept originator to its biosimilar on effectiveness and and the impact of shared decision making on retention and withdrawal rates. Ann Rheum Dis. 2018;77(suppl 2):1399.
Rajamani K, Choy E. Change in disease activity after switching etanercept (originator) to biosimilar (Benepali) is associated with active disease at baseline. Arthritis Rheumatol. 2018;70(suppl 10):2513.
Alten R, Jones H, Curiale C, Meng T, Lucchese L, Miglio C. Real world evidence on switching between etanercept and its biosimilar in rheumatic diseases. Ann Rheum Dis. 2018;77(suppl 2):961.
De Cock D, Kearsley-Fleet L, Davies R, et al. Biosimilar use in patients with juvenile idiopathic arthritis in a real-world setting in the United Kingdom. Ann Rheum Dis. 2018;77(suppl 2):485.
Madenidou AV, Jeffries A, Varughese S, et al. Switching patients with arthritis from etanercept (Enbrel) to the biosimilar drug, Benepali: a single-center retrospective observational study. Arthritis Rheumatol. 2018;70(suppl 10):2533.
Patel D, Ahmed TJ, Levy S, Rajak R, Sathananthan R, Horwood N. Analysis of rheumatoid arthritis patients who failed the switch from originator etanercept to biosimilar etanercept in Croydon. Rheumatology (UK). 2018;57:iii199.
Smith R, Fawthrop F. Similar experience of biosimilars: a review of Rotherham Hospital’s experience of switching from enbrel to benepali. Rheumatology (UK). 2018;57(suppl 3):iii64.
Waller J, Sullivan E, Piercy J, Black CM, Kachroo S. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence. 2017;11:519–30.
Aladul MI, Fitzpatrick RW, Chapman SR. Patients’ understanding and attitudes towards infliximab and etanercept biosimilars: result of a UK web-based survey. Biodrugs. 2017;31(5):439–46.
Moorkens E, Vulto AG, Huys I, et al. Policies for biosimilar uptake in Europe: an overview. PLoS One. 2017;12(12):e0190147.
Moots RJ, Xavier RM, Mok CC, et al. The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: results from a multinational, real-world clinical practice, non-interventional study. PLoS One. 2017;12(4):e0175207.
Tweehuysen L, van den Bemt BJF, van Ingen IL, et al. Subjective complaints as the main reason for biosimilar discontinuation after open-label transition from reference infliximab to biosimilar infliximab. Arthritis Rheumatol. 2018;70(1):60–8.
Boone NW, Liu L, Romberg-Camps MJ, et al. The nocebo effect challenges the non-medical infliximab switch in practice. Eur J Clin Pharmacol. 2018;74(5):655–61.
Acknowledgements
Funding
This review and the journal’s Rapid Service Fee were funded by Biogen International GmbH. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance were provided by Susanna Ryan of Springer Healthcare and were funded by Biogen International GmbH.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to the design of the review methodology, analysis of the data, and critical review and modification of the manuscript. All authors have approved the final version of the manuscript.
Disclosures
Hans C. Ebbers is an employee of Biogen International GmbH and may hold stock in Biogen. Burkhard Pieper is an employee of Biogen International GmbH and may hold stock in Biogen. Amine Issa is an employee of Biogen International GmbH and may hold stock in Biogen. Janet Addison is an employee of Biogen International GmbH and may hold stock in Biogen. Ulrich Freudensprung is an employee of Biogen International GmbH and may hold stock in Biogen. Mourad F. Rezk is an employee of Biogen International GmbH and may hold stock in Biogen.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analysed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8982353.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any non-commercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ebbers, H.C., Pieper, B., Issa, A. et al. Real-World Evidence on Etanercept Biosimilar SB4 in Etanercept-Naïve or Switching Patients: A Systematic Review. Rheumatol Ther 6, 317–338 (2019). https://doi.org/10.1007/s40744-019-00169-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-019-00169-4