Abstract
The introduction of effective systemic therapies has significantly changed the treatment of stage III and IV melanoma. Both immune checkpoint inhibitors and targeted therapies have improved recurrence-free survival in the adjuvant setting. Recent interest has sparked for neoadjuvant systemic therapy with immune checkpoint inhibitors. The intended benefit of pre-operative treatment with immunotherapy is amongst others to enable tailoring of the surgery and adjuvant systemic therapy according to the treatment response. Most importantly, recurrence-free survival might be improved by neoadjuvant systemic therapy over the current standard of care of surgery followed by adjuvant systemic therapy. The first phase I and II trials investigating anti-PD1 inhibitors, both as a single agent and in combination with anti-CTLA-4 inhibitors or other therapeutic agents, have shown promising results. Pathological complete response on neoadjuvant systemic therapy seems a valid surrogate endpoint for relapse-free and overall survival. Pathological complete response rates in these trials vary between 30 and 70%. The optimal dose with respect to efficacy and toxicity and the interval between systemic and surgical treatment remain important issues to address. Accumulating follow-up data and ongoing phase III studies must prove if neoadjuvant systemic therapy is superior to surgery followed by standard-of-care adjuvant therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The treatment of advanced (metastatic stage IV) melanoma with systemic (immuno)therapies has significantly improved survival. |
Phase I and II trials have proven the safety and effectiveness of neoadjuvant systemic therapy with immune checkpoint inhibitors. |
Neoadjuvant systemic therapy could potentially tailor the extent of surgery and/or any adjuvant systemic therapy, based on the pathologic response obtained. |
1 Introduction
The concept of neoadjuvant systemic therapy (NAST) is not novel, it has been used for different types of cancers for decades, with varying degrees of success. One of the most frequently listed goals of NAST is an increased efficacy leading to a better outcome, for example, less positive resection margins, less relapse and improved survival. Other goals of NAST that are currently pursued are: less extensive extent of surgery, more organ preservation and less morbidity. Examples for this include breast and colorectal cancer.
For breast cancer, NAST can convert the need for a breast amputation into the option to perform breast conservative surgery, without diminishing the survival chances by doing so [1]. However, NAST in breast cancer does not improve survival compared to adjuvant systemic therapy, the survival of NAST and adjuvant systemic therapy is equivalent [1, 2]. It is important to realize that this concerns NAST with chemotherapy in breast cancer and this might differ from immune checkpoint inhibitor (ICI)-based NAST, which has a completely different mechanism of action.
The oncology world has changed rigorously since the first positive result of an ICI therapy was demonstrated in melanoma. It was the anti-CTLA-4 inhibitor ipilimumab that was the first to improve survival for patients with stage IV melanoma, who were considered to have an infaust prognosis before [3]. Thereafter, anti-PD-L1 therapies (i.e. nivolumab, pembrolizumab) have been successfully brought to the clinic for a large diversity of cancer types [4,5,6]. Finally, the combination of anti-CTLA-4 and anti-PD-L1 has shown some of the highest benefits for a few cancer types, including melanoma, albeit at the cost of considerably more toxicity [7]. The blocking of novel checkpoints are currently being attempted, with anti-LAG-3 (i.e. relatlimab) the most recent to show a successful benefit [8].
Melanoma has been historically treated with surgery as much as possible because of the lack of effective systemic therapy for high-risk and stage IV melanoma prior to 2010. Therefore, effective NAST in melanoma remains in its early development. In this paper, we summarize the results obtained until now regarding the efficacy and safety of NAST in melanoma. Furthermore, we discuss the timing, specifics of intralesional therapies and future perspectives for NAST in melanoma.
2 Efficacy
2.1 History and Benchmark
First, before discussing the NAST results for melanoma, we must set the bar and create a benchmark to which we will later compare NAST. For the remainder of this paper, unless specifically described otherwise, we focus on patients with clinically detected (either by palpation or on imaging; macroscopic), resectable nodal stage III melanoma. Approximately, 10–20% of patients with melanoma are diagnosed yearly as such.
Historically, surgical resection through a formal therapeutic lymphadenectomy or therapeutic lymph node dissection (TLND) has always been the cornerstone of treatment for these patients. Despite this surgery with a curative intent, a considerable proportion of patients (between 20 and 80%) has been reported to progress to stage IV melanoma within months to years and subsequently succumb to the disease [9,10,11,12,13].
