Abstract
Background
Economic evaluation of one-time therapies during reimbursement decision-making is challenging due to uncertain long-term outcomes. The availability of 5-year outcome data from the ELIANA trial and real-world evidence of tisagenlecleucel, the first chimeric antigen receptor T-cell (CAR-T) therapy, presents an opportunity to re-evaluate the predictions of prior cost-effectiveness analyses (CEAs).
Objective
To conduct a systematic literature review (SLR) of prior CEAs of tisagenlecleucel for pediatric/young adult relapsed or refractory acute lymphoblastic leukemia (r/r ALL) and evaluate the impact of recently available 5-year efficacy data from ELIANA and advances in CAR-T manufacturing in an updated CEA model.
Methods
OVID MEDLINE/Embase and health technology assessment (HTA) databases were searched for full-text economic evaluations in English reporting cost-effectiveness results for tisagenlecleucel for r/r ALL. Evaluations with publicly reported incremental cost-effectiveness ratios (ICERs) were included in the SLR. Study screening and data abstraction were conducted following PRISMA guidelines. Data extracted included the country/currency, perspective, clinical trial evidence, model structures, long-term efficacy extrapolation approaches (i.e., overall survival [OS]), time horizon, discount rates, and outcomes (i.e., life years [LY], quality-adjusted LY [QALY], and ICERs). The CEA model reported in Wakase et al. was updated using 5-year OS data from ELIANA and the CAR-T infusion rate informed by real-world practice.
Results
Sixteen records corresponding to 15 unique studies were included in the SLR (11 publications and 5 HTA reports); all were conducted from the health care system perspective of the respective countries. Most studies found tisagenlecleucel to be cost effective, but all studies’ projected 3- and 5-year OS rates for tisagenlecleucel were lower than the observed 3- and 5-year rates, respectively, derived from 5-year ELIANA data. When applying updated OS projections from the most recent ELIANA data cut and higher infusion rates of 92.5% (per the real-world infusion rate)—96.0% (per the manufacturer success rate) to the CEA of Wakase et al., the associated QALYs for tisagenlecleucel increased from 11.6 to 14.6–15.0, and LYs increased from 13.3 to 17.0–17.5. Accordingly, the ICERs for tisagenlecleucel decreased from ¥2,035,071 to ¥1,787,988–¥1,789,048 versus blinatumomab and from ¥2,644,702 to ¥2,257,837–¥2,275,181 versus clofarabine combination therapy in the updated CEA model.
Conclusions and Relevance
Projections at launch of the likely cost effectiveness of tisagenlecleucel appear to have underestimated its ultimate economic value given more recent trial and real-world data. To balance uncertainty in initial valuation with the need to provide access to novel oncology therapies, payers can consider flexible reimbursement policies alongside ongoing assessments as new data emerge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In a systematic literature review, most economic assessments of tisagenlecleucel at launch projected it to be cost effective, yet consistently applied conservative long-term efficacy assumptions. |
Application of updated efficacy results to an example cost-effectiveness model revealed that prior economic assessments largely underestimated the value of tisagenlecleucel compared with observed 5-year data from ELIANA that are now available. |
To balance uncertainty in initial valuation with the need to provide access to novel oncology therapies, payers could establish interim coverage policies and value-based contracting, alongside ongoing assessments as new data emerge. |
1 Introduction
The past decade has brought remarkable innovation in cell and gene therapies aiming to provide long-term benefit through a single treatment. However, it is challenging to assess the value of novel, highly-active therapies at regulatory approval for reimbursement decision-making due to limited long-term data and frequent lack or infeasibility of head-to-head trials comparing them with standard of care (SOC) options [1]. If early data indicate promising long-term clinical benefit and curative potential for a disease with high unmet need, it creates urgency to develop solutions for timely access. In addition to accumulation of long-term data, several factors occurring after approval can impact cost effectiveness. The cellular therapy manufacturing and delivery processes are novel and continuously evolving, demonstrated by higher infusion success rates and shorter waiting times in the commercial setting than those observed in early clinical trials [2, 3]. Over time, the medical community’s accumulated experience contributes to the optimal utilization of these therapies, often among a population broader than that in clinical trials, and improved best practices for managing potential adverse events (AEs). Often, new and expanded indications pose additional challenges for cell and gene therapy reimbursement decisions.
