Abstract
Alzheimer’s disease (AD) is the primary cause of dementia in the elderly. It remains incurable and poses a huge socio-economic challenge for developed countries with an aging population. AD manifests by progressive decline in cognitive functions and alterations in behaviour, which are the result of the extensive degeneration of brain neurons. The AD pathogenic mechanism involves the accumulation of amyloid beta peptide (Aβ), an aggregating protein fragment that self-associates to form neurotoxic fibrils that trigger a cascade of cellular events leading to neuronal injury and death. Researchers from academia and the pharmaceutical industry have pursued a rational approach to AD drug discovery and targeted the amyloid cascade. Schemes have been devised to prevent the overproduction and accumulation of Aβ in the brain. The extensive efforts of the past 20 years have been translated into bringing new drugs to advanced clinical trials. The most progressed mechanism-based therapies to date consist of immunological interventions to clear Aβ oligomers, and pharmacological drugs to inhibit the secretase enzymes that produce Aβ, namely β-site amyloid precursor-cleaving enzyme (BACE) and γ-secretase. After giving an update on the development and current status of new AD therapeutics, this review will focus on BACE inhibitors and, in particular, will discuss the prospects of verubecestat (MK-8931), which has reached phase III clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Disease-modifying therapies for Alzheimer’s disease (AD) are under development. |
β-Site amyloid precursor-cleaving enzyme (BACE) inhibitors represent the most promising novel therapeutics for AD treatment. |
At least four BACE inhibitors are currently in phase II/III clinical trials, among which verubecestat is the most advanced. |
Verubecestat can effectively decrease the levels of amyloid beta (Aβ) in the cerebrospinal fluid of healthy volunteers and of patients with mild to moderate AD. |
Verubecestat is being tested in large cohorts of patients with mild cognitive impairment and with mild AD. |
Because of the numerous BACE substrates, the potential adverse effects of BACE inhibitors will have to be monitored carefully. |
1 Introduction
Age-related diseases are becoming prevalent among the developed countries in which populations are getting older. Alzheimer’s disease (AD), in particular, poses a heavy socio-economic burden upon our societies [1]. It is currently estimated that one in 20 people aged over 60 years will present with some form of dementia, and this figure can reach one in four among those aged over 85. Seventy percent of dementia cases are due to AD, a progressive degenerative disease of the brain that develops over several decades of life. It manifests as a decline of cognitive function and alterations in behaviour that result in the patients’ loss of autonomy and their need for permanent care and assistance. Although there is presently no cure for AD, and current therapies can only provide temporary relief from some of its symptoms, novel and promising mechanism-based therapies that aim to modify the course of the disease are undergoing clinical trials. These new therapeutic approaches are reviewed here, with particular focus on the β-site amyloid precursor-cleaving enzyme (BACE) inhibitor verubecestat.
2 The Pathophysiology of Alzheimer’s Disease (AD)
The definite diagnosis of AD is established at autopsy by the presence of pathological hallmarks in the brain of patients deceased with dementia [2, 3]. AD hallmarks consist of extracellular amyloid plaques, which accumulate in the brain parenchyma, and neurofibrillary tangles (NFT), which form within neuron bodies. Amyloid plaque deposition is the salient characteristic of AD, whereas NFT are also observed in other neurodegenerative diseases. Brain examination also indicates a major loss of grey matter that reflects extensive neuronal death. Plaques and tangles are principally localised in the hippocampus and frontal cortex, which are the brain regions associated with memory acquisition and retrieval. The essential component of amyloid plaques is a protein fragment termed amyloid beta (Aβ), which self-associates to form oligomers and aggregates [4]. Although the pathophysiology of AD is not fully understood, extensive research has demonstrated the toxicity of Aβ oligomers and their direct injury to neurons, including inhibition of long-term potentiation (LTP), synaptic damage, and apoptotic cell death [5, 6]. The NFT are composed of hyperphosphorylated tau protein that self-associates as paired helical filaments [7]. Tau hyperphosphorylation causes its dissociation from the neurons’ microtubules, with consequent loss of axon stability and cellular integrity, thus reduced viability. It is clear that the harmful combination of Aβ oligomers and hyperphosphorylated tau causes synaptic dysfunction and neuronal death in AD [8, 9]. The relationship between Aβ and tau is intricate and remains incompletely resolved. So far, experimental evidence supports that (1) Aβ fibrils promote tau phosphorylation and (2) tau phosphorylation mediates Aβ inhibition of LTP. In addition, it was recently shown that functional tau facilitates Aβ clearance and it was suggested that tau dysfunction could trigger Aβ oligomerisation and toxicity [10]. However, it is worth pointing out that tau is also associated with neurodegenerative diseases such as frontotemporal dementia, Pick’s disease, and other tauopathies that occur in the absence of Aβ pathology and which are caused by rare mutations in the tau gene, MAPT [11, 12]. Therefore, Aβ remains the primary pathological culprit in AD, and we will focus this review on the amyloidocentric therapeutic approaches that converge into preventing Aβ accumulation.
3 The Amyloid Cascade as the Basis for Rational Therapies in AD
3.1 Secretase Processing of the Amyloid Precursor Protein
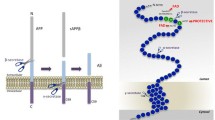
Aβ is a peptide fragment derived from cleavage processing of the amyloid precursor protein (APP), a membrane receptor involved in cell adhesion, synaptic plasticity and metal homeostasis [13–15]. APP is ubiquitously expressed, and alternative splicing of the gene produces two major variants: APP695, which is mostly expressed in neurons of the central nervous system (CNS), and APP751, which is principally expressed in the periphery. APP undergoes proteolytic processing by two alternative cellular pathways that can either generate or preclude formation of Aβ [16, 17] (refer to Fig. 1). When following the amyloidogenic pathway, APP undergoes cleavage in its juxtamembrane region by β-secretase, a proteolytic enzyme identified as BACE (or BACE1 for distinction from its homologue BACE2). Cleavage of APP by BACE1 generates the soluble β-cleaved APP fragment, sAPPβ, and a corresponding 99-amino-acid, membrane-tethered C-terminal fragment (C99) that is the direct precursor to Aβ. Subsequent processing of C99 by γ-secretase releases Aβ fragments of variable length that are secreted in the extracellular space, and concomitantly liberates the APP-intracellular domain (AICD) to allow its nuclear translocation together with transcription-active binding partners [18, 19]. γ-Secretase is a membrane-embedded catalytic complex composed of four subunits, among which presenilin represents the enzymatic entity [20]. Its mechanism involves sequential cuts that produce heterogeneous products [21]. Neuronal cells secrete Aβ peptides of 40 (Aβ40) and 42/43 (Aβ42/Aβ43) amino acids, in a ratio of approximately 95/5, with the longer peptides being more aggregating and cytotoxic. In the alternative non-amyloidogenic pathway, which is the default pathway for most cells apart from neurons, APP is cleaved by α-secretase, a Disintegrin and Metalloproteinase (ADAM; either ADAM10 or ADAM17) that sheds the large extra-cellular domain, sAPPα, following either a constitutive or a regulated pathway [22, 23]. Processing of the corresponding C-terminal fragment of 83 amino acids (C83) by γ-secretase generates small fragments termed p3, as well as AICD [17]. The regulated cellular trafficking of APP controls its co-localisation with either secretase, and is a major determinant to Aβ production.
Therapies targeting the amyloid cascade. The scheme depicts the amyloidogenic processing of the amyloid precursor protein, APP, and the targets of therapies in clinical trials. First, β-site APP-cleaving enzyme 1 (BACE1) cleaves APP to secrete the soluble β-cleaved APP fragment, sAPPβ, and produce the C-terminal membrane-tethered fragment, C99. The BACE inhibitors target this reaction. Next, cleavage of C99 by γ-secretase (γ-sec) produces amyloid beta (Aβ) peptides and releases the APP intracellular domain (AICD). γ-Secretase inhibitors (GSI) and γ-secretase modulators (GSM) are designed to interfere with this cleavage. Aβ can self-associate to form toxic oligomers. Immunotherapy targets either Aβ monomers or oligomers
3.2 Evidence for the Causative Role of the Amyloid Cascade in AD
The main risk factors associated with AD, which are aging, oxidative stress, metabolic diseases, and inflammation, are all known to increase BACE expression and activity and to favour the processing of APP through the amyloidogenic route [24–26]. Aβ is considered to be a normal product of cellular metabolism, which, when produced in limited amounts, is degraded through proteolytic mechanisms that involve the extracellular enzymes, insulin-degrading enzyme (IDE), neprilysin (NEP), and matrix metalloproteases (MMPs) [27]. However, when the levels of Aβ increase, in response to BACE1 elevation or by reason of defective clearance, it can exceed a threshold concentration beyond which it self-associates, undergoes a change in conformation, and forms insoluble oligomers that are resistant to degradation [28–30].
The causative role of the amyloid cascade in AD pathogenesis is further exemplified by the rare, familial-inherited forms of AD (FAD), which develop at an earlier age and follow an aggressive course (for reviews, see [31, 32]). FAD patients carry autosomal dominant missense mutations either in the APP gene, which modify the sites of processing by the secretases, or in the presenilin genes, which impact the structure and activity of these catalytic subunits of γ-secretase. Both APP and presenilin FAD mutations alter the processing of APP to promote amyloidogenesis. For instance, the Swedish double point mutation in APP increases cleavage by β-secretase, hence the rate of Aβ production [33]. Most of the other APP mutations alter the site of γ-secretase cleavage, leading to formation of longer and more aggregating Aβ peptides that are not only more toxic, but also harder to clear [34]. Another APP mutation (V715M) decreases Aβ40 production and increases formation of N-terminally truncated, aggregating, Aβ42 peptides [35]. The large majority of AD-causative mutations occur in the presenilins, and these also enhance the production of long Aβ variants [36]. Conversely, and in further support of the amyloid hypothesis, a very rare APP mutation that was identified in Icelandic families and which favours APP processing through the non-amyloidogenic pathway seems to protect its carriers from developing AD in their old age [37]. Furthermore, other genetic factors in AD can be linked to the amyloid cascade [38]. Such an example is the association of late-onset AD with rare mutations in the ADAM10 gene that attenuate α-secretase function, and thereby augment APP processing by β-secretase [39]. Polymorphisms in other genes that confer susceptibility for late-onset AD may also be connected to the amyloid cascade, considering that some of these genes control the trafficking of APP (SORL1, CD2AP) or BACE (BIN1) and APP endocytosis (PICALM), which may alter the colocalisation of APP and BACE. Some of the other genes are involved in lipid metabolism (APOE, ABCA7, CLU, PLD3), a cellular factor that affects cellular membrane composition, thus endocytosis and γ-secretase activity. Some genes (PRNP, ZCWPW1) mediate homeostasis of metals that are implicated in Aβ aggregation and in the generation of reactive oxygen radicals that promote BACE1 expression and Aβ production. Another group of AD susceptibility genes are linked to inflammation (TREM2, EPHA1, CLU, CR1), which also triggers Aβ production, and which mediates the clearance of Aβ and tau aggregates [40–42] (also refer to reference [43]).
In summary, there is a large amount of evidence to support the accumulation of Aβ in selected brain regions, through increased production and aggregation, and/or defective clearance of the amyloid peptide, as a factor underlying the causative mechanism in AD, and to lay the basis for a rational therapeutic intervention.
4 Progress and Challenges of Amyloidocentric Clinical Trials to Date
4.1 Immunotherapy Strategies to Clear Amyloid Beta (Aβ) Deposits and Oligomers
This is in principle the most straightforward approach; however, it has encountered considerable difficulties. Immunotherapy has been experimented with over the past 20 years, first with the aim of clearing amyloid plaques that were initially believed to cause neurodegeneration, and more recently with the purpose of removing toxic Aβ oligomers. The immunotherapy approach consists of either challenging the organism with Aβ or Aβ fragments to elicit an immune response that produces antibodies selective for Aβ (this is called active immunisation), or dispensing specific anti-Aβ monoclonal antibodies exogenously prepared in the laboratory (this is called passive immunisation). Immunotherapy has been so far successful in cancer therapy, with active immunotherapy being used in the development of preventive cancer vaccines, and monoclonal antibodies becoming widely used in cancer treatment. The development of AD immunotherapies has been more challenging than expected and has faced major problems with safety and efficacy.