For many years, adjuvant therapy had focused on the use of interferon (IFN), with varying schedules being examined, including high-dose (HD), intermediate-dose, low-dose, with or without an induction phase, short or longer maintenance and/or pegylated IFN. The results of all IFN trials were modest, as it did have a small benefit in terms of relapse-free survival (RFS), but limited to no effect on overall survival (OS) [14–24]. Therefore, the use of adjuvant IFN was varied across the world, with some countries where it would be considered standard-of-care (SoC) therapy and nearly all eligible patients receiving this therapy, whereas other countries, based on the same trial evidence, did not include adjuvant IFN in their guidelines and it would almost not be prescribed [25].
This all has changed radically during the last decade with the advent of ICI and BRAF-directed therapies. The 5-year OS of stage IIIB and IIIC melanoma used to be approximately 59% and 40%, respectively [10]. Updated OS data are not yet mature, but results from the pivotal phase III adjuvant trials have all reported improved RFS data.
2.2 Adjuvant ICI
The very first adjuvant trial of the modern era was the EORTC 18071 trial with HD ipilimumab. This trial used 10 mg/kg of adjuvant ipilimumab every 3 weeks (Q3W) for four courses and also included maintenance courses of ipilimumab every 12 weeks up to 3 years and compared this to placebo [26]. The trial demonstrated a significantly improved RFS, which translated into the same benefit for distant metastasis-free survival and OS, which was sustained even after a long-term follow-up [27]. This study was unique, as it showed a very clear effect of adjuvant therapy on RFS (± 10%) that translated into nearly exactly the same benefit for OS, which is currently much more difficult to demonstrate, as patients can be salvaged with an arsenal of therapies after progression. These effective therapeutic options were not yet available to the patients in the EORTC 18071 study, who were either treated as part of the study (adjuvant group) or not, as most could not (yet) access any effective drugs at that time outside of clinical trial(s), as BRAF-directed and other ICI therapies were not yet approved and thus not widely available.
Another thing that was different in the EORTC 18071 study was the choice of dose for ipilimumab, as after the trial was conducted, the dose for metastatic stage IV melanoma was determined at 3 mg/kg rather than 10 mg/kg, but in the adjuvant trial 10 mg/kg Q3W was used. It led to a significantly high rate of grade III/IV adverse events (AEs) that translated into premature termination of adjuvant therapy in nearly half of the patients [26]. Despite so many patients not completing the induction phase (or receiving any maintenance course(s) for that matter), the trial was still clearly positive in terms of efficacy.
Another trial, the North American Intergroup E1609 study, was originally designed to examine adjuvant ipilimumab at 10 mg/kg vs HD IFN; however, it was later amended to include a third arm with the more common dose of ipilimumab at 3 mg/kg. This trial demonstrated a significant benefit of adjuvant ipilimumab at 3 mg/kg over HD IFN. Ipilimumab 3 mg/kg was significantly less toxic than HD ipilimumab at 10 mg/kg [28].
The Checkmate 238 study was designed to compare adjuvant HD ipilimumab (10 mg/kg Q3W) vs nivolumab (3 mg/kg every 2 weeks). This trial too showed an improved efficacy of adjuvant nivolumab in terms of RFS benefit of approximately 10%, which was sustained with a long-term follow-up compared with an active control (HD ipilimumab) [29, 30]. Additionally, it was far less toxic, with approximately 15% of patients developing a grade 3/4 AE compared with 45% for HD ipilimumab.
These results were independently validated by a trial with another anti-PD-1 agent, pembrolizumab. The EORTC 1325/KN-054 study examined a fixed dose of pembrolizumab (200 mg Q3W, maximum of 17 adjuvant courses) vs a placebo in part 1 of the study. Part 2 of the trial consisted of a cross-over for patients who progressed on the placebo arm or re-treatment of patients on the adjuvant arm if they were more than 6 months after their last dose of adjuvant pembrolizumab. For now, we focus on the results of part 1 that showed an approximately 20% benefit in terms of RFS compared with placebo, which was again sustained after a long-term follow-up [31, 32]. Indirect modelling seems to indicate that the results obtained with another adjuvant anti-PD-1 agent are to be considered equivalent [33].