These features are evidenced in emerging data from tisagenlecleucel, the first chimeric antigen receptor T cell (CAR-T) therapy made available to patients when it was approved in the USA for B-cell precursor acute lymphoblastic leukemia (ALL) in children and young adults (aged up to 25 years) who were in their second or later relapse [4, 5]. B-cell ALL is one of the most common malignancies diagnosed in children and approximately 15% of pediatric and young adults with ALL ultimately relapse or experience refractory (r/r) disease after first-line treatment, which is associated with increased morbidity and mortality [6,7,8]. The approval of tisagenlecleucel was based on the pivotal single-arm, Phase II ELIANA trial, wherein 81% of infused patients achieved remission, accompanied by meaningful quality-of-life gains [9]. In contrast to prior SOC therapies for r/r ALL such as salvage chemotherapy (SC), tisagenlecleucel targets the tumor with high precision and can therefore be effective for aggressive disease where other therapies have failed [10].
Since the first approval of tisagenlecleucel in 2017, the success rate of manufacturing patients’ autologous CAR-T cells has improved, waiting times for infusion have decreased, and updates to the management of cytokine release syndrome and neurotoxicity have resulted in better tolerance and lower toxicity [11,12,13]. As it has been more than 5 years since the initial approval of tisagenlecleucel, we now have access to its related real-world and long-term trial data. Thus, there is an opportunity to evaluate how a one-time therapy, such as tisagenlecleucel, was initially evaluated by health technology assessment (HTA) agencies and in cost-effectiveness analyses (CEAs), to understand whether their assumptions were realistic and to determine whether modifications to existing approaches for assessing the value of one-time therapies are warranted.
Accordingly, we aimed to evaluate the impact of long-term data from ELIANA and evidence accumulated from real-world clinical practice on the economic value of tisagenlecleucel. First, we conducted a systematic literature review (SLR) of CEAs of tisagenlecleucel for ALL and summarized the clinical trial evidence used, long-term efficacy extrapolation approaches, and conclusions. Next, we conducted analyses incorporating long-term, 5-year efficacy data from ELIANA (data cutoff: November 17, 2022; median follow-up: 2.2 years; data on file, Novartis), as well as real-world data on improvements in CAR-T infusion rate, to understand their impact on the outcomes of a recent CEA of tisagenlecleucel for r/r ALL [14].
2 Methods
2.1 Systematic Literature Review
The methods for performing the SLR followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig. 1; refer to Supplemental Methods for the search strategy). A systematic literature search for full-text publications and reports describing economic evaluations of tisagenlecleucel for r/r ALL was conducted in OVID MEDLINE/Embase (March 16, 2023) and HTA databases where English reports were available (March 28, 2023) (a list of HTAs searched is in the Supplemental Methods). Records were excluded if not in English or did not report cost-effectiveness results of tisagenlecleucel in r/r ALL.
PRISMA flow diagram of studies and reports included in the systematic literature review (SLR). HTA health technology assessment, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SLR systematic literature review. aThe searches in OVID MEDLINE/Embase and the HTA databases were conducted on March 16, 2023 and March 28, 2023, respectively. bRecords were excluded if results were not separately reported for pediatric patients with relapsed/refractory acute lymphoblastic leukemia. cRecords were excluded if they were not full-text articles or HTA reports (e.g., note, letter, comment, case report, editorial, protocol, review, meta-analysis, or conference abstract). dRecords were excluded if results were not separately reported for the cost-effectiveness analysis. eRecords were excluded if the incremental cost-effectiveness ratio of tisagenlecleucel was not reported or was not publicly available
The following data elements were extracted: country, currency, perspectives; patient population; comparators; sources of clinical evidence; model structures; methods of long-term extrapolation of efficacy data; time horizon and discount rates; and outcomes including costs, life years (LYs), quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). The estimated overall survival (OS) rates with tisagenlecleucel in the first 5 years were extracted when available, which were compared with those observed using 5-year ELIANA data.
