Abstract
Background
Anti-drug antibodies (ADAs) to bococizumab were detected in > 40% of subjects in the SPIRE lipid-lowering trials. The risk of cross-reactivity between anti-bococizumab antibodies and other approved anti-proprotein convertase subtilisin/kexin type-9 (PCSK9) monoclonal antibodies (mAbs) was investigated using a single-assay approach.
Methods
Bococizumab immunogenicity was assessed in SPIRE-HR, a 52-week study. The highest ADA titer sample from each ADA-positive subject (n = 155) was tested in vitro for cross-reactivity to alirocumab and evolocumab using a novel ADA assay approach. Additional specificity tiers within the bococizumab ADA assay against each drug were validated using recombinant PCSK9 as a surrogate cross-reactive positive control. If the highest ADA titer sample showed cross-reactivity, additional samples from that subject were analyzed. Cross-reactivity was determined by the ability of alirocumab or evolocumab to inhibit the sample signal greater than or equal to the cross-reactivity cut-points.
Results
ADAs were detected in 44.0% (155/352) of bococizumab-treated subjects, and 27.0% also developed neutralizing antibodies (NAbs). Median ADA and NAb titers ranged from 276 to 526 and 8 to 12 over the course of the study, respectively. From 155 ADA-positive subjects tested for cross-reactivity, one (0.6%) subject showed weak cross-reactivity to both alirocumab and evolocumab. This cross-reactivity signal was transient (from Days 337 to 373) and undetectable at the last ADA-positive timepoint (Day 407).
Conclusion
A novel, single-assay approach was validated to assess the potential cross-reactivity of anti-bococizumab antibodies to alirocumab and evolocumab. In subjects who developed ADAs to bococizumab, the likelihood of clinically relevant cross-reactivity to marketed anti-PCSK9 mAbs is remote, based on the low frequency of cross-reactivity observed, which was weak in signal inhibition and transient in nature.
Clinical Trial Registration
The SPIRE-HR study is registered on ClinicalTrials.gov under the identifier NCT01968954.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9) has an essential role in the biology of low-density lipoprotein (LDL) cholesterol (LDL-C), and in the past few years monoclonal antibodies (mAbs) against PCSK9 have become an important addition to the physicians’ armamentarium for the management of dyslipidemia [1, 2]. PCSK9 binds to the LDL receptor, resulting in its degradation and an increase in circulating levels of LDL-C [3, 4]. The importance of PCSK9 has been demonstrated by studying genetic mutations. For example, loss-of-function mutations are associated with decreased LDL-C levels and reduced coronary heart disease events [5], whereas gain-of-function mutations in PCSK9 cause familial hypercholesterolemia (FH), a condition associated with high LDL-C levels and elevated cardiovascular risk [6, 7]. Antibodies targeted against PCSK9 result in a higher density of LDL receptors at the surface of hepatocytes and ultimately this lowers LDL-C [8, 9]. Two fully human anti-PCSK9 mAbs (alirocumab, evolocumab) are approved for use alongside dietary changes and other lipid-lowering agents to reduce LDL-C levels in patients with FH or atherosclerotic cardiovascular disease [10, 11].
Bococizumab is a humanized IgG2Δa mAb targeting the LDL receptor-binding domain of PCSK9 [12]; it has been studied in phase I–III clinical studies, and has been found to both lower LDL-C and reduce cardiovascular event rates [13,14,15,16,17,18,19,20,21]. In November 2016, clinical development of bococizumab was discontinued as a result of the emerging clinical profile observed from the SPIRE (Studies of PCSK9 Inhibition and the Reduction of vascular Events) phase III lipid-lowering program [15, 22]. The SPIRE program reported an unanticipated attenuation of LDL-C lowering over time, alongside a higher incidence of anti-drug antibodies (ADAs), and a higher rate of injection-site reactions than other agents in this drug class [15, 22]. Forty-eight percent of subjects in the SPIRE lipid-lowering studies had detectable ADAs to bococizumab and 29% also had neutralizing antibodies (NAbs) [15]. However, all biologics have the potential to be immunogenic, and although humanizing reduces the risk of an immune response, the potential for immunogenicity still exists for both humanized and fully human antibodies [23,24,25].