Because treatment for patients with stage IV metastatic melanoma had evolved towards the use of the combination of ipilimumab and nivolumab (IPI/NIVO), adjuvant studies were also set up to examine this. The first to report was the IMMUNED study that was a phase II adjuvant trial for patients with resected stage IV melanoma and had three arms: double placebo, adjuvant nivolumab (3 mg/kg every 2 weeks) and adjuvant IPI/NIVO (IPI 3 mg/kg and nivolumab 1 mg/kg Q3W for four courses, followed by nivolumab 1 mg/kg Q3W). This phase II trial showed a clear benefit of IPI/NIVO over nivolumab alone, which in turn showed improved survival compared with placebo [34].
However, to the surprise of many, the subsequent randomized phase III clinical trial Checkmate 915, comparing IPI/NIVO with nivolumab alone for resected stage IIIb–IV melanoma, turned out negative and thus the combination of IPI/NIVO is not used in the adjuvant setting for melanoma today [35]. Reasons for the lack of benefit in the Checkmate 915 study have only been speculated, but include amongst others the dose and schedule of adjuvant IPI/NIVO used by the trial. Where IPI/NIVO for patients with stage IV melanoma is typically dosed at ipilimumab 3 mg/kg and nivolumab 1 mg/kg Q3W for four courses, the Checkmate 915 study not only used the ‘flip dose’ of ipilimumab 1 mg/kg and nivolumab 3 mg/kg, but also at an interval of every 6 weeks.
2.3 Adjuvant BRAF-Directed Therapy
To complete the picture, we must also discuss the results for adjuvant BRAF-directed therapy. Approximately 40–50% of patients with melanoma harbor an activating BRAF mutation [36, 37]. For metastatic stage IV melanoma, safety and efficacy of combinations of BRAF and MEK inhibitors with vemurafenib and cobimetinib, dabrafenib and trametinib or encorafenib and binimetinib were demonstrated [38,39,40]. Only adjuvant therapy with dabrafenib and trametinib (D&T) was tested for patients with stage III melanoma BRAF V600E/K mutations in the adjuvant COMBI-AD study vs a placebo. This study showed an improved RFS of adjuvant D&T vs placebo, which, although its effect diminished slightly over time, still translated into a more than 15% RFS benefit after 5 years [41, 42].
Thus, currently, it is considered SoC to treat patients with clinically detected, resectable nodal stage III melanoma with TLND, followed by adjuvant systemic therapy that can be either anti-PD-1 (either nivolumab or pembrolizumab) or D&T for patients with a BRAF V600E/K mutation. Landmark 1-year and 2-year RFS is approximately 75% and 65% for patients after TLND and adjuvant anti-PD-1 [30, 32].
2.4 NAST
Until today, only phase I/II NAST trials have been conducted and reported for melanoma. There have been studies looking at NAST with anti-PD-1 alone and studies looking at the combination of IPI/NIVO (Table 1).
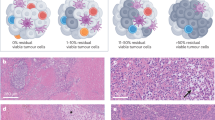
First, there is the study by Huang et al. that examined single-dose pembrolizumab NAST before surgery. It showed that 30% of patients could achieve a major pathologic response (MPR), which is either a pathologic complete response (pCR, no residual melanoma cells detected) or a near pCR (≤ 10% viable tumor cells left) [43].
Thereafter, a study by Amaria et al. showed similar results for NAST with nivolumab with 25% of patients developing a pCR [44]. However, the same study by Amaria et al. randomized patients between nivolumab alone or the combination of IPI/NIVO and showed a much higher pCR for the combination of 45% [44].
At the same time, the OpACIN study examined NAST IPI/NIVO vs adjuvant IPI/NIVO in a randomized phase Ib study. This study showed similar high overall response rates (ORRs) for the neoadjuvant combination of 78% [45]. Importantly, the OpACIN study was not designed to look at an improved efficacy of NAST vs adjuvant therapy, but did seem to indicate such in terms of improved RFS for NAST patients compared with adjuvant patients after a long-term follow-up [46]. A perhaps even more significant finding of the OpACIN study is the biomarker analysis, that showed a more extensive T-cell response for NAST patients compared with adjuvant patients, which might indicate a biologically driven, improved mechanism of action for NAST over adjuvant therapy [46].
The largest NAST study to date is the OpACIN-neo study, which examined three different schedules of IPI/NIVO, either ipilimumab 3 mg/kg plus nivolumab 1 mg/kg Q3W for two courses compared to the ‘flip-dose’ of ipilimumab 1 mg/kg plus nivolumab 3 mg/kg vs sequential treatment of ipilimumab 3 mg/kg followed by nivolumab 3 mg/kg. This study demonstrated significant overall response rates in all three arms of 65–80% with a MPR of 45–70% [46, 47]. This translated into a landmark 1-year RFS rate of 85%, which is sustained with a long-term follow-up [46, 47].
