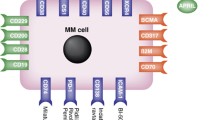
Abstract
The antibody–drug conjugate (ADC) is a combination of a cytotoxic agent and monoclonal antibodies (mAbs) through a stable specialized chemical linker. After ADC binds to the target antigen, the conjugate is internalized and toxin is released, leading to the death of a target cell. Lorvotuzumab mertansine, indatuximab ravtansine, and milatuzumab–doxorubicin are currently under clinical development for use in multiple myeloma (MM). Preliminary data from recent studies indicate that these agents induce responses in patients with relapsed and/or refractory MM and have an acceptable safety profile.
Similar content being viewed by others
References
Rajkumar SV. Treatment of multiple myeloma. Nat Rev Clin Oncol. 2011;8:479–91.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–71.
Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98.
Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–64.
Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–25.
Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM). Leukemia. 2010;24:1934–9.
Anderson KC. The 39th David A. Karnofsky Lecture: bench-to-bedside translation of targeted therapies in multiple myeloma. J Clin Oncol. 2012;30:445–52.
Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31.
Lonial S, Vij R, Harousseau JL, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol. 2012;30:1953–9.
Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: a phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2015;33 Suppl:8508 [abstract].
Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207–19.
Sherbenou DW, Behrens CR, Su Y, Wolf JL, Martin TG 3rd, Liu B. The development of potential antibody-based therapies for myeloma. Blood Rev. 2015;29:81–91.
Lutz RJ, Whiteman KR. Antibody-maytansinoid conjugates for the treatment of myeloma. mAbs. 2009;1:548–51.
Berdeja JG. Lorvotuzumab mertansine: antibody-drug-conjugate for CD56+ multiple myeloma. Front Biosci (Landmark Ed). 2014;19:163–70.
Tassone P, Gozzini A, Goldmacher V, et al. In vitro and in vivo activity of the maytansinoid immunoconjugate huN901-N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine against CD56+ multiple myeloma cells. Cancer Res. 2004;64:4629–36.
Erickson HK, Lambert JM. ADME of antibody-maytansinoid conjugates. AAPS J. 2012;14:799–805.
Ishitsuka K, Jimi S, Goldmacher VS, et al. Targeting CD56 by the maytansinoid immunoconjugate IMGN901 (huN901-DM1): a potential therapeutic modality implication against natural killer/T cell malignancy. Br J Haematol. 2008;141:129–31.
Bataille R, Jego G, Robillard N, et al. The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica. 2006;91:1234–40.
Chanan-Khan A, Wolf J, Garcia J, et al. Efficacy analysis from a phase I study of lorvotuzumab mertansine (IMGN901) used as monotherapy in patients with heavily pre-treated CD56-positive multiple myeloma. Blood (ASH Annual Meeting Abstracts). 2010;116:819;1962 [abstract].
Berdeja JG, Hernandez-Ilizaliturri F,Chanan-Khan A, et al. Phase I study of lorvotuzumab mertansine (LM, IMGN901) in combination with lenalidomide (Len) and dexamethasone (Dex) in patients with CD56-positive relapsed or relapsed/refractory multiple myeloma (MM). Blood (ASH Annual Meeting Abstracts). 2012;120:728 [abstract].
Lopus M, Oroudjev E, Wilson L, et al. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther. 2010;9(10):2689–99.
Issell BF, Crooke ST. Maytansine. Cancer Treat Rev. 1978;5:199–207.
Ikeda H, Hideshima T, Fulciniti M, et al. The monoclonal antibody nBT062 conjugated to cytotoxic maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin Cancer Res. 2009;15:4028–37.
Tassone P, Goldmacher VS, Neri P, et al. Cytotoxic activity of the maytansinoid immunoconjugate B-B4-DM1 against CD138+ multiple myeloma cells. Blood. 2004;104:3688–96.
Jagannath S, Chanan-Khan A, Heffner LT, et al. BT062, an antibody-drug conjugate directed against CD138, shows clinical activity in patients with relapsed or relapsed/refractory multiple myeloma. Blood. 2011;118:305 [abstract].
Heffner LT, Jagannath S, Zimmerman TM, et al. BT062, an antibody-drug conjugate directed against CD138, given weekly for 3 weeks in each 4 week cycle: safety and further evidence of clinical activity. Blood (ASH Annual Meeting Abstracts). 2012;120:4042 [abstract].
Kelly KR, Chanan-Khan A, Somlo G, et al. Indatuximab ravtansine (BT062) in combination with lenalidomide and low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma: clinical activity in len/dex-refractory patients. Blood (ASH Annual Meeting Abstracts). 2013;122:758 [abstract].
Kelly KR, Chanan-Khan A, Heffner LT, et al. Indatuximab ravtansine (BT062) in combination with lenalidomide and low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma: clinical activity in patients already exposed to lenalidomide and bortezomib [abstract]. Blood (ASH Annual Meeting Abstracts). 2014;124(21):4736 [abstract].
Sapra P, Stein R, Pickett JQ, et al. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res. 2005;11:5257–64.
Stein R, Griffiths GL, Cardillo T, et al. Therapeutic activity of a new antibody-drug immunoconjugate, IMMU-110, in preclinical studies targeted against multiple myeloma. J Clin Oncol. 2004;22 Suppl:6535 [abstract].
Stein R, Mattes MJ, Cardillo T, et al. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res. 2007;13:5556s–63s.
Govindan SV, Cardillo TM, Sharkey RM, et al. Milatuzumab-SN-38 conjugates for the treatment of CD74+ cancers. Mol Cancer Ther. 2013;12:968–78.
Kaufman JL, Niesvizky R, Stadtmauer EA, et al. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br J Haematol. 2013;163:478–86.
Davis RS. Fc receptor-like molecules. Annu Rev Immunol. 2007;25:525–60.
Elkins K, Zheng B, Go M, et al. FcRL5 as a target of antibody–drug conjugates for the treatment of multiple myeloma. Mol Cancer Ther. 2012;11:2222–32.
Ise T, Nagata S, Kreitman RJ, Wilson WH, Wayne AS, Stetler-Stevenson M, et al. Elevation of soluble CD307 (IRTA2/FcRH5) protein in the blood and expression on malignant cells of patients with multiple myeloma, chronic lymphocytic leukemia, and mantle cell lymphoma. Leukemia. 2007;21:169–74.
Tai Y-T, Mayes PA, Acharya C, et al. Novel afucosylated anti-B cell maturation antigen-monomethyl auristatin F antibody– drug conjugate (GSK2857916) induces potent and selective anti-multiple myeloma activity. Blood. 2014;123:3128–38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Pawel Robak and Tadeusz Robak have no conflicts of interest that are directly relevant to the content of this study. No writing assistance was utilized in the production of this manuscript.
Funding
The preparation of the manuscript was supported in part by a grant from the Medical University of Lodz (No503/1-093-01/503-11-001 and 503/8-093-01/503-81-001).
Rights and permissions
About this article
Cite this article
Robak, P., Robak, T. Management of Multiple Myeloma with Second-Generation Antibody-Drug Conjugates. BioDrugs 30, 87–93 (2016). https://doi.org/10.1007/s40259-016-0165-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-016-0165-6