During the SPIRE clinical trial program, approximately 16,000 subjects were treated with bococizumab [14, 15]. Given the high incidence of ADAs observed with bococizumab treatment [15], and the potential for ADAs to persist within an individual, it was important to assess whether anti-bococizumab antibodies could cross-react with other anti-PCSK9 mAbs if subjects were subsequently treated with these approved anti-PCSK9 agents. Cross-reactivity between anti-bococizumab antibodies and either alirocumab or evolocumab could potentially alter the efficacy and/or safety profile of these mAbs. This study therefore sought to assess experimentally whether ADAs against bococizumab would cross-react with alirocumab or evolocumab. Moreover, this study outlines a novel approach for assessing cross-reactivity against a biotherapeutic within the same target class using a single assay approach. This approach is an adaptation of the specificity assessment outlined in the draft US Food and Drug Administration (FDA) guidance document [26] and has been utilized to characterize gross epitope binding of ADAs (e.g., antibody–drug conjugates) [27,28,29]. While bococizumab was discontinued from clinical development, the conceptual approach taken to assess cross-reactivity may be applied to other biotherapeutics where cross-reactivity is a potential concern.
2 Methods
2.1 Study Design
Plasma samples from bococizumab-treated subjects enrolled in the SPIRE-HR study (ClinicalTrials.gov identifier NCT01968954) were analyzed for cross-reactivity to the anti-PCSK9 mAbs alirocumab and evolocumab. The study design and findings of the SPIRE-HR study have been published previously [15, 30]. Briefly, 711 statin (HMG-CoA reductase inhibitor)-treated subjects with primary hyperlipidemia or mixed dyslipidemia who were at high risk for cardiovascular events were randomized (1:1) and treated for 12 months with bi-monthly subcutaneous bococizumab (150 mg) or with placebo [15, 30]. All subjects provided written informed consent prior to participation in the study [15, 30]. The primary efficacy endpoint was percent change from baseline (%CFB) in LDL-C at Week 12 [15, 30]. In addition, the persistence of LDL-C response over the 12-month treatment period was also evaluated, as described elsewhere [15].
2.2 Assessment of Immunogenicity
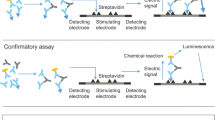
Bococizumab immunogenicity (presence of ADA and NAb) was assessed at eight timepoints throughout the study from randomization (Day 1) to the follow-up visit at Week 58 (Day 407). ADAs to bococizumab were determined using a validated bridging electrochemiluminescent (ECL) immunoassay using MesoScale Discovery (MSD) technology and following a three-tiered testing strategy of screen, confirm specificity to bococizumab, and titer as previously described [15]. Briefly, plasma samples, anti-bococizumab antibody-positive control (639.26G8.3D3.2C4 [2C4], Pfizer Inc, San Francisco, CA, USA), and a negative control plasma pool were incubated overnight with biotin (ThermoFisher Scientific, Waltham, MA)-labeled bococizumab and SULFO-TAG™ (Meso Scale Diagnostics, Rockville, MD)-labeled bococizumab. Following incubation, the ADA bridged with both biotin- and SULFO-TAG™-labeled bococizumab were captured on blocked MSD plates coated with streptavidin and detected using tripropylamine read buffer and an MSD imager [15]. Samples confirmed as positive for bococizumab ADA were further characterized for NAb using a competitive ECL immunoassay and a two-tiered testing strategy of screen and titer, as previously described [15]. ADA-positive samples had a titer ≥ 75 and NAb-positive samples had a titer ≥ 3. The overall inter-assay precision of the ADA assay was 1.32% for the positive control endpoint titer and 7.41% for the negative control. The inter-assay precision for the NAb assay was 10.0% for the positive control endpoint titer and ≤ 25.5% for the negative control.
2.3 Assessment of Cross-Reactivity
The highest ADA titer sample from each bococizumab ADA-positive subject was selected and tested for cross-reactivity to alirocumab and evolocumab. If the highest titer sample for a given subject cross-reacted to alirocumab or evolocumab, additional samples (baseline and all other ADA-positive samples) were also analyzed for cross-reactivity.