More recent developments include the PRADO extension cohort of the OpACIN-neo study. The aim of this extension cohort was to validate the safety and efficacy of the ‘flip-dose’ schedule and to determine if the index node could be used to determine response and tailor adjuvant treatment. Patients with clinically detected, resectable nodal stage III melanoma were treated with two courses of ipilimumab 1 mg/kg and nivolumab 3 mg/kg after which, rather than proceed immediately to TLND, they would undergo removal of the index node first. Based on the response, further treatment would be tailored. In the case of MPR, patients would have no further surgery or adjuvant systemic therapy. For patients with a partial response, they would undergo a TLND, but no further adjuvant systemic therapy. In patients with a non-response (> 50% viable tumor cells), TLND would be followed by adjuvant systemic therapy (either BRAF directed in the case of a BRAF mutation or anti-PD-1 for wild-type patients) and/or adjuvant radiotherapy to the nodal basin. First results have been presented and confirm an ORR of 71% and an MPR rate of 61% [48]. However, a longer follow-up is needed to determine if the omission of TLND was safe for patients with an MPR. Finally, a recent NAST study by Amaria et al. reported that the use of the anti-LAG-3 ICI relatlimab in combination with nivolumab demonstrated a 59% pCR rate [49].
2.5 INMC
The International Neo-Adjuvant Melanoma Consortium (INMC) has performed a pooled analysis of a number of the above-mentioned trials and demonstrated that pCR for NAST with ICIs is an excellent surrogate marker for RFS and OS [50].
3 Safety
The early NAST studies prioritized safety first. Safety consideration can be divided into two different items: AEs due to NAST or the failure to bring patients to (timely) surgery and thus potentially losing the ability to safely perform a potentially curative TLND.
With respect to the AEs, studies with single-agent anti-PD-1 have shown a favorable toxicity profile with 8–30% of patients presenting with grade ≥ 3 AEs [43, 44]. In contrast, NAST with IPI/NIVO combinations with the ipilimumab 3-mg/kg and nivolumab 1-mg/kg doses have shown significantly higher toxicity rates, with grade ≥ 3 AEs being seen in 73–90% of patients [44, 45]. Of note, this is a much higher rate of grade ≥ 3 AEs than patients with stage IV metastatic melanoma treated with the same schedule, which is noted at around 45% grade ≥ 3 AEs [7]. We can only speculate about the reasons for this, but one hypothesis is the increased efficacy and toxicity of this regime in NAST compared with patients with stage IV melanoma is due to the lesser immune suppressive status of the host immune system of those patients with earlier stage disease.
This has sparked investigators to examine different schedules of NAST to reduce toxicity. In this case, the OpACIN-neo study examined different schedules of the combination of IPI/NIVO. Either ipilimumab 3 mg/kg plus nivolumab 1 mg/kg Q3W for two courses compared to the ‘flip-dose’ of ipilimumab 1 mg/kg plus nivolumab 3 mg/kg vs sequential treatment of ipilimumab 3 mg/kg followed by nivolumab 3 mg/kg. The OpACIN-neo study concluded that efficacy was similar for all three treatment arms (albeit slightly less for the sequential arm vs the concurrent arms), but the toxicity was least (20% grade ≥ 3 AEs) for the ‘flip-dose’ arm of ipilimumab 1 mg/kg and nivolumab 3 mg/kg [47]. Investigators are using this schedule moving forward [48].
With respect to the failure to perform timely surgery and the loss of local control, the study with single-agent pembrolizumab did not see any delays or failures to perform timely surgery [43], whereas the Data Safety Monitoring Board terminated accrual of patients to single-agent nivolumab because of disease progression in 2/12 (17%) patients during NAST [44]. The first studies with the combination IPI/NIVO did not show any delays or failures [44, 45]. The PRADO extension cohort found progression during NAST in 7/99 patients and toxicity in 1/99 patients (total 8%).