To assess the results across multiple countries, the ICERs of tisagenlecleucel versus comparators in the base-case and sensitivity/scenario analyses were compared against the respective willingness-to-pay (WTP) thresholds in each country.
2.2 Assessing the Economic Value of Tisagenlecleucel Based on Long-term Data and Improved Infusion Rate
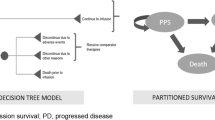
To assess the impact of long-term data, the CEA model reported in Wakase et al. [14] was updated using 5-year ELIANA data and the infusion rate informed by real-world practice. Wakase et al. was selected because the model was accessible to the authors for update. It was a partitioned survival model (PSM) evaluating the cost effectiveness of tisagenlecleucel in comparison with blinatumomab and clofarabine combination therapy for the treatment of r/r ALL from the perspective of a Japanese public health care payer. A leading decision-tree was applied to the tisagenlecleucel arm, which was separately followed by patients who received tisagenlecleucel infusion versus those who did not receive infusion for various reasons. Among those who received infusion, the efficacy, cost, and disutility inputs were based on the tisagenlecleucel-infused population. Patients who did not receive tisagenlecleucel infusion were assumed to have received blinatumomab, and the associated efficacy and costs were assumed to be the same as those directly assigned to blinatumomab.
All patients were distributed across the following partitioned health states: event-free survival (EFS), progressive disease, and death. The utilities were assumed to depend on health states only and to be independent of treatment. For patients who received tisagenlecleucel infusion, the observed OS and EFS data during the trial period were used to model OS and EFS until Year 5. For comparators, the observed OS during the trial period reported in respective trial publications was used, and then after the end of trial observation period the hazard ratios (HRs) versus tisagenlecleucel derived from the matching-adjusted indirect comparison (MAIC) analyses were used to project OS up to Year 5. EFS data were not publicly available and were thus estimated based on OS data, assuming a constant cumulative HR between OS and EFS. At the end of Year 5, living patients were assumed to be long-term survivors who would experience no additional relapses and whose risk of death was based on standard mortality ratio (SMR)-adjusted general population mortality. The model also assumed that the clinical benefits of subsequent hematopoietic stem cell transplant (HSCT) were captured in the EFS and OS estimations for all treatments as a result of using the direct trial data, whereas the costs and disutilities of the procedure were added separately. The base-case analysis was performed over a lifetime horizon with a monthly model cycle, with costs and effectiveness discounted 2% annually following the recommendations from the Japan HTA agency Center for Outcomes Research and Economic Evaluation for Health (C2H) [15].
The OS and EFS data originally used by Wakase et al. [14] for tisagenlecleucel were based on pooled trial data from an earlier data cut with a shorter follow-up time of ELIANA (ClinicalTrials.gov Identifier: NCT02435849), the Phase II ENSIGN trial (NCT02228096), and the Phase I/II B2101J study (NCT01626495). All three trials were single-arm studies without randomization and the enrolled patients had similar characteristics. These three trials enrolled pediatric or young adult patients (aged up to 25 years) with ALL who were primary refractory, chemo-refractory, relapsed after HSCT, chemotherapy resistant, or were otherwise ineligible for HSCT; 44–63% of patients had received prior HSCT and the number of prior regimens ranged from 1 to 9. In the updated model, the observed OS and EFS data were replaced with the 5-year ELIANA data. The longer follow-up data from ELIANA were immature for assessment of the cure assumption, thus the updated model kept the original assumption that patients alive after 5 years were long-term survivors, which was supported by literature [16,17,18]. The proportion of patients receiving tisagenlecleucel infusion in the model by Wakase et al. was updated from the trial-based to the real-world infusion rates of 92.5% and 96.0% (using the manufacturer success rate as the upper limit) [2], reflecting improvements in manufacturing and CAR-T delivery. All other CEA parameters remained the same as those in the original model. LYs, QALYs, and ICERs in the base-case analysis were estimated from the updated model constructed in Excel (Microsoft, Redmond, WA, USA). In addition, deterministic sensitivity analyses (DSAs) and probabilistic sensitivity analyses (PSAs) were performed again to reflect the updated model.