4.1.1 Active Immunisation
Initial experiments, in which the whole Aβ42 peptide was injected in an AD transgenic mouse model, supported that this intervention would be safe and effective, achieving protection against amyloid deposition in young mice and a decrease in brain amyloid burden in older mice, which also showed moderate improvement in spatial memory tasks [44–46]. Unfortunately, such promising results did not translate successfully when trialled in humans.
AN 1792 The Elan/Wyeth group reported that the phase I administration of AN 1792 (Aβ42 plus QS21 adjuvant) to patients with mild to moderate AD was well tolerated, but that only 53 % of patients showed evidence of an immune response. The following phase II trial was terminated because 6 % of those given the Aβ42-containing formulation developed meningoencephalitis due to a neuroinflammatory reaction [45]. Post-mortem histological examination proved that the immunisation had been successful at reducing Aβ amyloid plaque in the brain, had improved neurite dystrophy in the hippocampus, and had moderately reduced tau pathology [47]. However, no significant cognitive improvement was reported, probably because the patients selected for the study had already reached a too far advanced stage of the illness, with extensive neuronal loss [48]. A likely reason for the adverse effects of AN 1792 was the toxicity of administered Aβ42 peptide that caused an inflammatory reaction. Substituting Aβ42 for smaller fragments was then tested, and this established that the N-terminal region of Aβ, which binds to B cells, induced a humoral immunity response, whereas the mid and C-terminal regions of Aβ were predominantly presented to T cells and induced an inflammatory response. Further research in Aβ vaccine development then moved onto using N-terminal Aβ fragments and short peptides that are unlikely to activate T cells. Novartis, Janssen/Pfizer, Affiris, and AC Immune have begun clinical trials with peptides corresponding to the Aβ N-terminal region [49].
CAD106 Researchers of the Karolinska Institute (Sweden), with funding support from Novartis, reported data from a phase I trial on the safety and tolerability of CAD106 (an antigen derived from Aβ 1–6), which could produce an antibody response in a cohort of patients (aged 50–80 years) [50]. Phase IIa studies in patients with mild AD further confirmed that CAD106 vaccination was generally well-tolerated and elicited an immune response in ~64 % of patients, with only minor side-effects, except for one case of cerebral haemorrhage [51]. A phase II/III trial in people “at risk” of developing AD—which means they carry one or two copies of the apolipoprotein E gene ε4 allele (APOEε4)—has just started and will go on for the next 5 years as part of the Alzheimer Prevention Initiative [52].
ACC-001 After reporting favourable data on the safety and tolerability of ACC-001/PF-05236806 in a phase I trial, Janssen/Pfizer have discontinued their phase II trials in mild to moderate AD participants after the patients showed no improvement in cognitive function and suffered some adverse reactions [53] (https://trialbulletin.com/lib/entry/ct-00955409).
AD02 and AD04 Researchers at Affiris have screened peptide libraries to identify novel “affitopes” that elicit the production of antibodies that bind exclusively to Aβ N-terminus, are selective for Aβ aggregates, and do not bind to APP or other fragments. Preclinical trials of selected AD01 and AD02 antigens have been encouraging since immunised AD mice showed a reduction in amyloid burden and brain neuropathology, and a significant improvement in cognitive tasks [54]. A phase II study of AD02 in patients with early AD has shown a stabilisation of their cognitive function after 18 months of treatment. Surprisingly, 47 % of patients—mostly at the disease’s early stage—who were treated for 18 months with the AD04 control antigen, saw their hippocampal volume stabilise, and this was correlated with a stabilisation of cognitive function. Although the mechanism of AD04 remains to be elucidated, this study supports that a disease-modifying therapy capable of halting hippocampal degeneration could stop cognitive decline [55].
Overall, the potential problems associated with Aβ active immunisation are the lack of a response in some aged patients who have a defective immune system, and the production of self-antibodies against APP and its soluble fragment, sAPPα, the functions of which are not completely understood. If the second problem can be resolved, Aβ active immunisation might be of value as prevention in asymptomatic patients.
4.1.2 Passive Immunisation
Some encouraging preclinical data were first obtained in AD mouse models, which involved injections of antibodies produced against Aβ42. Some antibodies successfully crossed the blood–brain barrier, but only those that targeted an epitope within the N-terminal region of Aβ were able to bind to amyloid plaques and induce their clearance by activating microglia and inducing phagocytosis [56]. The efficacy of other exogenous Aβ antibodies that did not reach the CNS was attributed to an alternative mechanism, via which they acted as a peripheral sink that depleted Aβ from the blood circulation, and thereby increased its transfer from brain to plasma [57]. Monoclonal mAb266 proved to be effective at ameliorating cognitive impairments in AD mice without decreasing brain amyloid plaque burden, suggesting it worked by redistributing the soluble toxic Aβ oligomers from the CNS into the blood and cerobrospinal fluid (CSF) [58]. A number of clinical trials of passive immunisation treatment have been registered, which are summarised below [49].
Bapineuzumab Janssen/Pfizer’s antibody bapineuzumab (also called 3D6) is a humanised monoclonal antibody targeting Aβ 1–5, which was initially developed by Elan/Wyeth to target amyloid plaques. It has unfortunately failed to meet its clinical endpoints in human trials. In a phase II multicentre study, a group of patients with mild to moderate AD who were treated for 18 months showed no cognitive improvement, although the study remained inconclusive because of its lack of statistical power. Vasogenic oedema (water accumulation in the brain) was observed in 10 % of treated patients and was more frequent in APOEε4 carriers and in subjects being given the higher doses of antibody, possibly due to the removal of vascular amyloid [59]. Positron emission tomography (PET) imaging of Aβ indicated that bapineuzumab removed cortical fibrillar amyloid from the brain of treated patients [60], and CSF analysis showed a decrease of tau and phospho tau, two key signature markers in AD [61]. Two phase III trials in patients with mild to moderate AD, one study with over 1000 asymptomatic APOEε4 carriers and the other with 1000 non-APOEε4 carriers, showed no clinical improvement in either group, in spite of changes in CSF biomarkers in the cohort of APOEε4 carriers [62]. Of patients who received the antibody treatment, 0.9 % developed neoplasma, with a fatal outcome. Brain image analysis showed that the drug had hit its target and decreased fibrillary amyloid, and thus it might be beneficial as prevention in asymptomatic patients [63]. A study at Novartis with two models of AD transgenic mice has shown that mAb3D6, a murine equivalent of bapineuzumab, can clear amyloid pathology, but cannot rescue cognitive deficits, and even aggravates neuronal dysfunction [64]. The mechanism is unclear, but electrophysiology indicates neuronal hyperactivity and unusual synchrony, which may be caused by cross-reactivity with APP and other APP-derived fragments.
Solanezumab Monoclonal antibody solanezumab (LY2062430) from Eli Lilly has provided more promising results and remains in clinical trials. It is directed to the mid region of Aβ and targets the soluble monomer. Early clinical trials have shown that it is usually well-tolerated, although most treated patients complained of headaches. A 12-week phase II study in patients with AD has shown an increase of Aβ42 in the CSF, suggesting that this antibody depletes peripheral Aβ and displaces Aβ associated with amyloid plaques [65]. Two 80-week phase III trials (EXPEDITION1 and EXPEDITION2) in mild to moderate AD have failed to meet their cognitive and functional endpoints [66]. A first partial analysis of the data suggested a greater cognitive decline in the patients given the drug compared with those treated with the placebo, leading to interruption of the trials. Also 0.9 % of the patients who received solanezumab experienced side effects, such as brain oedema and microhaemorrhages [66]. Follow-up analysis of the clinical outcomes has now established some slight but significant benefits of solanezumab treatment in patients with mild cognitive impairment (MCI), and found that these benefits increase over time [67]. Lilly has thus extended to 3.5 years its EXPEDITION3 trial, which involves about 2000 patients with mild AD [68]. Further trials also include asymptomatic “at-risk” patients as part of the Anti-Amyloid Treatment for Asymptomatic Alzheimer’s Disease (A4) and the Dominantly Inherited Alzheimer Network (DIAN) initiatives.
Cremazumab This humanised monoclonal Immunoglobulin G4 (IgG4) antibody, developed by AC Immune/Genentech/Roche, targets aggregated Aβ, including oligomeric and fibrillar species and amyloid plaques, and has been selected for not inducing microglia activation [69]. After successful phase I trials, a phase II multidose trial in mild to moderate AD patients for 68 weeks has failed to meet its clinical endpoints, but has shown a trend for a modest cognitive improvement in the patients with mild AD who received the highest dose of the drug. Two other phase II studies remain ongoing, and cremazumab is also being tested in preventive trials (Alzheimer Prevention Initiative) in people “at risk” (APOEe4 homozygotes) and in carriers of the E280A PS1 FAD mutation in a Colombian kindred [49].
Gantenerumab Roche/Genentech has conducted trials of gantenerumab (RO4909832), an IgG1 against Aβ N-terminus that specifically targets Aβ fibrils. Results showed efficacy at reducing amyloid burden in the brain, but also serious adverse effects such as inflammation or vasogenic oedema in some patients [70]. At the end of 2014, Roche announced the termination of a phase III trial of gantenerumab in patients with prodromal AD (SCarlet RoAD trial), although biological changes indicated that the drug had hit its target and was able to decrease brain amyloid in a number of patients [71].
Aducanumab Biogen is conducting trials of aducanumab (BIIB037), a human IgG1 antibody that was identified by screening natural human antibodies from healthy aged donors, and which targets a conformational epitope of Aβ. Data from a phase Ib study in patients with prodromal and mild AD (PRIME) showed that injections of this antibody could reduce amyloid burden at 26 weeks, and even further at 54 weeks, with a slower rate of cognitive decline compared with patients receiving a placebo (data presented at the International Conference on Alzheimer’s and Parkinson’s Diseases 2015 [72]). Phase III trials are being carried out in 150 centres worldwide, and are recruiting patients with prodromal and mild AD.
Summary After many disappointments, mostly due to the selection of patients at a far too-advanced stage of illness for clinical trials, passive immunisation is starting to offer better promise, and results of preventive treatments are awaited with interest. Janssen/Pfizer (AAB-003), Lilly (N3pG-Aβ), and Astra-Zeneca (MEDI814) have also begun phase I trials of their anti-Aβ in patients with mild to moderate AD. However, the cost of the treatment and the frequency of reported serious adverse effects might limit the clinical applications of passive Aβ immunotherapy in AD.
4.2 Targeting γ-Secretase to Prevent or Modulate Aβ Formation
Targeting the secretases has been considered as a primary choice of intervention because proteolytic enzymes can be inhibited by small molecules that can translate into cost-effective orally available drugs. Successes of the antiretroviral medications targeting the HIV protease and of the inhibitors of circulating enzymes such as renin and kallikrein have demonstrated the feasibility of this therapeutic approach.
4.2.1 γ-Secretase Inhibitors
γ-Secretase inhibitors (GSI) were first developed without knowledge of the identity of the target enzyme. Biochemical evidence supported that the enzyme responsible for the γ-secretase cleavage of APP was an aspartyl protease [73, 74], spurring the design of transition-state analogues of this class of enzymes based on the sequence of the APP cleavage site. Hydroxyisosteres, such as L-685,458 [75], difluoroketones, such as DFK-167 [76], hydroxylether ureas, such as WPE-III31C [77], and pepstatin derivatives that contain a statinoic acid isostere [78] were shown to inhibit γ-secretase activity at low or sub-micromolar concentrations, in vitro and in cellular assays. Demonstration that these inhibitors interacted with key aspartate residues within transmembrane domains of the presenilins established the function of these proteins as novel intramembranous aspartyl proteases, and provided a more substantial bases for rational drug design [79–82]. High-throughput screening of non-targeted drug libraries to identify compounds capable of inhibiting Aβ production from cellular models provided other classes of GSI. Following this approach, Elan discovered a hit dipeptide derivative that was further refined into the lead compound N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) [83], and Dupont (now Bristol Myers Squibb) identified the benzodiazepine derivative, Compound E [82]. Both of these compounds work through an allosteric mechanism and do not directly engage with the γ-secretase active site aspartates. Because such compounds were discovered through cellular screen, they had already passed the test of membrane permeability and had the advantage of a better bioavailability over the substrate analogues. DAPT was the first GSI reported to be orally active and to lower brain Aβ levels in an AD mouse model [83].