Interestingly, a consideration for all pivotal phase III adjuvant trials is the fact that they reported only on patients who have successfully completed the screening phase. A report by Bloemendal et al. demonstrated that 18% of patients were excluded from their adjuvant dendritic cell trial because of progression detected during the screening phase [51]. Importantly, this was exclusively related to patients with clinically detected, nodal stage 3 melanoma (unlike other adjuvant trials that also include sentinel node-positive patients), all of whom had also been initially staged prior to their TLND. This rate of progression after TLND, prior to commencing adjuvant systemic therapy, needs to be considered when looking at the comparison with NAST and taking the progression during NAST into the balance.
4 Timing
What is the optimal duration of NAST prior to surgery? This is a difficult question to answer, as it has not been prospectively investigated in any randomized approach by any trial (yet). Huang et al. showed that with only a single dose of pembrolizumab already a 30% MPR could be achieved within 3 weeks [43]. However, one could hypothesize that the maximum reduction achievable by NAST might not yet be reached if one performs the surgery too soon. Others have used 12 weeks (four full courses of IPI/NIVO Q3W) and did not see better outcomes than what were seen after only two courses of IPI/NIVO (6 weeks) in the OpACIN or OpACIN-neo studies [44, 45, 47]. An excessively lengthy interval between starting NAST and performing surgery might create the opportunity for resistance to occur. Thus, empirically, it seems that the optimal duration of NAST is somewhere between 3 and 12 weeks, with most studies currently moving forward with a 6-week interval.
5 Future
5.1 INMC Principles
The INMC mission is to bring together key stakeholders across multiple disciplines including medical oncology, surgical oncology, pathology, radiology and translational research from institutions around the world with the goal of creating an organized approach into the investigation of neoadjuvant treatment in melanoma. Through this mechanism and with a comprehensive approach to maximizing collaborative opportunities amongst investigators and institutions, the INMC seeks to advance treatment for patients with melanoma.
Potential benefits of NAST according to the INMC include:
-
1.
Improving RFS and distant metastasis-free survival, with the ultimate goal to improve OS compared to SoC adjuvant therapy.
-
2.
Identifying a cohort of patients who have drug-responsive disease and might be treated with less extensive surgery, and possible without surgery at all.
-
3.
Identifying a cohort of patients with a favorable prognosis who may not require adjuvant radiotherapy and/or systemic therapy, and a tailored follow-up.
-
4.
The NAST response might provide important prognostic/predictive and toxicity information, and help direct the choice of adjuvant therapy.
-
5.
Identifying patients with resistant disease to direct towards clinical trials of novel therapies or new drug combinations.
-
6.
Reducing tumor burden to facilitate resection and potentially lessening the morbidity of resection.
-
7.
A model for drug development.
-
8.
Exploring biomarkers of response and resistance with the provision of unique high-value specimens collected routinely in the NAST paradigm, including sequential tissue and blood specimens before, during and after NAST.
-
9.
No delay in initiating effective systemic therapy.
5.2 Phase II Trials
There is currently a large volume of different phase II NAST trials ongoing, examining a wide range of different systemic agents, intralesional therapies and combinations. Examples include the DONIMI study (NCT04133948), which is examining the added value of the histone deacetylase inhibitor domatinostat together with ICIs. This trial demonstrates the use of the NAST platform as a model for drug development (Table 1).
5.3 Intralesional
Another area of interest within NAST is the use of locoregional therapies, specifically intralesional therapies. Melanoma is known to be able to present with a unique type of metastases that are satellite or in-transit metastases, which usually present as cutaneous or subcutaneous lesions between the scar of the original melanoma and the regional nodal basin. Approximately 4–8% of patients with melanoma develop such lesions [52]. The fact that these lesions are easily accessible for locoregional therapies makes them attractive for application. A number of locoregional therapies have shown their efficacy for patients with multiple, bulky and/or quickly recurrent in-transit metastases, such as isolated limb perfusion, isolated limb infusion, electrochemotherapy, CO2 laser, and more recently, the oncolytic virus talimogene laherparepvec (T-VEC) [53,54,55,56,57,58,59].
A randomized NAST trial with T-VEC vs SoC surgery demonstrated both an improved RFS (29.5% vs 16.5%) and OS (88.9% vs 77.4%), which was sustained after 3 years of follow-up [60]. The pCR rate was 17.1% for patients taking T-VEC [60]. Although this study was positive, it cannot compete with the results achieved above with NAST with the use of ICIs. Therefore, for example, the combination of T-VEC and nivolumab is currently being studied as NAST in the NIVEC study (NCT04330430).