3 Results
3.1 SLR of CEAs of Tisagenlecleucel for r/r ALL
3.1.1 Search Results
A total of 219 publications and 18 HTA reports were identified and screened for eligibility (Fig. 1). Sixteen records corresponding to 15 unique studies were included in the SLR, comprising 11 publications [14, 19,20,21,22,23,24,25,26,27,28] and 5 HTA reports [29,30,31,32,33]. Whittington et al. [28] reflected the same results as a 2018 report from the Institute for Clinical and Economic Review [30] and were consolidated (henceforth referred to as the ICER report). The other HTA reports included a 2021 report from the C2H in Japan [33], a 2019 report from the Canadian Agency for Drugs and Technologies in Health (CADTH) [29], a 2018 report from the National Institute for Health and Care Excellence (NICE) in the UK [31], and a 2019 report from Scottish Medicines Consortium (SMC) [32]. The 2019 report from the Medical Services Advisory Committee (MSAC) in Australia [34] was excluded because ICER results were not publicly available.
3.1.2 Overview of the Included Studies
Three studies were conducted in the USA (Sakar et al. [26], Lin et al. [27], and ICER [28, 30]); two in the UK (NICE [31] and SMC [32]); two in Canada (Furzer et al. [25] and CADTH [29]); two in Japan (Wakase et al. [14] and C2H [33]); and one each in Australia (Gye et al. [19]), Ireland (Carey et al. [20]), Singapore (Wang et al. [21]), Switzerland (Moradi-Lakeh et al. [22]), the Netherlands (Thielen et al. [23]), and Spain (Ribera-Santasusana et al. [24]) (Table S1). Eleven studies considered pediatric and young adult patients up to age 23–25 years, and 4 studies only considered pediatric patients with r/r ALL.
All evaluations were conducted from the health care system perspectives of the respective countries, either nationalized or private. The reports from the Netherlands and USA (Sarkar et al.) also included the societal perspective. As comparators with tisagenlecleucel, 11 studies included blinatumomab, 7 included SC, 7 included clofarabine (as combination therapy [Clo-C], monotherapy [Clo-M], or both), and 1 included inotuzumab ozogamicin. Salvage chemotherapy was typically based on fludarabine, cytarabine, and idarubicin (FLA-IDA). Additionally, the comparator in two evaluations was the regional SOC, defined in Furzer et al. as any treatment a tisagenlecleucel-eligible patient received at their physician’s discretion, including chemotherapy with follow-up HSCT, and in Sarkar et al. as Clo-C followed by HSCT.
The model structure in 8 studies was a PSM preceded by a decision tree. The PSM part reflects the health states of EFS or PFS, progressed/relapsed disease, and death. Lin et al. used a Markov structure and the ICER report used a semi-Markov structure preceded by a decision tree. Thielen et al., Ribera-Santasusana et al., and the CADTH report used a PSM without a decision tree, while Furzer et al. and Sarkar et al. used a microsimulation approach.
3.1.3 Efficacy Sources for Tisagenlecleucel and Long-term OS Extrapolation Approaches
The majority of studies (12) used data from ELIANA, ENSIGN, and the B2101J study (Table 1). Two evaluations used data from ELIANA and ENSIGN (Gye et al. and Carey et al.), and one (Sarkar et al.) used ELIANA data only. In addition, in the CADTH report, the company submission used pooled data from all 3 sources, but the re-analysis by CADTH used only ELIANA data. Nine evaluations used efficacy inputs per the tisagenlecleucel-infused populations in clinical trials, 3 used efficacy inputs from the intent-to-treat (ITT) population, and 3 did not report this information.