Parallel studies with neuronal cultures derived from PS1 knockout mouse embryos revealed that γ-secretase activity was required to mediate Notch signalling during brain development, through the release of the Notch intracellular domain (NICD) that signals gene transcription [84]. Notch signalling controls cell–cell interactions, cell differentiation, and cell-fate decision in the brain and in many body tissues, not only during development but also throughout life [85]. Therefore, the future of GSI development in AD therapy appeared to be precarious, although it was argued that Notch signalling might not play an essential role in aging adults. However, serious side effects of the DAPT-derived LY-411,575 were observed in mice treated with the compound for 15 days, as the mice showed decreased maturation of peripheral B cells and altered intestinal tissue morphology caused by inhibition of Notch signalling [86]. From then on, research focussed on finding “Notch-sparing” GSI.
Semagacestat Researchers at Lilly produced the carboxamide-containing GSI LY-350139 (semagacestat) with marginal selectivity for APP against Notch. Acute dosing and short treatments with this compound lowered Aβ in the plasma, CSF, and brain in animal models, and in the plasma and CSF of humans [87, 88]. Unfortunately, a phase II trial in patients with mild or moderate AD did not lower Aβ levels in CSF, but exposed some side effects of the drug, consisting of skin lesions and intestinal toxicity [89]. A phase III trial in AD patients that was initially planned to last for 21 months was abruptly terminated because cognitive decline worsened in the group treated with semagacestat compared with the group given a placebo. In addition, serious adverse reactions were observed, including gastrointestinal bleeding, skin cancers, and decreased resistance to infection due to immunosuppression, all of which were incumbent to defective peripheral Notch processing.
Avagacestat Bristol Myers Squibb developed BMS-708163 (avagacestat), a carboxamide-substituted sulfonamide with nearly 200-fold selectivity for APP over Notch, and which proved to be effective at lowering Aβ in brain, plasma, and CSF of rats and dogs [90] and in the plasma and CSF of humans [91]. Phase I safety trials in different age groups, including patients with MCI, indicated that the drug was safe and capable of decreasing Aβ levels in plasma [92, 93]. A 24-week phase II trial in patients with mild to moderate AD showed that the highest doses of avagacestat worsened the patients’ cognitive decline and caused a range of adverse secondary effects [94]. Trials in AD were thus terminated. A multicentre phase II trial conducted in patients with MCI and who tested positive for CSF AD biomarkers showed no benefit of the drug, as the patients receiving avagacestat progressed to dementia at the same rate as those given a placebo, with no significant effect on brain atrophy, PET amyloid imaging, or CSF biomarkers [95]. Gastrointestinal, cutaneous, and reversible renal adverse effects (probably related to Notch inhibition) were more frequently observed in patients treated with the drug.
Begacestat The thiophene sulfonamide derivative GSI-953 (begacestat), which has been developed by Wyeth, has encountered similar problems. After performing favourably in preclinical studies, where AD transgenic mice showed reduced Aβ levels in the brain, plasma, and CSF, and improved behaviour in contextual fear-conditioning tests, the drug entered clinical trials. However, phase I results showed no significant effect on CSF Aβ levels and led to discontinuation of human trials because the initial reduction in plasma levels of Aβs followed with an increase [96]. Determining the right dosage of the drug may be critical because some GSI show a biphasic curve and increase Aβ formation at low concentrations [97].
Summary Taken together, trials of GSI in humans have shown no benefit on cognitive function and have demonstrated a range of adverse effects caused by inhibition of Notch signalling.
4.2.2 γ-Secretase Modulators (GSM)
The evidence of the toxicity of drugs that block the activity of γ-secretase called for a new approach to targeting γ-secretase. Novel classes of molecules termed γ-secretase modulators (GSM) have been developed that can modulate the enzyme’s activity, alter its kinetics through an allosteric mechanism, and enhance the production of shorter, less aggregating Aβ peptides, whilst not compromising the release of the Notch NICD fragment or the cleavage of other γ-secretase substrates. γ-Secretase is a multimolecular complex that behaves like proteolytic machinery and digests receptors’ transmembrane domains by a series of cuts. A first endoproteolytic cleavage on the cytosolic side of the membrane facilitates the release of the transcriptionally active cytosolic domains of receptors [20]. Then serial cleavages by a carboxyl-peptidase-like activity progress along one side of the membrane domain α-helix, beginning on its C-terminal end and cutting at every third amino-acid residue [21]. Its cleavage of APP creates Aβ fragments of 49, 46, 43 and 40 amino acids, with only the shorter ones being released from the membrane. FAD mutations in APP impair the first cleavage and cause it to shift, resulting in the production of Aβ fragments of 48, 45, and 42 amino acids. The FAD presenilin mutations also modify γ-secretase by decreasing the rate of the enzyme’s kinetics, and cause processing termination at Aβ43 or Aβ42 [98]. In contrast, some modulators can accelerate the carboxypeptidase reaction, leading to the formation of shorter Aβ peptides, Aβ38 and Aβ37 [99].
Non-steroidal Anti-inflammatory Drugs and Tarenflurbil The first class of molecules that were discovered to modulate the γ-secretase cleavage of APP in vitro was the non-steroidal anti-inflammatory drugs (NSAIDs). Ibuprofen, indomethacin, and sulindac sulphide were found to inhibit production of Aβ42 in cellular and transgenic mouse models, with no effect on Notch processing [100]. Tarenflurbil, a flurbiprofen enantiomer that does not affect cyclo-oxygenase I (COX-1) activity, was tested for 6 months in patients with mild AD, but provided no benefit on cognitive decline or daily living activities, probably due to its very low binding affinity [101].
CHF5074 The tarenflurbil analogue produced by Chiesi, CHF5074, has shown promise so far. It can decrease brain amyloid plaque burden and Aβ levels, and also rescue cognitive deficits in AD transgenic mice. Phase I clinical trials showed it to be safe and well-tolerated upon a single administration, but a phase II study has revealed some adverse effects upon 40-week treatment in patients who received high doses of the compound, with rare cases of worsened cognitive decline and, more commonly, gastrointestinal upset. Some patients, who tolerated the drug and reached 64 weeks of treatment, showed a significant improvement, with lesser cognitive decline than those given a placebo. This progress was particularly evident in APOEe4 carriers. CSF analysis indicated a decrease in the neuroinflammation markers, tumour necrosis factor-α and soluble CD40 after 12 weeks of dosing, supporting the multi-target mechanism of the drug, and CHF5074 has since been considered to be primarily a microglial modulator rather than a GSM [Alzheimer’s Association International Conference (AAIC) 2013; oral presentation O3-06-5; presented July 14, 2013]. The drug has now been licensed to CereSpir Inc. and has progressed into phase III.
Summary and Future perspectives The search for GSM remains active, and the new information recently obtained about the structure of γ-secretase will assist in designing specific GSM [102, 103]. Novel proteins found to interact with C99, or presenilin, and which can modify the processing of APP by γ-secretase, such as TMP 21 [104], the transient receptor potential canonical TRPC 6 [105], and the γ-secretase activating protein (GSAP) [106] may also open new therapeutic avenues, although the effect of the latter on γ-secretase activity and Aβ production remains controversial [107]. However, targeting γ-secretase remains a difficult task because the concept of intramembrane proteolysis is rather novel, and the mechanism of γ-secretase that combines endoproteolytic and carboxypeptidase activities is poorly understood. In addition, the enzymatic activity is contained within a complex comprising at least four subunits, and the expression of subunit homologues, alternative splice variants, and post-translational modifications can potentially generate a diversity of proteolytic complexes with different kinetics and substrate preferences [108]. Therefore, further basic research in the biology of γ-secretase is needed to advance the design of specific inhibitors and modulators.
4.3 BACE Inhibition as a Strategy to Prevent and Control Aβ Formation
Considering the challenges encountered with γ-secretase inhibition, most of the field has shifted focus to β-secretase inhibitors. BACE1 was identified over 15 years ago as the gene responsible for β-secretase activity [109]. Ablation of the BACE1 gene in mice did not cause any major defect in embryonic or early-life development, and the mice reached adulthood with no anatomical abnormalities or overt phenotype [110, 111]. This encouraged the rapid development of BACE1 inhibitors. Reports that BACE1 levels were increased in the brains of AD patients further prompted intensive research into the therapeutic application of BACE1 inhibitors [112–115]. BACE1 is the founder of a new subclass of membrane-tethered aspartyl proteases, with sequence homology to renin, and cathepsin D. The three-dimensional structure of the BACE1 catalytic domain was determined to facilitate the design of inhibitors [116]. It revealed an extended catalytic that posed a challenge for designing high-affinity inhibitors that would be small enough to penetrate through cellular membranes and to cross the blood–brain barrier. In addition, the close homology of BACE1 with BACE2 has further complicated the design of specific BACE1 inhibitors.
Indeed, BACE2 shares 45 % sequence identity and 75 % homology with BACE1, including a close structural similarity in the active site region [117–120]. However, the two enzymes differ by their cleavage specificity. BACE2 was ruled out as a β-secretase candidate because it cleaves APP at an alternative site, close to the cleavage site of α-secretase [117, 121, 122]. The patterns of expression of BACE1 and BACE2 also differ considerably, with BACE1 being principally expressed in the brain where little or no BACE2 is detectable [123, 124]. BACE2 knockout mice display an increased mass of pancreatic β cells and alterations in coat colour that are consistent with hypopigmentation. Physiological and biochemical examinations of these mice have revealed that BACE2 controls the proliferation and function of β cells in the pancreas by cleaving the type I transmembrane protein TMEM27 [125]. BACE2 knockout mice show increased insulin levels and improved glucose metabolism, which supports further investigations to determine if BACE2 inhibition could be beneficial to patients with type 2 diabetes. BACE2 also plays a role in the regulation of melanosomes by cleaving the premelanosome protein PMEL [126], thus BACE2 inhibition may increase photosensitivity in treated patients. Other substrates for BACE2 have also been uncovered by proteomic analysis [127]. Most inhibitors under current development inhibit both BACE1 and BACE2; therefore the potential secondary effects of BACE2 inhibition will have to be taken into consideration.
Hundreds of compounds that potently inhibit BACE1 in vitro have been described, and we have reviewed them previously [128, 129]. Here, we will only summarise key information and the latest progress in the development of BACE1 inhibitors.
4.3.1 Peptidomimetics
The initial approach to designing BACE1 inhibitors was to synthesise transition-state analogues based on the APP sequence, where the peptide bond at the cleavage site is replaced with a non-cleavable isostere such as hydroxyethylene, hydroxyethylamine, or statine/norstatine groups, and then improve their pharmacological properties by chemical substitution and structure refinement, according to a strategy that has been successful in producing efficacious inhibitors for other aspartyl proteases [130]. This approach produced large and polar molecules, with a high binding affinity for BACE1, but unfavourable pharmacological properties and poor brain penetration. Another hurdle has been that some of the substrate analogues and peptidomimetics that were able to pass across cellular membranes and to reach their target faced the problem of efflux by the P-glycoproteins (PgP) that control the blood–brain barrier. These compounds had to be administered together with a PgP inhibitor to demonstrate their efficacy in reducing Aβ formation and amyloid deposition in the brain in animal studies [116, 131–133]. Thus these were unlikely to be applied in the clinic.