Cross-reactivity to evolocumab or alirocumab was assessed by validating additional specificity tiers within the bococizumab ADA assay against each drug. These specificity tiers were performed in a similar manner to the bococizumab confirmatory assay, in which samples were diluted in the presence of excess bococizumab or buffer control. Inhibition of signal in the presence of spiked bococizumab versus buffer control confirms specificity of the response. Samples analyzed in the specificity assays for cross-reactivity were independently diluted in the presence of 40 µg/mL of alirocumab (Praluent®, Sanofi-Aventis, Bridgewater, NJ, USA) or evolocumab (Repatha®, Amgen, Thousand Oaks, CA, USA) and the subsequent assay signals were compared with samples diluted in buffer control to calculate percent inhibition. During method development, it was found that signal inhibition was saturated at 40 µg/mL for both alirocumab and evolocumab (data not shown) and this concentration was selected to be consistent with the concentration of bococizumab used in the confirmatory tier. Specificity cut-points for alirocumab and evolocumab were determined using 50 samples from placebo-treated subjects from the SPIRE-HR study performed over three independent runs and calculated based on a 99.9% confidence interval [31]. The cross-reactivity cut-points (CR-CPs) for alirocumab and evolocumab were determined to be 18.0% and 16.9%, respectively. Bococizumab ADA-positive samples were classified as cross-reactive to alirocumab or evolocumab if the sample signal inhibition in the presence of either drug was greater than or equal to the corresponding CR-CP.
In order to determine the suitability of the method for assessing cross-reactivity between anti-PCSK9 mAbs, recombinant PCSK9 (rPCSK9, Pfizer Inc) was used as a surrogate cross-reactive positive control because an antibody-positive control capable of binding to all three anti-PCSK9 mAbs was not available. The selection of rPCSK9 was based on the fact that bridging ADA assay formats are susceptible to false positivity by multimeric soluble targets, which can mimic an ADA response by bridging the labeled drugs [32]. PCSK9 is known to self-associate to form dimers and trimers [33], and a concentration-dependent increase in the assay signal with an aggregated form of rPCSK9 was demonstrated in the bococizumab ADA screening assay (Fig. 1a), and this response was inhibited by all three anti-PCSK9 mAbs (Fig. 1b). In addition, only bococizumab was able to inhibit the signals generated by the two anti-bococizumab-positive controls (2C4 and 3 mAb pool [637.23G8.H1, 637.10F2.E2.H4, and 2C4], Pfizer Inc); signal inhibition was not observed with either alirocumab or evolocumab (Fig. 1b). Collectively, these observations demonstrate the specificity of the assay and that all three anti-PCSK9 mAbs can compete with rPCSK9 and, as such, rPCSK9 can be used as a surrogate positive control in the cross-reactivity assay.
a Concentration-dependent bridging by rPCSK9 in the anti-bococizumab antibody assay, and b specificity of the assay controls to bococizumab, alirocumab, and evolocumab. mAb monoclonal antibody, PC positive control, RLU relative luminescence units, rPCSK9 recombinant proprotein convertase subtilisin/kexin type-9
2.4 Immunogenicity Data Analysis
Immunogenicity data were analyzed using descriptive statistics. Statistical calculations were performed using SAS® software (version 9.3 or above; SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 SPIRE-HR Immunogenicity Data
In total, 356 subjects in the SPIRE-HR study received at least one dose of bococizumab, and 295 (82.9%) subjects completed the 52-week treatment period. The prevalence of pre-existing ADA to bococizumab was 0.9% for the bococizumab-treated subjects (3/338). None of these subjects showed a treatment-boosted ADA response (defined as a > 3-fold dilution increase in titer from baseline) following bococizumab administration. In bococizumab-treated subjects, the overall incidence of ADA during the study was 44.0% (155/352), and 27.0% of subjects (61.3% ADA-positive subjects) also developed NAbs. The median (first quartile [Q1], third quartile [Q3]) time to first ADA and NAb detection was Day 168 (85, 174) and Day 166 (85, 170), respectively. However, ADAs and NAbs were first detected as early as Day 29. In addition, 67.1% (104/155) of ADA-positive subjects had ADAs that persisted for ≥ 16 weeks (≥ 112 days). The time course of ADA and NAb titers observed during the study is shown in Fig. 2. The median ADA and NAb titers ranged from 276 to 526 and from 8 to 12, respectively. The titers for the ADA samples tested for cross-reactivity to alirocumab or evolocumab are shown in Fig. 3. These samples had a median (Q1, Q3) titer value of 553 (212, 1833), representing samples collected from Day 29 to Day 415. In addition, 81 of the 155 highest-titer ADA-positive samples were also NAb-positive (52.3%) (Fig. 3).