5.4 NADINA
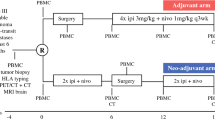
A phase III randomized trial to answer the question if NAST is superior to TLND followed by SoC adjuvant systemic therapy has commenced. This NADINA trial (NCT04949113) is randomizing patients with clinically detected, resectable nodal stage III melanoma (with up to a maximum of three satellite/in-transit metastases) to upfront TLND (plus resection of all satellite/in-transit metastases) followed by a maximum of a year of adjuvant nivolumab 480 mg every 4 weeks) or NAST with two courses of IPI/NIVO (ipilimumab 80 mg, nivolumab 240 mg Q3W). After NAST, patients will proceed to undergo surgery and based on the response, it will be determined how patients will continue their disease management. In the case of a non-response, patients will be eligible to receive either 11 courses of adjuvant nivolumab (480 mg every 4 weeks) or 46 weeks of adjuvant dabrafenib and trametinib (only for those patients whose melanomas harbor a BRAF V600E/K mutation). Patients with a pCR, near pCR and pathologic partial response (pPR) will not receive any further adjuvant therapy, but continue in the follow-up. The trial has started its accrual in 2021.
6 Conclusions
Treatment of clinically detected, resectable nodal stage III melanoma with NAST with the use of ICIs is safe and effective. It might even be superior to the current SoC TLND and adjuvant systemic therapy. Neoadjuvant systemic therapy also offers other potential benefits, such as the ability to tailor surgery with decreasing morbidity and adjuvant therapy based on the response to NAST.
References
Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94:1189–200.
Wolff AC, Davidson NE. Primary systemic therapy in operable breast cancer. J Clin Oncol. 2000;18:1558–69.
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32.
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34.
Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24–34.
Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67:472–92.
Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206.
Madu MF, Schopman JHH, Berger DMS, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244–51.
Madu MF, Wouters MW, Klop WM, et al. Clinical prognostic markers in stage IIIB melanoma. Ann Surg Oncol. 2016;23:4195–202.
van der Ploeg AP, van Akkooi AC, Schmitz PI, et al. Therapeutic surgical management of palpable melanoma groin metastases: superficial or combined superficial and deep groin lymph node dissection. Ann Surg Oncol. 2011;18:3300–8.
Agarwala SS, Lee SJ, Flaherty K, et al. Randomized phase III trial of high-dose interferon alfa-2b (HDI) for 4 weeks induction only in patients with intermediate- and high-risk melanoma (Intergroup trial E 1697). J Clin Oncol. 2011;29(Suppl):8505.
Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–26.
Flaherty LE, Othus M, Atkins MB, et al. Southwest Oncology Group S0008: a phase III trial of high-dose interferon alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma: an intergroup study of cancer and leukemia Group B, Children’s Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. J Clin Oncol. 2014;32:3771–8.
Grob JJ, Dreno B, de la Salmoniere P, et al. Randomised trial of interferon alpha-2a as adjuvant therapy in resected primary melanoma thicker than 1.5 mm without clinically detectable node metastases. French Cooperative Group on Melanoma. Lancet. 1998;351:1905–10.
Hauschild A, Weichenthal M, Rass K, et al. Efficacy of low-dose interferon {alpha}2a 18 versus 60 months of treatment in patients with primary melanoma of ≥ 1.5 mm tumor thickness: results of a randomized phase III DeCOG trial. J Clin Oncol. 2010;28:841–6.
Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–58.
Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–80.
Kirkwood JM, Manola J, Ibrahim J, et al. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–7.
Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17.
Mocellin S, Lens MB, Pasquali S, et al. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev. 2013;18;(6):CD008955.
Wheatley K, Ives N, Hancock B, et al. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29:241–52.
Harries M, Mohr P, Grange F, et al. Treatment patterns and outcomes of Stage IIIB/IIIC melanoma in France, Germany and the UK: a retrospective and prospective observational study (MELABIS). Int J Clin Pract. 2017;71:e12946.
Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–30.
Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer. 2019;119:1–10.
Tarhini AA, Lee SJ, Hodi FS, et al. A phase III randomized study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma (US Intergroup E1609): preliminary safety and efficacy of the ipilimumab arms. J Clin Oncol. 2017;35(15_Suppl):9500.
Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–35.
Ascierto PA, Del Vecchio M, Mandala M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:1465–77.
Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–801.
Eggermont AMM, Blank CU, Mandala M, et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 Trial. J Clin Oncol. 2020;38:3925–36.