CTS-21166 The hydroxyethylene isostere substrate analogue CTS-21166/ASP1702, which was developed by Ghosh and colleagues, proved to potently inhibit BACE1 in vitro with an inhibitory constant (K i) of 1 nM, and was among the first BACE1 inhibitors to be successfully trialled in AD transgenic mice [134]. Intravenous injections in Tg2576 mice decreased Aβ levels by 70 % in plasma and by 55 % in the brain (Table 1). Further preclinical trials demonstrated brain penetration in non-transgenic animals, including mice, rats, dogs, and monkeys, and showed that single and multiple doses of the compound given to rats could lower Aβ in the brain and CSF by up to 50 % [135]. A phase I clinical trial, in which CTS-21166 was given intravenously to healthy male volunteers at doses up to 225 mg, proved that the drug could decrease Aβ levels in plasma in a dose-dependent manner, with an effect lasting for up to 72 h. A second phase I study, where participants received an oral liquid solution of the compound, consolidated these results. These phase I trials proved that CTS-21166 was well-tolerated and attested for the first time that a BACE1 inhibitor could lower Aβ production in humans, although this was only demonstrated in plasma [130]. There is no other trial of this drug currently registered in the USA. The group of Ghosh has also produced GRL-8234, a hydroxyethylamine peptidomimetic with a high affinity for BACE1, good selectivity, and blood–brain barrier permeability, and showed it could lower Aβ levels in the brain of AD transgenic mice [136]. Chronic administration of GRL-8234 for 6 months produced exciting results as, not only could this decrease brain amyloid burden in treated animals, but it could also rescue cognitive deficits. This provided the first proof-of-concept that BACE1 inhibition could ameliorate cognitive performance in an AD model, and has fostered the further development of BACE1 inhibitors.
4.3.2 Small Molecules and Fragment-Based BACE1 Inhibitors
As an alternative approach to substrate analogues, pharmaceutical companies have screened libraries of small molecules, either by virtual simulation—where the chemical entities are tested for docking into a model of the BACE1 catalytic site—or by using high-throughput in vitro assays to identify new warheads capable of altering BACE1 enzymatic activity. The hits obtained from these screens provided lead compounds that were further refined to improve potency and specificity and to demarcate each company’s intellectual property space (for a review see [137]). This approach had the advantage of providing small molecules, with better bioavailability than the peptidomimetics, but also had the disadvantage of producing inhibitors that might block only part of the BACE active site and thus lack specificity for BACE1 relative to BACE2 or to the ubiquitous lysosomal protease cathepsin D. These disadvantages were the key factors in discontinuing the clinical trials of some BACE1 inhibitors.
LY2811376 Eli Lilly and Co. designed the cyclic isothiourea derivative LY2811376, which provided very encouraging results in animal studies. Testing in AD mice and beagle dogs showed dose-dependent reductions in plasma and CSF of Aβ peptides and of the APP-derived products produced by BACE1 cleavage, C99, and sAPPβ [138]. Results from a phase I clinical trial in healthy volunteers indicated that LY2811376 could lower CSF Aβ by up to 55 %, a finding that was for the first time observed in humans and that provided the evidence that the drug had engaged with its target. However, the clinical trials of LY2811376 were terminated as a preventative measure when retinal toxicity was observed in parallel toxicological studies in animals. Rats chronically treated for 3 months with LY2811376 developed an accumulation of lipofuscin that led to cellular degeneration in the retinal epithelium, and to a lesser extent, in the brain neurons and glia [138]. Lipofuscin consists of autofluorescent granule pigments that build up with aging in the lysosomes. Because a similar phenomenon was also observed in the BACE1 knockout mice, it was concluded that LY2811376 toxicity was caused by the lack of specificity of the drug, which also inhibits cathepsin D.
LY2886721 Lilly’s next-generation small molecule BACE inhibitor LY2886721 appeared to be more potent and more specific for BACE1 in preclinical testing [139]. Four double blind, placebo-controlled Phase 1 studies, among which three included in healthy volunteers (NCT01133405, NCT01227252, NCT01534273) and one included both healthy volunteers and AD patients (NCT01807026), and two open label studies in healthy volunteers (NCT01367262 and NCT01775904) were carried out to test the safety and efficacy of ascending doses of the drug. These indicated that the drug was well-tolerated and capable of decreasing levels of Aβ40, Aβ42, and sAPPβ in CSF by up to 74 %. The phase II study NCT01561430 was set up to trial the drug in MCI patients, but this had to be discontinued because of liver toxicity that was attributed to an off-target effect of the drug [140].
AZD3839 Astra-Zeneca reported promising preclinical data of the efficacy and in vivo performance of the aminoisoindole derivative AZD3839 [141]. In vitro studies established the selectivity of the compound to be three orders of magnitude greater for BACE1 compared with cathepsin D, and 14-fold versus BACE2. Oral dosing of AZD3839 in AD transgenic mice, in guinea pigs, and in monkeys demonstrated dose-dependent decreases in the levels of Aβ in brain, CSF, and plasma. The safety and pharmacological properties of the drug were tested in a phase I trial in 54 healthy volunteers (NCT01348737) [142]. A dose-dependent decrease was observed in the levels of plasma Aβ40 and Aβ42, which reached a maximum effect of 55 % in those given the highest dose of 300 mg. These data are consistent with findings in animal studies. However, a concern was raised regarding the safety of the drug because one patient experienced a moderate adverse event, three reported events of presyncope, and others reported milder effects, including dizziness and headaches. Cardiac electrophysiology examination indicated that the drug caused arrhythmia, with a dose-dependent prolongation of the QT interval [143]. This long QT syndrome adverse effect is attributed to the inhibition of potassium ion channel activity. With the consideration that doses of AZD3839 that do not disrupt the heart electrical activity can only marginally decrease plasma Aβ levels, the clinical trials of AZD3839 were terminated.
BI-1147560/VTP37948 Boerhinger-Ingelheim has collaborated with Vitae Pharmaceuticals to test VTP37948 in phase I trials. The drug had given hope in preclinical testing. Initial phase I trials in healthy volunteers gave promising results, with a single-dose treatment decreasing Aβ levels in CSF by 80 % [144]. Further phase I trials that involved 10-day multiple dosing in healthy young and aged volunteers in Germany and in Belgium (NCT02254161) were placed on hold in the first quarter of 2015 because of skin reactions in some of the study participants. Another phase I trial and phase II/III trials have now been terminated.
NB-360 Novartis has also several BACE inhibitors in their pipeline. The cyclic sulfoxide hydroxyethylamine derivative, NB-04 proved to be a potent BACE inhibitor in vitro and capable of reducing Aβ levels in the brain of AD mice and in the CSF of beagle dogs [145]. It could not be advanced into clinical trials because of PgP efflux issues. Based on these results and on the failures of other BACE inhibitors, Novartis have produced NB-360, a new-generation BACE inhibitor with an amino-1,4-oxazine core that provided enhanced pharmacological properties and resulted in improved potency, selectivity, and brain penetration [146]. NB-360’s pharmacokinetics are also favourable, as it does not bind to plasma proteins and it is slowly cleared from the blood, as demonstrated in several animal models, including dogs. Preclinical safety and toxicity studies are ongoing.
5 BACE Inhibitors in Clinical Development
5.1 Verubecestat
The joint efforts and expertise of the chemists and biologists at Merck/Schering-Plough have led to the discovery of the potent BACE inhibitor SCH-900931/MK-8931, recently renamed verubecestat. The chemists used a fragment-based discovery and a fragment combination approach to produce a series of iminothiadiazine dioxide derivatives capable of binding to the BACE1 active site. The compounds were validated as BACE1 inhibitors in an APP substrate cleavage assay, and verubecestat was further selected based on its ability to reduce Aβ levels in the CSF of AD transgenic mice. It is a potent inhibitor of both BACE1 and BACE2, with a Ki for BACE1 equal to 1.75 nM, and a Ki for BACE2 equal to 0.37 nM (patent US8729071 B2, compound 25). Chronic oral administration of verubecestat to APP transgenic TgCRND8 mice prior to amyloid plaque deposition resulted in a considerable reduction of Aβ accumulation in the brain compared with mice treated with vehicle only [147]. The patent covering the compound also states that Aβ levels dropped by 49 % in the brain cortex of rats at 3 h after receiving a dose of 10 mg/kg of the drug. Preclinical characterisation of the drug has recently been reported, including its dose-dependent reduction in levels of Aβ in plasma, CSF, and brain of rats and cynomolgus monkeys [148]. An oral dose of 10 mg/kg caused a drop of 80 % of CSF Aβ40 at 12 h, which lasted for up to 24 h. A 72 % decrease of Aβ was observed in the brain cortex at 4 h after dosing. The potential toxicity of the drug has also been thoroughly investigated, and it is predicted to be low because verubecestat does not activate cytochrome P450, a major inducer of liver toxicity, and it is a weak inhibitor of hERG, which controls the electrical activity of the heart.
The drug has been tested in clinical trials for the past 4 years, and has now moved to phase III. Impressive data have been reported for several phase I studies. A two-part phase I trial that was conducted in 88 healthy volunteers, aged 18–45 years, proved that verubecestat was well-tolerated and produced a profound and lasting decrease of Aβ in the CSF [149, 150]. In a randomised, double-blind, placebo-controlled, single ascending dose study, a dose-dependent decrease in levels of Aβ40 in the CSF was observed at 36 h after administration, with a 21 % reduction in those given a 20-mg dose, a 75 % reduction in those given a 100-mg dose, and a 92 % reduction in those who received 550 mg of the drug. In a multiple ascending dose study, the participants were administered daily doses of the drug for 14 days. CSF Aβ40 levels, which were measured at the end of the study and compared with the baseline, were reduced by as much as 32 % for the 10-mg dose, 91 % for the 150-mg dose, and 94 % for the 250-mg dose. A phase Ib clinical trial was also conducted to test the safety, tolerability, and pharmacodynamics of the drug in AD participants (NCT01496170) [151]. Thirty patients, aged 50–85 years, with a history of cognitive decline and who were classified as mild to moderate AD, were administered either 12, 40, or 60-mg daily doses of verubecestat or a placebo for 7 days. Dose-dependent, sustained reductions in Aβ40, Aβ42, and sAPPβ in CSF by up to nearly 90 % were observed at 36 h after the last dose. The data were consistent between AD patients and healthy controls, suggesting that brain amyloid load does not impair the drug efficacy [152]. A study carried out in Japan corroborated the data obtained in the USA, and showed a sustained 88 % reduction in baseline levels of CSF Aβ40 at 24 h after oral administration of a 450-mg single dose of verubecestat [153].
The challenge remains now to demonstrate the cognitive benefits of the drug in MCI, mild AD, and “at-risk” patients. Merck has now registered two phase II/III clinical trials. The EPOCH study began in 2012 with a phase II trial in 400 patients with mild to moderate AD, who received a daily oral dose of 12, 40, or 60 mg of verubecestat, or a placebo, to assess the safety and efficacy of the drug over an 18-month treatment period (NCT01739348). There has been no report of adverse reactions so far, but the highest dose has been dropped to 40 mg in the extension of the study, which is being continued for up to 5 years. The EPOCH phase III has also begun, which involves 1560 additional participants who are dosed daily with 12 or 40 mg of the drug. The primary outcomes of the EPOCH study will be the observation at 18 months of a change from baseline in psychometric tests that measure cognitive function (Alzheimer’s Disease Assessment Scale-Cognition, ADAS-Cog score) and performance in daily activities (Alzheimer's Disease Cooperative Study Activities of Daily Living, ADCS-ADL score). If the drug proves to be beneficial, the trial will be extended to 5 years. Secondary outcomes will also be measured, which include the Clinical Dementia Rating Sum of Boxes (CDR-SB) score to obtain a precise rating of dementia stage, Mini-Mental State Examination (MMSE) score, changes in hippocampal volume determined by magnetic resonance imaging (MRI), changes in CSF tau, and changes in brain amyloid load determined by PET imaging. The final data will be collected mid 2017, and the study is expected to be completed by July 2019. Another phase III study, called β Amyloid Production and Effects on Cognition Study (APECS), is still recruiting. This aims to test daily treatment with 12 mg or 40 mg of verubecestat in 1500 participants with prodromal AD, for an initial period of 2 years, which could be extended to an additional 5 years (NCT01953601). The participants should be aged 50–85 years, have a history of progressive memory decline, and test positive for AD PET biomarkers, but not meet the criteria for dementia. The outcomes of this study are the same as those of the EPOCH trial. APECS is due to be completed in March 2021, and since it started in 2013, interim 24-month data may become available very soon.