Among the 15 studies, before applying the long-term survival extrapolation, 7 explored standard parametric models, spline models, and mixture cures, whereas 3 only explored standard parametric models and 5 only used observed data. Typically, statistical fit, visual fit, and/or clinical assessment were used to select the best fitting model. All studies used a lifetime horizon (i.e., 70–88 years). In 13 studies, SMRs were applied to extrapolate long-term survival after 3–5 years. The majority of studies used a SMR of 9.1 sourced from a Canadian cohort study in childhood cancer patients who had survived at least 5 years [17], whereas 3 studies used SMRs of 15.5, 15.2 and 20.8, respectively, obtained from studies investigating ALL survivors specifically [17, 35, 36].
3.1.4 Overall Survival Estimates for Tisagenlecleucel in the Identified CEAs Compared with 5-year ELIANA Data
The OS rates for tisagenlecleucel in the first 5 years, as predicted by the included evaluations and as observed in ELIANA with 5-year data, are summarized in Table 2. Overall, the 3- and 5-year OS rates were available and extracted from 8 of the 15 studies. Of these 8 studies, 3 reported the OS rates among the ITT population and 5 reported the OS rates among infused patients only.
In the 5-year ELIANA data, over 30% of patients were still under follow-up at Year 5. The number of patients at risk of death among the ITT population (n = 98) was 66, 43, and 33 at Years 1, 3, and 5, respectively; among infused patients (n = 80), the numbers were 61, 43, and 25, respectively. The survival rate of infused patients in the 5-year ELIANA data was higher than that of infused patients in the previous data cut used in the CEA by Wakase et al. (3-year OS: 63.8% vs 62.8%; EFS: 49.2% vs 47.6%).
Across all 8 studies with available information, the 3- and 5-year OS rates among infused patients (ranging from 18.8 to 63.4% at Year 3 and from 7.2 to 44.2% at Year 5) were lower than those observed in the ELIANA 5-year data, which were 63.8% and 56.0%, respectively. After applying the real-world infusion rate of 92.5–96.0% to the infused efficacy observed in the ELIANA 5-year data, the OS rates estimated among all intended for tisagenlecleucel infusion were 59.0–61.3% at Year 3 and 51.8–53.8% at Year 5, higher than those predicted by the included CEAs.
3.1.5 Results of the Economic Evaluations
The results of the included studies’ CEA analyses are summarized in Table 3. Among the included CEA studies, the efficacy inputs of comparators were generally sourced from the respective clinical trials and the cost components accounted for comparators were similar, including drug acquisition and administration costs, hospitalization costs during treatment, AE costs, disease management costs, and subsequent treatment costs. However, the specific considerations varied across studies. The incremental QALYs for tisagenlecleucel in the base-case analyses ranged widely from − 0.61 in Lin et al. to 10.77 in Thielen et al. In Lin et al., the assumed 5-year relapse-free survival (RFS) was overly conservative at 0%; in Thielen et al., all patients were assumed to receive tisagenlecleucel infusion. The company submission to C2H included incremental QALYs of 8.56–9.55 but was revised in the re-analyses by C2H to 6.64–8.57. The company submission to CADTH included incremental QALYs of 8.61–11.74 but was revised in the re-analyses by CADTH to 7.94–10.60.
Among the US studies, the base-case ICERs per QALY gained ranged from USD $45,871 in the ICER report to $61,000–$184,000 in Lin et al. (Table 3). The company submission to C2H estimated generally lower ICERs per QALY gained (¥1,994,592–¥2,087,581) than the re-analysis by C2H (¥2,184,285–¥2,747,550) [33]. This was potentially due to more conservative assumptions for long-term extrapolation of survival in the C2H re-analysis. The CADTH report estimated generally lower ICERs per QALY gained (CAD $51,295–$54,393) than Furzer et al. (CAD $71,000–$281,000) due to more conservative assumptions for the QALYs for tisagenlecleucel in the latter. The two UK CEAs arrived at similar ICERs per QALY gained, ranging from £18,392–£25,404 in the NICE report and £25,238 in the SMC report.