Weber JS, Ascierto PA, Middleton MR, et al. Indirect treatment comparison of nivolumab versus placebo as adjuvant treatment for resected melanoma. Eur J Cancer. 2021;158:225–33.
Zimmer L, Livingstone E, Hassel JC, et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;395:1558–68.
Long GV, Schadendorf D, Del Vecchio M, et al. Adjuvant therapy with nivolumab (NIVO) combined with ipilimumab (IPI) vs NIVO alone in patients (pts) with resected stage IIIB-D/IV melanoma (CheckMate 915). Cancer Res. 2021;81(13_Suppl):CT004.
Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. 2017;548:234–8.
Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–80.
Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–88.
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16.
Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–15.
Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–23.
Dummer R, Hauschild A, Santinami M, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. 2020;383:1139–48.
Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25:454–61.
Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24:1649–54.
Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24:1655–61.
Rozeman EA, Hoefsmit EP, Reijers ILM, et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat Med. 2021;27:256–63.
Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20:948–60.
Blank CU, Reijers ILM, Pennington T, et al. First safety and efficacy results of PRADO: A phase II study of personalized response-driven surgery and adjuvant therapy after neoadjuvant ipilimumab (IPI) and nivolumab (NIVO) in resectable stage III melanoma. J Clin Oncol. 2020;38(15_Suppl):10002.
Amaria R, Postow M, Tetzlaff MT, et al. Neoadjuvant and adjuvant nivolumab (nivo) with anti-LAG3 antibody relatlimab (rela) for patients (pts) with resectable clinical stage III melanoma. J Clin Oncol. 2021;39(15_Suppl):9502.
Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. 2021;27:301–9.
Bloemendal M, van Willigen WW, Bol KF, et al. Early recurrence in completely resected IIIB and IIIC melanoma warrants restaging prior to adjuvant therapy. Ann Surg Oncol. 2019;26:3945–52.
Read RL, Haydu L, Saw RP, et al. In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol. 2015;22:475–81.
Madu MF, Deken MM, van der Hage JA, et al. Isolated limb perfusion for melanoma is safe and effective in elderly patients. Ann Surg Oncol. 2017;4:1997–2005.
Kroon HM, Coventry BJ, Henderson MA, et al. Evaluation of the efficacy and toxicity of upper extremity isolated limb infusion chemotherapy for melanoma: an Australian multi-center study. Eur J Surg Oncol. 2019;45:832–7.
Nan Tie E, Henderson MA, Gyorki DE. Management of in-transit melanoma metastases: a review. ANZ J Surg. 2019;89:647–52.
Campana LG, Mocellin S, Basso M, et al. Bleomycin-based electrochemotherapy: clinical outcome from a single institution’s experience with 52 patients. Ann Surg Oncol. 2009;16:191–9.
Testori A, Faries MB, Thompson JF, et al. Local and intralesional therapy of in-transit melanoma metastases. J Surg Oncol. 2011;104:391–6.
van Jarwaarde JA, Wessels R, Nieweg OE, et al. CO2 laser treatment for regional cutaneous malignant melanoma metastases. Dermatol Surg. 2015;41:78–82.
Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–8.
Dummer R, Gyorki DE, Hyngstrom J, et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage IIIB-IVM1a melanoma: a randomized, open-label, phase 2 trial. Nat Med. 2021;27:1789–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Conflict of interest
Alexander C.J. van Akkooi reports advisory board and consultancy honoraria (all paid to institute and unrelated to current work) from Amgen, Bristol-Myers Squibb, Novartis, 4SC, Merck-Pfizer, MSD-Merck, Pierre Fabre, Sanofi and Sirius Medical, and research grants from Amgen and Merck-Pfizer (all paid to the institute and unrelated to the current work). Michel W.J.M. Wouters declares a research grant from Novartis. Lisanne P. Zijlker declares that she has no conflicts of interest that might be relevant to the contents of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data included in this review are available from peer-reviewed and published articles.
Code availability
Not applicable.
Authors’ contributions
AvA has drafted this manuscript at the invitation of the editors of Springer Healthcare. AvA, LZ and MW were involved in the revision and final approval of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
van Akkooi, A.C.J., Zijlker, L.P. & Wouters, M.W.J.M. Neoadjuvant Immune Checkpoint Inhibitor Therapy in Melanoma: Efficacy, Safety and Timing. BioDrugs 36, 373–380 (2022). https://doi.org/10.1007/s40259-022-00525-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00525-x