In summary, verubecestat is the most advanced BACE1 inhibitor in clinical trials today. Its high potency and its selectivity for BACE1 against cathepsin D limit off-target effects. Preclinical trials, as well as phase I and II, have met their objectives. The design of phase II/III trials, involving patients with moderate cognitive impairment and MCI, supported by thorough clinical investigations and by state-of-the-art methodologies to monitor biomarkers of the disease progression, represents the best possible set up to achieve a positive outcome. Results of these trials will provide the ultimate test of the effectiveness of BACE1 inhibitors in AD therapy. The equal specificity of verubecestat for BACE1 and BACE2, and the diversity of BACE1 substrates remain potential sources of mechanism-based toxicity, especially during prolonged treatment.
5.2 Other Promising BACE Inhibitors
Merck has been leading the field of BACE inhibitors for the last 5 years; however, three other companies are currently determined to catch up and are expediting the recruitment of participants for phase II and phase III trials of their proprietary compounds. The timelines for completion of these trials are compared with that of verubecestat in Fig. 2. The three following drugs are the direct competitors of verubecestat.
5.2.1 AZD3293
Astra-Zeneca is now testing AZD3293/LY-3314814 in human trials in collaboration with Lilly. This double-spiro amino imidazole derivative, which was designed from a fragment-based screening approach, is an orally available and brain penetrant BACE inhibitor with a high potency [in vitro concentration inhibiting enzyme activity by 50 % (IC50) = 0.2 nM]. It has a 25,000-fold selectivity for BACE against cathepsin D, but it inhibits BACE2 and BACE1 equally. It has been proved efficacious at inhibiting Aβ40 secretion in various neuronal systems. Experiments in animals have demonstrated dose-dependent decreases of Aβ40, Aβ42, and sAPPβ levels in the brain and plasma of AD mice and in the brain, plasma, and CSF of guinea pigs and dogs [154, 155].
Phase I safety and efficacy clinical trials began in late 2012, with 72 young healthy volunteers being administered 1- to 1000-mg single doses of the drug (NCT01739647). Other phase I trials were carried out to assess the safety and pharmacodynamics of the drug. Trials in the USA (NCT01795339) and in Japan (NCT02005211), in which healthy participants and AD patients were given single (15, 50 or 150 mg) or multiple doses (15 or 50 mg) of the compound or placebo to evaluate tolerability and effects on plasma and CSF biomarkers indicated that the drug caused no serious adverse effect. A strong, dose-dependent reduction of CSF Aβ levels was observed in both healthy volunteers and AD patients [156, 157]. In the study carried out in Japan with young and elderly volunteers, a single 50-mg dose of AZD3293 could lower plasma levels of both Aβ40 and Aβ42 by ~75 %. Daily dosing for 14 days resulted in similar >75 % decreases of Aβ40 and Aβ42 in plasma and CSF [158]. A phase II/III trial began in December 2014, and is due to end in May 2019 and to be fully completed by 2021 (NCT02245737). This is a 24-month multicentre, randomised, double-blind study in patients with early AD (AMARANTH study) that is expected to involve 2202 participants over 15 countries. The primary measured outcome will be a change from baseline in the Clinical Dementia Rating (CDR) score observed at 24 months. The patients are also being assessed for changes in psychometric tests that consist of cognitive score (ADAS-Cog) and functional activities in daily living (ADCS-ADL), and for changes in brain volume identified by MRI. A series of AD biomarkers will be measured, including CSF levels of Aβ42, Aβ40, total tau, and phospho tau, as well as brain PET imaging of amyloid accumulation and glucose metabolism.
5.2.2 E2609
This small oral BACE inhibitor developed by Eisai Co. Ltd was reported to decrease Aβ levels in the plasma, brain, and CSF of animal models, including rats, guinea pigs, and rhesus monkeys [159]. Biogen is conducting the clinical development of the drug. Phase I trials have shown that the drug is well-tolerated, has a prolonged half-life, and can effectively lower Aβ levels in plasma by up to 90 % [160] and in CSF by 71 % after 100-mg daily dosing for 14 days [161]. The drug was proved to engage with its target since it was also found to decrease BACE1 enzymatic activity in the CSF by up to 99 %, in a dose-correlated manner, without changing the levels of BACE1 protein [162]. Eight phase I clinical trials have been completed in Japan and in the USA, involving elderly healthy participants, and patients with MCI and moderate AD (among which are NCT02207790, NCT01294540, NCT01511783, and NCT01600859). A phase II placebo-controlled, double-blind, randomised, proof-of-concept study is currently being conducted in the USA that involves 700 patients, including those with MCI and mild to moderate AD. The primary outcome of the study is to establish the safety and tolerability of the drug given as a daily dose regimen, and to evaluate its cognitive benefits in MCI patients after 18 months of treatment, as defined from changes from baseline in the derived Alzheimer’s Disease Composite Score (NCT02322021). Data to be recorded are (1) changes in hippocampal atrophy at 12 and 18 months; (2) changes in CSF Aβ levels at 4 weeks and 18 months; and (3) changes in Alzheimer’s Disease Composite Score in patients with mild to moderate AD at 18 months. The study is due to be completed by January 2018.
5.2.3 JNJ-54861911
Data recently reported by Janssen/Johnson & Johnson indicate that they are also at the forefront of the competition to bring a BACE inhibitor into the clinic. They have registered 14 clinical trials for the compound JNJ-54861911. Phase I trials in a group of healthy participants aged 55–85 years have been recently reported [163]. A single dose of the compound efficiently decreased Aβ levels in a dose-dependent manner, with a maximum effect being recorded in plasma at 3–4 h and in CSF at 6–8 h. Up to 95 % reductions in CSF Aβ levels were observed after 14 days of daily dosing. sAPPβ was also decreased, with a concomitant two- to threefold increase in sAPPα. Janssen has just completed a study in Europe and is finishing a study in Japan, both of which involve participants with MCI who received 10 or 50 mg of the drug, or a placebo, daily for 4 weeks in order to investigate the outcomes of the treatment on the products of APP processing in plasma and CSF (NCT01978548 and NCT02360657). They are also carrying on a multicentre study to test the safety and tolerability of the drug in 100 patients with early AD who are receiving a daily dose of 10 or 50 mg of JNJ-54861911 for 10 months (NCT02260674). The efficacy of the drug on the levels of Aβ and APP fragments in plasma and CSF is also being analysed at 1 and 6 months. This study has already been extended for 12 months (NCT02406027). Janssen has also begun phase II/III studies in Europe, USA, Canada, Mexico, and Australia, with focus being placed on prevention and early intervention. A 54-month study has started with patients aged 60–85 years classified as at risk of developing AD because they have a family history of dementia (first-degree relative) or they are APOEε4 allele carriers or they have tested positive for Aβ accumulation in the brain by CSF analysis or PET imaging (NCT02569398). The participants are given a daily oral dose of JNJ-54861911 (either 5 mg or 25 mg) or a placebo. The primary outcome measurement will be the finding of a change from baseline in cognitive assessment tests at the end of the study. A battery of psychometric and psychological tests will be performed, and the patients will be carefully monitored for adverse reactions. Changes in AD imaging biomarkers will be recorded.
5.3 Potential Problems Associated with BACE Inhibition
Although the first reports on BACE1 knockout mice stated that these animals had no overt phenotypes, further examination has revealed subtle abnormalities in behaviour, physiology, histochemistry, and electrophysiology. An increased rate of perinatal mortality, a smaller adult size, hyperactive behaviour, and some alterations in the inactivation of voltage-gated sodium channels were recorded [164]. Furthermore, the evidence of hypomyelination in the central and peripheral nervous systems of these mice revealed that the membrane-bound isoforms of neuregulin 1 (Nrg1) were substrates of BACE1 [165, 166]. BACE1 is required to activate the Nrg1/erbB cascade that regulates the thickness of myelin sheath during neuronal development early in life. BACE1 expression is also needed in both neurons and Schwann cells to mediate remyelination upon injury [167]. Considering that Nrg1 is an established susceptibility gene for schizophrenia and other psychotic disorders, the behaviour of the BACE1−/− mice was also thoroughly examined, and this revealed “schizophrenia-like” phenotypes associated with defective Nrg1-erbB4 signalling [168]. BACE1 cleavage also activates the function of Nrg1 in muscle-spindle formation, which underlies proper motor coordination [169]. In addition, BACE1 is also implicated in the regulation of voltage-gated sodium channels by cleaving their regulatory β subunits [170]. Proteomics analyses have demonstrated that three dozen neuronal type I integral proteins are misprocessed in the absence of BACE1 expression [127, 171, 172]. BACE1 substrates include cell adhesion molecules and receptors required for axonal growth and guidance, regulatory molecules involved in synaptic plasticity and in the activity of sodium and potassium ion channels, as well as proteins that contribute to inflammation and repair processes. Therefore, it can be expected that blocking BACE1 activity will give rise to an array of mechanism-based side effects. As an example, BACE inhibitors were shown to cause retinal toxicity in animal models by a mechanism that entails misprocessing of vascular endothelial growth factor receptor 1 and defective lysosomal function [173]. Also, because the active sites of BACE1 and BACE2 are structurally very similar, most BACE inhibitors inhibit equally both proteases. BACE2 is principally expressed in the periphery, and plays an important role in the pancreas, where it controls the proliferation and activity of β cells [127]. It is also required for the processing of the melanocyte protein PMEL that controls skin pigmentation [174]. Therefore, potential adverse reactions will have to be cautiously monitored in patients receiving BACE inhibitors, and ideally the drugs should be dosed to restrict Aβ production to non-toxic levels, while sustaining some degree of BACE activity to carry on the cleavage of various BACE1 and BACE2 substrates. Newly discovered cleavages of APP upstream from the BACE1 cleavage site, which are mediated by MMPs and that influence subsequent processing by α- and β-secretases, will also have to be taken into consideration [175, 176]. The biological importance of the so-called “eta-secretase” (η-secretase; MMP-7), which acts in tandem with either BACE1 or α-secretase to generate fragments that modify LTP, is under scrutiny and may well complicate the applications of BACE inhibitors [175]. The membrane-type 5 matrix metalloproteinase (MT5-MMP), which was shown to promote the amyloidogenic processing of APP in an AD mouse model, might offer an alternative therapeutic target [176].
5.4 Rationale for the Continued Use of BACE Inhibitors in AD Therapy
Because the clinical trials of novel therapies based on the amyloid cascade have so far provided a succession of disappointing and negative results, the question of the validity of this approach has again been raised. However, the amyloid hypothesis cannot be denied from the results of unsuitable drug trials. Each failed trial has taught us important lessons to move forward. First, we now know that the choice of the right cohort of participants is primordial and that an intervention in patients with advanced AD is unfortunately unlikely to succeed. The recent progress in the development of diagnostic tools has helped to better understand the disease progression and it has highlighted that Aβ begins to accumulate in the brain years before the occurrence of AD symptoms [177–180]. Therefore, a therapeutic intervention should ideally be initiated in asymptomatic people who report memory complaints and who test positive for AD imaging and CSF biomarkers, and in those who carry a genetic risk for developing AD, such as the APOEε4 allele. Also, safety trials should be conducted over several months to detect potential adverse effects of the drug. And it is evident that an orally available drug will be easier to dose and administer than an antibody. Based on these considerations, BACE inhibitors remain strong candidates in future AD therapy.