3.2 Analysis of Cross-Reactivity to Alirocumab and Evolocumab
Of the 155 ADA-positive subjects treated with bococizumab, cross-reactivity to alirocumab and evolocumab was detected in one subject (0.65%), as indicated by the percent signal inhibition above the CR-CP in the respective assays (Fig. 4a). This subject, who demonstrated cross-reactivity to both alirocumab and evolocumab, had neither pre-existing ADA to bococizumab nor pre-existing cross-reactivity to either drug at baseline. Analysis of all additional ADA-positive samples from this subject showed cross-reactivity to both alirocumab and evolocumab on Days 337 and 373 (Fig. 4b). However, cross-reactivity to either drug was no longer detected by Day 407, when the subject was still ADA-positive but NAb-negative. The cross-reactive signal inhibition ranged from 24.2 to 35.5% for either drug (Fig. 4b). This percentage signal inhibition was much weaker than the 72.8–75.2% signal inhibition observed for bococizumab assessed during the confirmation tier.
For the one subject who displayed in vitro cross-reactivity, anti-bococizumab antibodies were detected from Day 337 to the follow-up visit on Day 407. ADA responses were also characterized as neutralizing on Days 337 and 373 but not Day 407. In this subject, an initial robust reduction in LDL-C was demonstrated, with a Week 12 %CFB of − 82.2%, compared with mean %CFB of − 58.8% for ADA-negative subjects (Fig. 5). The LDL-C-lowering response in this subject who displayed cross-reactivity was slightly attenuated following the detection of ADA, which was further characterized as neutralizing.
4 Discussion
Within the past few decades, advances in the field of molecular biology have enabled antibody therapies to be designed and engineered towards target molecules of interest, in order to improve the management of therapeutic conditions such as cancer, inflammatory diseases, autoimmune diseases, and more recently dyslipidemia [8, 23, 34]. Despite efforts to humanize the engineered protein, immunogenicity against mAbs is increasingly recognized as a possible mechanism to explain treatment failure or reduced efficacy. Bococizumab was terminated in late phase III clinical development, partly due to a high incidence of ADA and the attenuation of efficacy in subjects with high ADA titers [15]. Although bococizumab is not approved for clinical use, approximately 16,000 patients were treated with bococizumab in the phase III studies [14, 15]. It was therefore important to determine whether anti-bococizumab antibodies in ADA-positive subjects would cross-react with marketed anti-PCSK9 mAbs (alirocumab or evolocumab) if subjects were subsequently treated.
Consistent with the pooled immunogenicity results reported for the six lipid-lowering SPIRE studies [15], treatment-induced ADAs were observed in 44.0% of subjects in the SPIRE-HR study, and 27.0% of subjects also developed NAbs. In addition, 67.1% of ADA-positive subjects had ADAs that persisted for at least 16 weeks. Using samples from these bococizumab-treated subjects, we demonstrate experimentally that ADAs generated in response to bococizumab treatment are unlikely to cross-react with other anti-PCSK9 mAbs. Using a novel single-assay approach for assessing cross-reactivity against a biotherapeutic agent within the same target class, only one of the 155 ADA-positive subjects (0.65%) showed detectable cross-reactivity to alirocumab and evolocumab, and this cross-reactivity was both transient in nature and weak in signal. In the one subject who showed in vitro cross-reactivity to alirocumab and evolocumab, a slight attenuation in LDL-C response was observed, which coincided with the detection of NAbs to bococizumab. These results suggest that subjects who have previously received bococizumab may be treated for high LDL-C with an appropriate anti-PCSK9 mAbs, regardless of the presence of ADAs to bococizumab.
Although there was a need to investigate the possibility, cross-reactivity between anti-bococizumab antibodies and alirocumab or evolocumab was not anticipated, based on the differences in the structural properties of the anti-PCSK9 mAbs. As such, ADAs to bococizumab were not expected to recognize alirocumab or evolocumab, and this was confirmed by the present study. This low risk of clinically relevant cross-reactivity within the anti-PCSK9 therapeutic class is also consistent with the literature from the tumor necrosis factor (TNF) inhibitors [35,36,37]. Immunogenicity is a known contributing factor to the loss of clinical response to anti-TNF biologics [38,39,40]. However, studies have suggested that developing antibodies to one anti-TNF inhibitor does not necessarily translate to clinically relevant cross-reactivity, as a similar clinical response has been reported to a secondary TNF inhibitor for subjects who develop antibodies to a first TNF inhibitor compared with subjects who are treatment-naïve [35, 37].