Overall, 13 of the 15 studies found tisagenlecleucel to be cost effective for r/r ALL under their country-specific WTP thresholds in the base case (Fig. 2). Among these 13 studies, Lin et al. and Furzer et al. each included three scenarios assuming different efficacy for tisagenlecleucel: 0%, 20%, and 40% RFS rates in Lin et al. and low cure rates of 10%, 20%, and 40% in Furzer et al. Tisagenlecleucel was projected to be cost effective under the most reasonable, although still conservative, scenarios per its true efficacy (i.e., a 40% RFS rate in Lin et al. and 40% cure rate in Furzer et al.). The two studies that did not find tisagenlecleucel cost effective, Gye et al. and Carey et al., both reported fewer discounted QALYs for tisagenlecleucel than other studies (i.e., 5.36 and 4.33, respectively, vs 8.29–16.76 in other studies when reasonable scenarios were used in Lin et al. and Furzer et al.). This may be due to the higher benefit discount rates used in both studies (5.0% in Gye et al. and 4.0% in Carey et al. vs 1.5–3.5% in others) and a higher SMR in Carey et al. (15.5 vs 9.1 applied in most studies). In addition, Gye et al. and Carey et al. used WTP thresholds of AUD $50,000/USD $33,454 and €45,000/USD $49,352, respectively, which were lower than thresholds for other countries ranging from USD $52,725–$150,000, except Spain (USD $32,901). The WTP thresholds that should be used are subject to debate and historical influence. If twice the per-capita country-specific gross domestic product were instead used as the threshold, all studies found tisagenlecleucel to be cost effective (eFig. 1).
Cost effectiveness of tisagenlecleucel for r/r ALL compared with other therapies, based on WTP threshold of the country. The dots represent the comparison of the base-case incremental cost-effectiveness ratio against the respective WTP threshold while the ranges represent the comparison of incremental cost-effectiveness ratios from sensitivity/scenario analyses against the WTP threshold. The red dashed line represents the WTP threshold where incremental cost-effectiveness ratios on the left of this line reflect that tisagenlecleucel was found to be cost-effective at the study country’s WTP for a comparator. C2H Center for Outcomes Research and Economic Evaluation for Health, CADTH Canadian Agency for Drugs and Technologies in Health, Com comparator, ICER Institute for Clinical and Economic Review, NICE National Institute of Health and Care Excellence, RFS relapse-free survival, r/r ALL relapsed/refractory acute lymphoblastic leukemia, SMC Scottish Medicines Consortium, WTP willingness to pay. a In Furzer et al. 2020, a cure state was included to account for the limited long-term survival information currently available for tisagenlecleucel. The base-case estimates used a range of cure rates from 10 to 40% for those offered treatment based on expert opinion. b In Lin et al. 2018, three scenarios that cover a broad range of plausible long-term outcomes on the basis of observed variance and expert opinion were evaluated: 0%, 20% and 40% 5-year RFS rates without hematopoietic stem-cell transplantation and tisagenlecleucel as a bridge to transplantation under a 0% transplantation-free 5-year RFS scenario. c In Lin et al. 2018, tisagenlecleucel was dominated by blinatumomab in the base-case analysis and all scenario analyses for the scenario applying 0% as the 5-year RFS rate. d In Moradi-Lakeh et al. 2021 and Wakase et al. 2022 the lowest sensitivity/scenario analysis result was tisagenlecleucel being dominant over comparators. e In CADTH 2019, only the results of price-reduction scenario analyses were reported. f In SMC 2019, only the results of selected sensitivity analyses were reported. The lowest sensitivity/scenario analysis result was not available
3.2 Economic Value of Tisagenlecleucel Based on Long-term Data and Improved Infusion Rate
We updated the model used in Wakase et al. with the 5-year OS and EFS data observed among tisagenlecleucel-infused patients in ELIANA, which yielded improvements in the modeled OS versus that derived from the pooled tisagenlecleucel data of the prior data cut (Fig. 3). When applying the most recent OS projections and an infusion rate of 92.5–96% to the CEA of Wakase et al., the associated QALYs for tisagenlecleucel increased by 3.0–3.4 years and LYs increased by 3.7–4.2 years. The ICERs of tisagenlecleucel decreased from ¥2,035,071 to ¥1,787,988−¥1,789,048 versus blinatumomab and from ¥2,644,702 to ¥2,257,837−¥2,275,181 versus clofarabine combination therapy (table inset, Fig. 3). Even if the infusion rate remained unchanged (i.e., 84% based on pooled ELIANA, ENSIGN, and B2101J data), the QALYs and LYs for tisagenlecleucel still increased by 1.9 and 2.5, respectively.