Little study has been done to assess the pathology associated with BACE1 elevation, which happens in a large proportion of patients with sporadic AD, and also particularly in patients with MCI [181]. Considering the number and variety of BACE substrates, adverse consequences of high BACE1 activity in the brain, apart from the overproduction of Aβ, should therefore be expected. A study has shown that overexpression of BACE1 in transgenic mice caused increased processing of voltage-gated sodium channel β subunits, which resulted in decreased cell surface expression of the sodium channels, hence impaired propagation of action potentials and epileptic phenotypes [170, 182]. Other potential effects of BACE1 elevation in AD merit further investigation. Indeed, it was shown that the direct C-terminal product of APP cleavage by BACE1, C99, accumulates in the brain earlier than Aβ and significantly contributes to neurodegeneration and cognitive alterations in transgenic mice [183]. In addition, Aβ toxicity itself induces BACE1 expression, as evidenced from its accumulation in dystrophic neurites surrounding amyloid plaques [112, 184]. Therefore, BACE1 inhibition will provide a means to break this vicious cycle of BACE1 elevation and Aβ toxicity, and could also prevent the deleterious effects of BACE1 excess activity in the cleavage of its other substrates.
The risks associated with BACE1 inhibition have been essentially assessed from the observation of BACE1 knockout mice, or from wild-type animals treated with levels of inhibitors that completely get rid of BACE1 activity. For BACE1-targeted therapy to be safe, it will be important to monitor the levels of BACE1 activity and to determine its normal range in the CSF and/or blood cells so that BACE inhibitors could be used to restore these levels, but not completely remove BACE1 activity. It may also be beneficial to use a combined therapy in patients with mild to moderate AD, with a BACE inhibitor being administered in conjunction with an antibody targeting Aβ, or tau. Indeed, recent studies in mice have shown that passive tau immunotherapy can successfully reduce tau pathology and ameliorate cognitive deficits in mouse models [185] [186], and some tau antibodies are now being tested in phase I clinical trials. Inhibitors of glycogen-synthase kinase-3β (GSK-3β), the major kinase responsible for tau hyperphosphorylation, have also emerged as promising drug candidates in AD [187, 188]. GSK-3β has been proposed as the possible link between Aβ and tau [189]. It was also shown to repress BACE1 expression [190]. Recently, dual inhibitors that consist of an arm targeting BACE1 and another arm that targets GSK-3β have been described that possess favourable pharmacological properties and can cross the blood–brain barrier [191, 192]. These attest that the search for new therapeutics in AD is still progressing.
6 Conclusions
Despite the failure of early BACE inhibitors that lacked potency and specificity, a new generation of BACE inhibitors provides strong candidates in AD therapy. BACE1 inhibition represents a rational and promising approach to break the pathological cycle of amyloid toxicity. The design of clinical trials in AD has made considerable progress and is now supported by diagnostic tools that permit selection of patients at the prodromal stage of AD, who are those most likely to benefit from this treatment. Verubecestat is the most advanced among the new BACE inhibitors, and some interim results of its phase III trials may soon become available. Several other compounds are also entering phase II/III trials. It may be expected that by the next decade, BACE inhibitors will be used in the clinic for AD therapy. However, research must continue to improve understanding of the biology of BACE1, as a prerequisite to evaluate, prevent, or compensate for the potential adverse effects of BACE1 inhibition.
References
Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88:640–51.
Perl DP. Neuropathology of Alzheimer’s Disease. Mt Sinai J Med. 2010;77:32–42.
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189.
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–9.
Sheng M, Sabatini BL, Südhof TC. Synapses and Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2012;4:pii: a005777.
Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–57.
Iqbal K, Liu F, Gong C-X. Tau and neurodegenerative disease: the story so far. Nat Rev Neurol. 2016;12:15–27.
Mondragón-Rodríguez S, Perry G, Zhu X, Boehm J. Amyloid beta and tau proteins as therapeutic targets for Alzheimer’s disease treatment: rethinking the current strategy. Int J Alzheimers Dis. 2012;2012:630182.
Mudher A, Lovestone S. Alzheimer’s disease—do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–6.
Lonskaya I, Hebron M, Chen W, Schachter J, Moussa C. Tau deletion impairs intracellular β-amyloid-42 clearance and leads to more extracellular plaque deposition in gene transfer models. Mol Neurodegen. 2014;9:46.
Dubey J, Ratnakaran N, Koushika SP. Neurodegeneration and microtubule dynamics: death by a thousand cuts. Front Cell Neurosci. 2015;9:343.
Kovacs GG. Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol. 2015;41:3–23.
Kang J, Lemaire H-G, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K-H, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–6.
Nhan HS, Chiang K, Koo EH. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol. 2015;129:1–19.
Nalivaeva NN, Turner AJ. The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett. 2013;587:2046–54.
O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204.
Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2012:2.
Pardossi-Piquard R, Checler F. The physiology of the beta-amyloid precursor protein intracellular domain AICD. J Neurochem. 2012;120(Suppl 1):109–24.
Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–20.
Iwatsubo T. The gamma-secretase complex: machinery for intramembrane proteolysis. Curr Opin Neurobiol. 2004;14:379–83.
Tomita T. Molecular mechanism of intramembrane proteolysis by γ-secretase. J. Biochem. 2014;156:195–201.
Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, et al. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci USA. 1999;96:3922–7.
Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, et al. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–7.
Chami L, Checler F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and beta-amyloid production in Alzheimer’s disease. Mol Neurodegen. 2012;7:52.
Tamagno E, Guglielmotto M, Monteleone D, Vercelli A, Tabaton M. Transcriptional and post-transcriptional regulation of β-secretase. IUBMB Life. 2012;64:943–50.
Tan J, Li QX, Ciccotosto G, Crouch PJ, Culvenor JG, White AR, et al. Mild oxidative stress induces redistribution of BACE1 in non-apoptotic conditions and promotes the amyloidogenic processing of Alzheimer’s disease amyloid precursor protein. PloS One. 2013;8:e61246.
Baranello RJ, Bharani KL, Padmaraju V, Chopra N, Lahiri DK, Greig NH, et al. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr Alzheimer Res. 2015;12:32–46.
Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12.
Haass C, Hung AY, Schlossmacher MG, Oltersdorf T, Teplow DB, Selkoe DJ. Normal cellular processing of the β-amyloid precursor protein results in the secretion of the amyloid β peptide and related molecules. Ann N Y Acad Sci. 1993;695(1):109–16.
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5.
Schellenberg G, Montine T. The genetics and neuropathology of Alzheimer’s disease. Acta Neuropathol. 2012;124:305–23.
Tanzi RE, Kovacs DM, Kim T-W, Moir RD, Guenette SY, Wasco W. REVIEWThe Gene Defects Responsible for Familial Alzheimer’s Disease. Neurobiol Dis. 1996;3:159–68.
Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, et al. Mutation of the [beta]-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature. 1992;360:672–4.
Herl L, Thomas AV, Lill CM, Banks M, Deng A, Jones PB, et al. Mutations in amyloid precursor protein affect its interactions with presenilin/γ-secretase. Mol Cell Neurosci. 2009;41:166–74.
Ancolio K, Dumanchin C, Barelli H, Warter JM, Brice A, Campion D, et al. Unusual phenotypic alteration of β amyloid precursor protein (βAPP) maturation by a new Val-715 – > Met betaAPP-770 mutation responsible for probable early-onset Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:4119–24.
Czech C, Tremp G, Pradier L. Presenilins and Alzheimer’s disease: biological functions and pathogenic mechanisms. Progr Neurobiol. 2000;60:363–84.
Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–9.
Lambert J-C, Amouyel P. Genetics of Alzheimer’s disease: new evidences for an old hypothesis? Curr Opin Genet Dev. 2011;21:295–301.
Kim M, Suh J, Romano D, Truong MH, Mullin K, Hooli B, et al. Potential late-onset Alzheimer’s disease-associated mutations in the ADAM10 gene attenuate alpha-secretase activity. Hum Mol Genet. 2009;18:3987–96.
Lambert J-C, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat enet. 2013;45:1452–8.
Calero M, Gómez-Ramos A, Calero O, Soriano E, Avila J, Medina M. Additional mechanisms conferring genetic susceptibility to Alzheimer’s disease. Front Cell Neurosci. 2015;9:138.
Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77:43–51.
Fagan T. Alzheimer’s GWAS Hits Point to Endosomes, Synapses. Published 13/11/2015. http://www.alzforum.org/news/conference-coverage/alzheimers-gwas-hits-point-endosomes-synapses.
Ingram DK. Vaccine development for Alzheimer’s disease: a shot of good news. Trends Neurosci. 2001;24:305–7.
Solomon B. Immunological approaches as therapy for Alzheimer’s disease. Expert Opin Biol Ther. 2002;2:907–17.
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7.
Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle M-B, Maurage C-A, et al. Beneficial effect of human anti-amyloid-β active immunization on neurite morphology and tau pathology. Brain. 2010;133:1312–27.
Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–62.
Wisniewski T, Goñi F. Immunotherapeutic Approaches for Alzheimer’s Disease. Neuron. 2015;85:1162–76.
Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, et al. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2015;11:597–604.
Farlow MR, Andreasen N, Riviere M-E, Vostiar I, Vitaliti A, Sovago J, et al. Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers Res Ther. 2015;7(1):23.
Alzforum website. Therapeutics, CAD106. http://www.alzforum.org/therapeutics/cad106. Accessed 03 March 2016.
Arai H, Suzuki H, Yoshiyama T. Vanutide Cridificar and the QS-21 adjuvant in Japanese subjects with mild to moderate Alzheimer’s disease: results from two phase 2 studies. Curr Alzheimer Res. 2015;12:242–54.
Mandler M, Santic R, Gruber P, Cinar Y, Pichler D, Funke SA, et al. Tailoring the antibody response to aggregated aβ using novel alzheimer-vaccines. PLoS One. 2015;10:e0115237.
Bock J. Breakthrough in Alzheimer’s Disease: AFFiRiS halted clinical progression in Alzheimer patients upon treatment with AD04 in a phase II clinical study. AFFiRiS website; 2014. http://www.affiris.com/pdf/presse_medien/pressemeldungen/14_Final_PK_PhaseII_E_0603.pdf. Accessed 03 March 2016.
Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9.
DeMattos RB, Bales KR, Cummins DJ, Dodart J-C, Paul SM, Holtzman DM. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–5.
Dodart J-C, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, et al. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat Neurosci. 2002;5:452–7.
Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–70.
Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-β load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–72.
Blennow K, Zetterberg H, Rinne JO, et al. EFfect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate alzheimer disease. Arch Neurol. 2012;69:1002–10.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–33.
Liu E, Schmidt ME, Margolin R, Sperling R, Koeppe R, Mason NS, Klunk WE, Mathis CA, Salloway S, Fox NC, Hill DL, Les AS, Collins P, Gregg KM, Di J, Lu Y, Tudor IC, Wyman BT, Booth K, Broome S, Yuen E, Grundman M, Brashear HR, Bapineuzumab 301 and 302 Clinical Trial Investigators. Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology. 2015;85:692–700.
Busche MA, Grienberger C, Keskin AD, Song B, Neumann U, Staufenbiel M, et al. Decreased amyloid-β and increased neuronal hyperactivity by immunotherapy in Alzheimer’s models. Nat Neurosci. 2015;18:1725–7.
Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement. 2012;8:261–71.
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 Trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–21.
Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, et al. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement. 2015;pii: S1552-5260:02148-2.
Gandy S, Sano M. Alzheimer disease: Solanezumab: prospects for meaningful interventions in AD? Nat Rev Neurol. 2015;11:669–70.
Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci. 2012;32:9677–89.
Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69:198–207.
Roche. Roche provides update on gantenerumab development programme. Roche website; 19/12/2014. http://www.roche.com/media/store/releases/med-cor-2014-12-19b.htm. Accessed 03 March 2016.
Strobel G. Biogen antibody buoyed by phase 1 data and hungry investors. Alzforum website 25/03/2015. http://www.alzforum.org/news/conference-coverage/biogen-antibody-buoyed-phase-1-data-and-hungry-investors. Accessed 03 March 2016.
Evin G, Cappai R, Li QX, Culvenor JG, Small DH, Beyreuther K, et al. Candidate gamma-secretases in the generation of the carboxyl terminus of the Alzheimer’s disease beta A4 amyloid: possible involvement of cathepsin D. Biochemistry. 1995;34:14185–92.