While our findings showed a lack of cross-reactivity between anti-bococizumab antibodies and alirocumab or evolocumab, determination of ADA cross-reactivity is challenging. Immunogenicity data are highly dependent on the sensitivity, specificity, and drug tolerance of the assay used [41], as well as other factors such as the study population or the ‘cut-points’ for defining a positive response. Therefore, the interpretation of cross-reactivity results should be made in the context of the assay capabilities and the experimental design. In assessing cross-reactivity using two independent methods for each biotherapeutic, a common cross-reactive positive control is needed to ensure similar method characteristics. For example, when assessing cross-reactivity for biosimilars, the positive control would be expected to cross-react with both the innovator and biosimilar drug to demonstrate and establish similar assay performance, if independent ADA assays for the innovator and biosimilar drug were used. When examining cross-reactivity across different biotherapeutics within the same class, a cross-reactive positive control may be limited or not feasible based on homology of the biotherapeutics. In the present study, the bridging ADA assay format allowed us to use the binding target as a surrogate cross-reactive positive control. This novel approach allowed assessment of cross-reactivity within the context of a single validated assay and helped minimize the problems associated with assessing cross-reactivity in separate assays. Thus, this method may be applicable to other biotherapeutics where cross-reactivity of ADAs may be a potential concern, especially for those within the same therapeutic class. One potential limitation of the present study is that evaluation of cross-reactivity was not conducted in a clinical switchover design. This assessment would require a clinical study where subjects who are ADA-positive to bococizumab were subsequently dosed with alirocumab or evolocumab, then efficacy and tolerability determined. However, such a switchover study would not be ethical given that bococizumab is no longer in clinical development [22]. As noted earlier, studies within the TNF inhibitor class have indicated that ADAs do not necessarily limit the response to a second biologic within the same class [35, 37], and therefore clinically relevant cross-reactivity would not be anticipated for anti-PCSK9 mAbs.
5 Conclusion
A novel, single-assay approach was used to assess the potential cross-reactivity of anti-bococizumab antibodies to alirocumab and evolocumab. In subjects who previously developed ADAs to bococizumab, the likelihood of clinically relevant cross-reactivity to the two marketed anti-PCSK9 mAbs is remote or absent based on the low frequency of cross-reactivity observed in this study, which was both weak in signal inhibition and transient in nature.
Data Availability
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
Stein EA. What role will proprotein convertase subtilisin/kexin type 9 inhibitors play in hyperlipidemia management? Curr Opin Endocrinol Diabetes Obes. 2016;23:97–105. https://doi.org/10.1097/MED.0000000000000242
Scherer DJ, Nelson AJ, Psaltis PJ, Nicholls SJ. Targeting low-density lipoprotein cholesterol with PCSK9 inhibitors. Intern Med J. 2017;47:856–65. https://doi.org/10.1111/imj.13451.
Seidah NG, Awan Z, Chrétien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014;114:1022–36. https://doi.org/10.1161/CIRCRESAHA.114.301621.
Vincent J. Reducing elevated plasma LDL cholesterol: the central role of the LDL receptor. Clin Pharmacol Ther. 2014;96:3–7. https://doi.org/10.1038/clpt.2014.95.
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72. https://doi.org/10.1002/humu.20882.
Abifadel M, Rabes JP, Devillers M, Munnich A, Erlich D, Junien C, et al. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat. 2009;30:520–9.https://doi.org/10.1002/humu.20882.
Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6. https://doi.org/10.1038/ng1161.
Catapano AL, Papadopoulos N. The safety of therapeutic monoclonal antibodies: implications for cardiovascular disease and targeting the PCSK9 pathway. Atherosclerosis. 2013;228:18–28. https://doi.org/10.1016/j.atherosclerosis.2013.01.044.
Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol. 2014;11:563–75. https://doi.org/10.1038/nrcardio.2014.84.
Amgen. REPATHA™ (evolocumab) U.S. prescribing information. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125522s000lbl.pdf. Accessed 19 Sept 2018.
Sanofi. PRALUENT™ (alirocumab) prescribing information. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125559Orig1s000lbledt.pdf. Accessed 19 Sept 2018.