Higaki J, Catalano R, Guzzetta AW, Quon D, Navé J-F, Tarnus C, et al. Processing of β-amyloid precursor protein by cathepsin D. J Biol Chem. 1996;271:31885–93.
Shearman MS, Beher D, Clarke EE, Lewis HD, Harrison T, Hunt P, et al. L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid beta-protein precursor gamma-secretase activity. Biochemistry. 2000;39:8698–704.
Wolfe MS, Citron M, Diehl TS, Xia W, Donkor IO, Selkoe DJ. A substrate-based difluoro ketone selectively inhibits Alzheimer’s gamma-secretase activity. J Med Chem. 1998;41:6–9.
Esler WP, Kimberly WT, Ostaszewski BL, Ye W, Diehl TS, Selkoe DJ, et al. Activity-dependent isolation of the presenilin- gamma -secretase complex reveals nicastrin and a gamma substrate. Proc Natl Acad Sci USA. 2002;5(99):2720–5.
Tian G, Sobotka-Briner CD, Zysk J, Liu X, Birr C, Sylvester MA, et al. Linear non-competitive inhibition of solubilized human gamma-secretase by pepstatin A methylester, L685458, sulfonamides, and benzodiazepines. J Biol Chem. 2002;277:31499–505.
Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–94.
Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–7.
Evin G, Sharples RA, Weidemann A, Reinhard FB, Carbone V, Culvenor JG, et al. Aspartyl protease inhibitor pepstatin binds to the presenilins of Alzheimer’s disease. Biochemistry. 2001;40:8359–68.
Seiffert D, Bradley JD, Rominger CM, Rominger DH, Yang F, Meredith JE, et al. Presenilin-1 and -2 are molecular targets for γ-secretase inhibitors. J Biol Chem. 2000;275:34086–91.
Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–81.
De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–22.
Kopan R. Notch signaling. Cold Spring Harb Perspect Biol. 2012;4:a011213.
Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, et al. Chronic treatment with the γ-secretase inhibitor LY-411,575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–82.
Henley DB, May PC, Dean RA, Siemers ER. Development of semagacestat (LY450139), a functional γ-secretase inhibitor, for the treatment of Alzheimer’s disease. Expert Opin Pharmacother. 2009;10:1657–64.
Bateman RJ, Siemers ER, Mawuenyega KG, Wen G, Browning KR, Sigurdson WC, et al. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann Neurol. 2009;66:48–54.
Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, et al. Phase II safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer’s disease. Arch Neurol. 2008;65:1031–8.
Gillman KW, Starrett JE, Parker MF, Xie K, Bronson JJ, Marcin LR, et al. Discovery and evaluation of BMS-708163, a potent, selective and orally bioavailable γ-secretase inhibitor. ACS Med Chem Lett. 2010;1:120–4.
Albright CF, Dockens RC, Meredith JE, Olson RE, Slemmon R, Lentz KA, et al. Pharmacodynamics of selective inhibition of γ-secretase by avagacestat. J Pharmacol Exp Ther. 2013;344:686–95.
Tong G, Wang J-S, Sverdlov O, Huang S-P, Slemmon R, Croop R, et al. Multicenter, randomized, double-blind, placebo-controlled, single-ascending dose study of the oral γ-secretase inhibitor BMS-708163 (avagacestat): tolerability profile, pharmacokinetic parameters, and pharmacodynamic markers. Clin Ther. 2012;34:654–67.
Tong G, Wang J-S, Sverdlov O, Huang S-P, Slemmon R, Croop R, et al. A contrast in safety, pharmacokinetics and pharmacodynamics across age groups after a single 50 mg oral dose of the γ-secretase inhibitor avagacestat. Br J Clin Pharmacol. 2013;75(1):136–45.
Coric V, van Dyck CH, Salloway S, et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate alzheimer disease. Arch Neurol. 2012;69:1430–40.
Coric V, Salloway S, van Dyck CH, et al. Targeting prodromal alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol. 2015;72:1324–33.
Martone RL, Zhou H, Atchison K, Comery T, Xu JZ, Huang X, et al. Begacestat (GSI-953): a novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein γ-secretase for the treatment of Alzheimer’s disease. J Pharmacol Exp Ther. 2009;331:598–608.
Niva C, Parkinson J, Olsson F, van Schaick E, Lundkvist J, Visser SG. Has inhibition of Aβ production adequately been tested as therapeutic approach in mild AD? A model-based meta-analysis of γ-secretase inhibitor data. Eur J Clin Pharmacol. 2013;69:1247–60.
Szaruga M, Veugelen S, Benurwar M, Lismont S, Sepulveda-Falla D, Lleo A, et al. Qualitative changes in human γ-secretase underlie familial Alzheimer’s disease. J Exp Med. 2015;212:2003–13.
Chávez-Gutiérrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, et al. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31:2261–74.
Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. A subset of NSAIDs lower amyloidogenic A[beta]42 independently of cyclooxygenase activity. Nature. 2001;414:212–6.
Green RC, Schneider LS, Amato DA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–64.
Takagi-Niidome S, Sasaki T, Osawa S, Sato T, Morishima K, Cai T, et al. Cooperative roles of hydrophilic loop 1 and the C-terminus of presenilin 1 in the substrate-gating mechanism of γ-secretase. J Neurosci. 2015;35:2646–56.
X-c Bai, Yan C, Yang G, Lu P, Ma D, Sun L, et al. An atomic structure of human γ-secretase. Nature. 2015;525:212–7.
Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, et al. TMP21 is a presenilin complex component that modulates γ-secretase but not ε-secretase activity. Nature. 2006;440:1208–12.
Wang J, Lu R, Yang J, Li H, He Z, Jing N, et al. TRPC6 specifically interacts with APP to inhibit its cleavage by γ-secretase and reduce Aβ production. Nat Commun. 2015;6:8876.
He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467:95–8.
Hussain I, Fabrègue J, Anderes L, Ousson S, Borlat F, Eligert V, et al. The role of γ-secretase activating protein (GSAP) and imatinib in the regulation of γ-secretase activity and amyloid-β generation. J Biol Chem. 2013;288:2521–31.
De Strooper B. Chávez Gutiérrez L. Learning by failing: ideas and concepts to tackle γ-secretases in Alzheimer’s disease and beyond. Annu Rev Pharmacol Toxicol. 2015;55:419–37.
Vassar R. The β-secretase, BACE. J Mol Neurosci. 2001;17:157–70.
Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, et al. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum Mol Genet. 2001;10:1317–24.
Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, et al. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–2.
Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O’Connor T, et al. β-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer’s disease pathogenesis. J Neurosci. 2007;27:3639–49.
Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–6.
Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–9.
Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, et al. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2004;101:3632–7.
Hong L, Koelsch G, Lin X, Wu S, Terzyan S, Ghosh A, et al. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science. 2000;290:150–3.
Farzan M, Schnitzler CE, Vasilieva N, Leung D, Choe H. BACE2, a beta -secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proc Natl Acad Sci USA. 2000;97:9712–7.
Acquati F, Accarino M, Nucci C, Fumagalli P, Jovine L, Ottolenghi S, et al. The gene encoding DRAP (BACE2), a glycosylated transmembrane protein of the aspartic protease family, maps to the Down critical region. FEBS Lett. 2000;468:59–64.
Saunders AJ, Kim T-W, Tanzi RE. BACE Maps to Chromosome 11 and a BACE homolog, BACE2, reside in the obligate Down syndrome region of chromosome 21. Science. 1999;286:1255.
Hussain I, Powell DJ, Howlett DR, Chapman GA, Gilmour L, Murdock PR, et al. ASP1 (BACE2) cleaves the amyloid precursor protein at the β-secretase site. Mol Cell Neurosci. 2000;16:609–19.
Fluhrer R, Capell A, Westmeyer G, Willem M, Hartung B, Condron MM, et al. A non-amyloidogenic function of BACE-2 in the secretory pathway. J Neurochem. 2002;81:1011–20.
Yan R, Han P, Miao H, Greengard P, Xu H. The transmembrane domain of the Alzheimer’s β-secretase (BACE1) determines its late Golgi localization and access to β-amyloid precursor protein (APP) substrate. J Biol Chem. 2001;276:36788–96.
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–41.
Bennett BD, Babu-Khan S, Loeloff R, Louis JC, Curran E, Citron M, et al. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem. 2000;275:20647–51.
Esterházy D, Stützer I, Wang H, Rechsteiner Markus P, Beauchamp J, Döbeli H, et al. Bace2 is a β cell-enriched protease that regulates pancreatic β cell function and mass. Cell Metabol. 2011;14:365–77.
Rochin L, Hurbain I, Serneels L, Fort C, Watt B, Leblanc P, et al. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc Natl Acad Sci USA. 2013;110:10658–63.
Stützer I, Selevsek N, Esterházy D, Schmidt A, Aebersold R, Stoffel M. Systematic proteomic analysis identifies β-site amyloid precursor protein cleaving enzyme 2 and 1 (BACE2 and BACE1) substrates in pancreatic β-cells. J Biol Chem. 2013;288:10536–47.
Evin G, Lessene G, Wilkins S. BACE inhibitors as potential drugs for the treatment of Alzheimer’s disease: focus on bioactivity. Recent Pat CNS Drug Discov. 2011;6:91–106.
Evin G, Kenche VB. BACE inhibitors as potential therapeutics for Alzheimer’s disease. Recent Pat CNS Drug Discov. 2007;2:188–99.
Ghosh AK, Brindisi M, Tang J. Developing β-secretase inhibitors for treatment of Alzheimer’s disease. J Neurochem. 2012;120:71–83.
Park H, Lee S. Determination of the active site protonation state of β-secretase from molecular dynamics simulation and docking experiment: implications for structure-based inhibitor design. J Am Chem Soc. 2003;125:16416–22.
McGaughey GB, Holloway MK. Structure-guided design of β-secretase (BACE-1) inhibitors. Expert Opin Drug Discov. 2007;2:1129–38.
Hussain I, Hawkins J, Harrison D, Hille C, Wayne G, Cutler L, et al. Oral administration of a potent and selective non-peptidic BACE-1 inhibitor decreases β-cleavage of amyloid precursor protein and amyloid-beta production in vivo. J Neurochem. 2007;100:802–9.
Ghosh AK, Kumaragurubaran N, Hong L, Kulkarni SS, Xu X, Chang W, et al. Design, synthesis, and X-ray structure of potent memapsin 2 (β-secretase) inhibitors with isophthalamide derivatives as the P2-P3-ligands. J Med Chem. 2007;17(50):2399–407.
Yu J, Koelsch G, Li A, Turner RT, Bilcer GM, Grove C, et al. In vivo efficacy of BACE-1 inhibitor CTS21166 (ASP1702) in rat CNS compartments. Alzheimers Dement. 2009;5:P430–1.
Chang WP, Huang X, Downs D, Cirrito JR, Koelsch G, Holtzman DM, et al. Beta-secretase inhibitor GRL-8234 rescues age-related cognitive decline in APP transgenic mice. FASEB J. 2011;25:775–84.
Oehlrich D, Prokopcova H, Gijsen HJM. The evolution of amidine-based brain penetrant BACE1 inhibitors. Bioorg Med Chem Lett. 2014;24:2033–45.
May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, et al. Robust central reduction of amyloid-beta in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci. 2011;31:16507–16.
May P, Boggs L, Brier R, Calligaro D, Citron M, Day T, et al. Preclinical characterization of LY2886721: A BACE1 inhibitor in clinical development for early Alzheimer’s disease. Alzheimers Dement. 2012;8:P95.
May PC, Willis BA, Lowe SL, Dean RA, Monk SA, Cocke PJ, et al. The potent BACE1 inhibitor LY2886721 elicits robust central aβ pharmacodynamic responses in mice, dogs, and humans. J Neurosci. 2015;35:1199–210.
Jeppsson F, Eketjall S, Janson J, Karlstrom S, Gustavsson S, Olsson L-L, et al. Discovery of AZD3839, a potent and selective BACE1 inhibitor clinical candidate for the treatment of Alzheimer disease. J Biol Chem. 2012;287:41245–57.