Liang H, Chaparro-Riggers J, Strop P, Geng T, Sutton JE, Tsai D, et al. Proprotein convertase substilisin/kexin type 9 antagonism reduces low-density lipoprotein cholesterol in statin-treated hypercholesterolemic nonhuman primates. J Pharmacol Exp Ther. 2012;340:228–36. https://doi.org/10.1124/jpet.111.187419.
Ballantyne CM, Neutel J, Cropp A, Duggan W, Wang EQ, Plowchalk D, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115:1212–21. https://doi.org/10.1016/j.amjcard.2015.02.006.
Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376:1527–39. https://doi.org/10.1056/NEJMoa1701488.
Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, Jukema JW, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. 2017;376:1517–26. https://doi.org/10.1056/NEJMoa1614062.
Wang EQ, Plotka A, Salageanu J, Sattler C, Yunis C. Pharmacokinetics and pharmacodynamics of bococizumab, a monoclonal antibody to PCSK9, after single subcutaneous injection at three sites [NCT 02043301]. Cardiovasc Ther. 2017;35:e12278. https://doi.org/10.1111/1755-5922.12278.
Levisetti M, Joh T, Wan H, Liang H, Forgues P, Gumbiner B, et al. A phase I randomized study of a specifically engineered, pH-sensitive PCSK9 inhibitor RN317 (PF-05335810) in hypercholesterolemic subjects on statin therapy. Clin Transl Sci. 2017;10:3–11. https://doi.org/10.1111/cts.12430.
Yokote K, Kanada S, Matsuoka O, Sekino H, Imai K, Tabira J, et al. Efficacy and safety of bococizumab (RN316/PF-04950615), a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, in hypercholesterolemic Japanese subjects receiving a stable dose of atorvastatin or treatment-naive- results from a randomized, placebo-controlled, dose-ranging study. Circ J. 2017;81:1496–505. https://doi.org/10.1253/circj.CJ-16-1310.
Fazio S, Robertson DG, Joh T, Wan H, Riel T, Forgues P, et al. Effects of 12 weeks of treatment with intravenously administered bococizumab, a humanized monoclonal antibody blocking proprotein convertase subtilisin/kexin type 9, in hypercholesterolemic subjects on high-dose statin. Cardiovasc Ther. 2018;36:e12308. https://doi.org/10.1111/1755-5922.12308.
Gumbiner B, Joh T, Liang H, Wan H, Levisetti M, Vana AM, et al. The effects of single- and multiple-dose administration of bococizumab (RN316/PF-04950615), a humanized IgG2Deltaa monoclonal antibody binding proprotein convertase subtilisin/kexin type 9, in hypercholesterolemic subjects treated with and without atorvastatin: results from four phase I studies. Cardiovasc Ther. 2018;36:e12309. https://doi.org/10.1111/1755-5922.12309.
Wang EQ, Plotka A, Salageanu J, Baltrukonis D, Mridha K, Frederich R, et al. Comparative pharmacokinetics and pharmacodynamics of bococizumab following a single subcutaneous injection using drug substance manufactured at two sites or administration via two different devices. Clin Pharmacol Drug Dev. 2019;8:40–8. https://doi.org/10.1002/cpdd.454.
Pfizer Inc. Pfizer discontinues global development of bococizumab, its investigational PCSK9 inhibitor. 2016. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_discontinues_global_development_of_bococizumab_its_investigational_pcsk9_inhibitor. Accessed 5 Dec 2016.
Foltz IN, Karow M, Wasserman SM. Evolution and emergence of therapeutic monoclonal antibodies: what cardiologists need to know. Circulation. 2013;127:2222–30. https://doi.org/10.1161/CIRCULATIONAHA.113.002033.
van Brummelen EM, Ros W, Wolbink G, Beijnen JH, Schellens JH. Antidrug antibody formation in oncology: clinical relevance and challenges. Oncologist. 2016;21:1260–8. https://doi.org/10.1634/theoncologist.2016-0061.
Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9:767–74. https://doi.org/10.1038/nrd3229.
Food and Drug Administration (FDA). Assay development and validation for immunogenicity testing of therapeutic protein products. Guidance for industry (draft). 2016. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM192750.pdf. Accessed 20 Apr 2017.
Hock MB, Thudium KE, Carrasco-Triguero M, Schwabe NF. Immunogenicity of antibody drug conjugates: bioanalytical methods and monitoring strategy for a novel therapeutic modality. AAPS J. 2015;17:35–43. https://doi.org/10.1208/s12248-014-9684-6.
Stevenson L, Amaravadi L, Myler H, Salazar-Fontana L, Gorovits B, Kirshner S, et al. 2014 White Paper on recent issues in bioanalysis: a full immersion in bioanalysis (part 3—LBA and immunogenicity). Bioanalysis. 2014;6:3355–68. https://doi.org/10.4155/bio.14.283.
Hoofring SA, Lopez R, Hock MB, Kaliyaperumal A, Patel SK, Swanson SJ, et al. Immunogenicity testing strategy and bioanalytical assays for antibody-drug conjugates. Bioanalysis. 2013;5:1041–55. https://doi.org/10.4155/bio.13.10.
Ridker PM, Amarenco P, Brunell R, Glynn RJ, Jukema JW, Kastelein JJ, et al. Evaluating bococizumab, a monoclonal antibody to PCSK9, on lipid levels and clinical events in broad patient groups with and without prior cardiovascular events: rationale and design of the Studies of PCSK9 Inhibition and the Reduction of vascular Events (SPIRE) Lipid Lowering and SPIRE Cardiovascular Outcomes trials. Am Heart J. 2016;178:135–44. https://doi.org/10.1016/j.ahj.2016.05.010.
Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48:1267–81. https://doi.org/10.1016/j.jpba.2008.09.020.
Zhong ZD, Clements-Egan A, Gorovits B, Maia M, Sumner G, Theobald V, et al. Drug target interference in immunogenicity assays: recommendations and mitigation strategies. AAPS J. 2017;19:1564–75. https://doi.org/10.1208/s12248-017-0148-7.
Fan D, Yancey PG, Qiu S, Ding L, Weeber EJ, Linton MF, et al. Self-association of human PCSK9 correlates with its LDLR-degrading activity. Biochemistry. 2008;47:1631–9. https://doi.org/10.1021/bi7016359.
Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–33. https://doi.org/10.1111/j.1476-5381.2009.00190.x.
Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis. 2010;69:817–21. https://doi.org/10.1136/ard.2009.112847.
Smolen JS, Kay J, Doyle MK, Landewe R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210–21. https://doi.org/10.1016/S0140-6736(09)60506-7.
Jamnitski A, Bartelds GM, Nurmohamed MT, van Schouwenburg PA, van Schaardenburg D, Stapel SO, et al. The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann Rheum Dis. 2011;70:284–8. https://doi.org/10.1136/ard.2010.135111.
Thomas SS, Borazan N, Barroso N, Duan L, Taroumian S, Kretzmann B, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. BioDrugs. 2015;29:241–58. https://doi.org/10.1007/s40259-015-0134-5.
Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1739–45. https://doi.org/10.1136/ard.2008.092833.
Pascual-Salcedo D, Plasencia C, Ramiro S, Nuno L, Bonilla G, Nagore D, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology (Oxford). 2011;50:1445–52. https://doi.org/10.1093/rheumatology/ker124.
Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga A, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 2014;16:658–73. https://doi.org/10.1208/s12248-014-9599-2.
Acknowledgements
Medical writing support was provided by Karen Burrows, MPhil, CMPP, of Engage Scientific Solutions (Horsham, UK) and was funded by Pfizer.
Author information
Authors and Affiliations
Contributions
All authors were involved in the conception and design of the study, or in data collection or data analysis. All authors were involved in interpretation of the data. All authors were involved in drafting the article and in critical revision of the article. All authors approved the final approval version of the manuscript to be published and take responsibility for the data.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
The SPIRE-HR study was funded by Pfizer.
Conflict of interest
Ellen Q. Wang, Carla Yunis, Pamela F. Schwartz, and Daniel Baltrukonis are full-time employees of Pfizer. Jack F. Bukowski and Charles L. Shear were employees of Pfizer at the time the study was conducted. Paul M. Ridker has acted as a consultant to Pfizer in relation to the SPIRE clinical trial program. Paul M. Ridker has received additional research grant support from Novartis, Amgen, and Kowa, and is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to AstraZeneca and Siemens.
Additional information
Jack F. Bukowski and Charles L. Shear were full-time employees at Pfizer during the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, E.Q., Bukowski, J.F., Yunis, C. et al. Assessing the Potential Risk of Cross-Reactivity Between Anti-Bococizumab Antibodies and Other Anti-PCSK9 Monoclonal Antibodies. BioDrugs 33, 571–579 (2019). https://doi.org/10.1007/s40259-019-00375-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-019-00375-0