Quartino A, Huledal G, Sparve E, Lüttgen M, Bueters T, Karlsson P, et al. Population pharmacokinetic and pharmacodynamic analysis of plasma Aβ40 and Aβ42 following single oral doses of the BACE1 inhibitor AZD3839 to healthy volunteers. Clin Pharmacol Drug Dev. 2014;3:396–405.
Sparve E, Quartino AL, Lüttgen M, Tunblad K, Gårdlund AT, Fälting J, et al. Prediction and modeling of effects on the QTc interval for clinical safety margin assessment, based on single-ascending-dose study data with AZD3839. J Pharmacol Exp Therapeut. 2014;350:469–78.
Vitae. Positive top-line results achieved from two phase 1 clinical trials of BACE inhibitor BI1181181/VTP-37948 in Alzheimer’s disease. Viate Pharmaceuticals website. http://ir.vitaepharma.com/phoenix.zhtml?c=219654&p=irol-newsArticle&ID=1981037. 23 Oct 2013.
Rueeger H, Lueoend R, Machauer R, Veenstra SJ, Jacobson LH, Staufenbiel M, et al. Discovery of cyclic sulfoxide hydroxyethylamines as potent and selective β-site APP-cleaving enzyme 1 (BACE1) inhibitors: structure based design and in vivo reduction of amyloid β-peptides. Bioorg Med Chem Lett. 2013;23:5300–6.
Neumann U, Rueeger H, Machauer R, Veenstra S, Lueoend R, Tintelnot-Blomley M, et al. A novel BACE inhibitor NB-360 shows a superior pharmacological profile and robust reduction of amyloid-β and neuroinflammation in APP transgenic mice. Mol Neurodegener. 2015;10:44.
Hyde L, Chen X, Stahl L, Sondey M, Scott J, Cumming J, et al. Chronic BACE inhibition dramatically slows the rate of Aβ accumulation and the development of amyloid plaques in young TgCRND8 mice. Alzheimers Dement. 2012;8:P188.
Kennedy M, Scott J, Cantu C, Chen X, Kuvelkar R, Werner B, et al. Preclinical profile of MK-8931, a structurally novel, centrally-active, β-secretase (BACE1) inhibitor for the treatment of Alzheimer’s disease. 12th International Conference AD/PD, Nice, France, March 18–22, 2015. Neurodegen Dis. 2015;15(Suppl. 1):290.
Forman M, Tseng J, Palcza J, Leempoels J, Ramael S, Krishna G, et al. The novel BACE inhibitor MK-8931 dramatically lowers CSF Aβ peptides in healthy subjects: results from a rising single dose study (PL02.004). Neurol. 2012;78:PL02.004.
Forman M, Palcza J, Tseng J, Leempoels J, Ramael S, Han D, et al. The novel BACE inhibitor MK-8931 dramatically lowers cerebrospinal fluid Aβ peptides in healthy subjects following single- and multiple-dose administration. Alzheimers Dementia. 2012;8:P704.
Forman M, Kleijn H-J, Dockendorf M, Palcza J, Tseng J, Canales C, et al. The novel BACE inhibitor MK-8931 dramatically lowers CSF β-amyloid in patients with mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2013;9:P139.
Stone J, Kleijn HJ, Dockendorf M, Ma L, Palcza J, Tseng J, et al. Consistency of BACE inhibitor-mediated brain amyloid production inhibition by MK-8931 in Alzheimer’s disease patients and healthy young adults. Alzheimers Dement. 2013;9:P690–1.
Min K, Forman M, Dockendorf M, Palcza J, Soni P, Ma L, et al. A study to evaluate the pharmacokinetics and pharmacodynamics of single and multiple oral doses of the novel BACE inhibitor MK-8931 in Japanese subjects. Alzheimers Dement. 2012;8:P186.
Haeberlein SB, Cebers G, Höglund K, Salter H, Eketjäll S, Bogstedt A, et al. AZD3293, a potent and selective orally active, brain-permeable BACE1 inhibitor. Alzheimers Dement. 2013;9:P813.
Eketjäll SJJ, Kaspersson K, Bogstedt A, Jeppsson F, Fälting J, Haeberlein SB, Kugler AR, Alexander RC, Cebers G. AZD3293: A novel, orally active BACE1 inhibitor with high potency and permeability and markedly slow off-rate kinetics. J Alzheimers Dis. 2016;40:1109–23.
Höglund K, Salter H, Zetterberg H, Andreason U, Olsson T, Alexander R, et al. Monitoring the soluble amyloid precursor protein alpha (sAPPα) and beta (sAPPβ) fragments in plasma and CSF from healthy individuals treated with BACE inhibitor AZD3293 in a multiple ascending dose study: pharmacokinetic and pharmacodynamic correlate. Alzheimers Dement. 2014;10:P447.
Alexander R, Budd S, Russell M, Kugler A, Cebers G, Ye N, et al. AZD3293 A novel BACE1 inhibitor: safety, tolerability, and effects on plasma and CSF Aβ peptides following single- and multiple-dose administration. Neurobiol Aging. 2014;35(Supplement 1):S2.
Astra Zeneca. A Two-part single and multiple dose study to assess the safety, pharmacokinetics and effects of AZD3293 in healthy Japanese young and elderly volunteers. NIH, Clinical Trialsgov 2013. https://clinicaltrials.gov/ct2/show/results?term=AZD3293&rank=9§=X5430126%29. Accessed 03 March 2016.
Fukushima T, Osada Y, Ishibashi A, Lucas F. Novel BACE1 inhibitor, E2609, lowers Abeta levels in the brain, cerebrospinal fluid and plasma in rats and guinea pigs. Alzheimers Dement. 2012;8:P223–4.
Lai R, Albala B, Kaplow JM, Aluri J, Yen M, Satlin A. First-in-human study of E2609, a novel BACE1 inhibitor, demonstrates prolonged reductions in plasma β-amyloid levels after single dosing. Alzheimers Dement. 2012;8:P96.
Albala B, Kaplow JM, Lai R, Matijevic M, Aluri J, Satlin A. CSF amyloid lowering in human volunteers after 14 days oral administration of the novel BACE1 inhibitor E2609. Alzheimers Dement. 2013;8:S743.
Bernier F, Sato Y, Matijevic M, Desmond H, McGrath S, Burns L, et al. Clinical study of E2609, a novel BACE1 inhibitor, demonstrates target engagement and inhibition of BACE1 activity in CSF. Alzheimers Dement. 2013;9:P886.
Timmers M, Van Broeck B, Slemmon J, et al. Profiling the dynamics of CSF and plasma Aβ reduction with JNJ-54861911, an oral BACE inhibitor. In: 12th international conference on Alzheimer’s and Parkinson’s diseases and related neurological disorders (AD/PD) Nice (France) Mar 2015.
Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho IE, Marjaux E, Craessaerts K, Roebroek AJ, Schwake M, D’Hooge R, Bach P, Kalinke U, Moechars D, Alzheimer C, Reiss K, Saftig P, De Strooper B. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem. 2005;280:30797–806.
Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the β-secretase BACE1. Science. 2006;314:664–6.
Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–5.
Hu X, Hu J, Dai L, Trapp B, Yan R. Axonal and Schwann cell BACE1 is equally required for remyelination of peripheral nerves. J Neurosci. 2015;35:3806–14.
Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci USA. 2008;105:5585–90.
Cheret C, Willem M, Fricker FR, Wende H, Wulf-Goldenberg A, Tahirovic S, et al. Bace1 and Neuregulin-1 cooperate to control formation and maintenance of muscle spindles. EMBO J. 2013;32:2015–28.
Kim D, Carey B, Wang H, Ingano L, Binshtok A, Wertz M, et al. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9:755–64.
Zhou L, Barao S, Laga M, Bockstael K, Borgers M, Gijsen H, et al. The neural cell adhesion molecules L1 and CHL1 are cleaved by BACE1 protease in vivo. J Biol Chem. 2012;287(31):25927–40.
Kuhn P-H, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, et al. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012;31:3157–68.
Cai J, Qi X, Kociok N, Skosyrski S, Emilio A, Ruan Q, et al. β-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol Med. 2012;4:980–91.
Rochin L, Hurbain I, Serneels L, Fort C, Watt B, Leblanc P, et al. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc Natl Acad Sci USA. 2013;110:10658–63.
Willem M, Tahirovic S, Busche MA, Ovsepian SV, Chafai M, Kootar S, et al. eta-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015;526:443–7.
Baranger K, Marchalant Y, Bonnet AE, Crouzin N, Carrete A, Paumier JM, et al. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer’s disease. Cell Mol Life Sci. 2016;73:217–36.
Blennow K. The past and the future of Alzheimer’s disease CSF biomarkers—a journey towards validated biochemical tests covering the whole spectra of molecular events. Front Neurosci. 2015;9:345.
Snyder PJ, Kahle-Wrobleski K, Brannan S, Miller DS, Schindler RJ, DeSanti S, et al. Assessing cognition and function in Alzheimer’s disease clinical trials: do we have the right tools? Alzheimers Dement. 2014;10:853–60.
Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, et al. Aβ; and cognitive change: examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimers Dement. 2014;10:743–51.
Cash DM, Rohrer JD, Ryan NS, Ourselin S, Fox NC. Imaging endpoints for clinical trials in Alzheimer’s disease. Alzheimers Res Ther. 2014;6:87.
Cheng X, He P, Lee T, Yao H, Li R, Shen Y. High activities of BACE1 in brains with mild cognitive impairment. Am J Pathol. 2014;184:141–7.
Kim DY, Gersbacher MT, Inquimbert P, Kovacs DM. Reduced sodium channel Nav1.1 levels in BACE1-null mice. J Biol Chem. 2011;286:8106–16.
Lauritzen I, Pardossi-Piquard R, Bauer C, Brigham E, Abraham J-D, Ranaldi S, et al. The β-secretase-derived C-terminal fragment of βAPP, C99, but not Aβ, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J Neurosci. 2012;32:16243–55.
Kandalepas PC, Sadleir KR, Eimer WA, Zhao J, Nicholson DA, Vassar R. The Alzheimer’s β-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol. 2013;126:329–52.
Castillo-Carranza DL, Gerson JE, Sengupta U, Guerrero-Munoz MJ, Lasagna-Reeves CA, Kayed R. Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J Alzheimers Dis. 2014;40(Suppl 1):S97–111.
Sigurdsson EM. Tau immunotherapy and imaging. Neurodegener Dis. 2014;13:103–6.
Georgievska B, Sandin J, Doherty J, Mörtberg A, Neelissen J, Andersson A, et al. AZD1080, a novel GSK3 inhibitor, rescues synaptic plasticity deficits in rodent brain and exhibits peripheral target engagement in humans. J Neurochem. 2013;125:446–56.
Miguel M, Jesus A. Glycogen synthase kinase-3 (GSK-3) inhibitors for the treatment of Alzheimers disease. Curr Pharm Des. 2010;16:2790–8.
Llorens-Martin M, Jurado J, Hernandez F, Avila J. GSK-3β, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. 2014;7:46.
Ly PTT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, et al. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Invest. 2013;123(1):224–35.
Di Martino RMC, De Simone A, Andrisano V, Bisignano P, Bisi A, Gobbi S, et al. Versatility of the curcumin scaffold: discovery of potent and balanced dual BACE-1 and GSK-3β inhibitors. J Med Chem. 2016;59:531–44.
Prati F, De Simone A, Armirotti A, Summa M, Pizzirani D, Scarpelli R, et al. 3,4-Dihydro-1,3,5-triazin-2(1H)-ones as the first dual BACE-1/GSK-3β fragment hits against Alzheimer’s disease. ACS Chem Neurosci. 2015;6:1665–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Genevieve Evin has no conflicts of interest that are directly relevant to the contents of this publication.
Funding
No sources of funding were used to assist in the preparation of this study.
Rights and permissions
About this article
Cite this article
Evin, G. Future Therapeutics in Alzheimer’s Disease: Development Status of BACE Inhibitors. BioDrugs 30, 173–194 (2016). https://doi.org/10.1007/s40259-016-0168-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-016-0168-3