The DSA results of the updated model, reflecting updated efficacy and current infusion rate (eFig. 2 [infusion rate of 96%] and eFig. 3 [infusion rate of 92.5%]), suggested that the ICERs of tisagenlecleucel versus both comparators were reduced compared to the original Wakase et al. model. Similar to the original model, when productivity gain was considered, tisagenlecleucel dominated both comparators. The largest ICER across all DSAs decreased from ¥2,885,485 to ¥2,549,724- ¥2,551,493 versus blinatumomab and decreased from ¥3,756,251 to ¥3,223,428- ¥3,248,597 versus clofarabine combination therapy. Across all other DSAs, the ICER were reduced and the degree of reduction ranged from ¥142,113 to ¥594,428 versus blinatumomab and from ¥242,491 to ¥971,266 versus clofarabine combination therapy. At a threshold of ¥7,500,000 per QALY gained [37, 38], the probability of tisagenlecleucel being cost effective remained 100% compared to both comparators in the PSA analysis of the updated model, considering either rate of infusion (eFig. 4 [infusion rate of 96%] and eFig. 5 [infusion rate of 92.5%]).
4 Discussion
In the SLR of prior economic analyses of tisagenlecleucel for r/r ALL, there were large variations in the results that were partially attributable to the differences in the extrapolation approach used to project long-term efficacy. All studies used some type of extrapolation mechanism based on trial data, particularly ELIANA, but some incorporated a cure assumption for patients in remission at 5 years while others did not. Additionally, some assumptions explored were overly conservative relative to the observed long-term data, such as low cure rates or RFS. This heterogeneity is reflected in the wide ranges of LYs (7.13–20.60) and QALYs (2.96–16.76) of tisagenlecleucel reported by the studies, which can greatly impact the value assessment. The variations in the models’ efficacy inputs largely stemmed from the lack of long-term efficacy data for tisagenlecleucel and differing willingness to accept uncertainties for new CAR-T therapies.
Although the majority of CEAs found that tisagenlecleucel was cost effective under their country’s WTP, its value was likely still underestimated in many studies. Specifically, both the LYs and QALYs for tisagenlecleucel substantially increased when applying efficacy data from the 5-year ELIANA data cut to the model of Wakase et al., whether or not improvements in the CAR-T infusion rates were considered. Notably, the 5-year ELIANA data revealed that the best extrapolation approach to predict the observed OS of tisagenlecleucel was to fit all standard parametric survival models and spline models and then calculate the average weighted by Akaike information criterion of each model. This approach incorporates spline models, which can provide more flexibility in curve fitting compared to standard parametric survival models and may better reflect long-term extrapolation.
Real-world evidence has consistently confirmed the efficacy of tisagenlecleucel in r/r ALL as observed in ELIANA. For example, a 2022 real-world study reported that the 1-year OS of 185 patients with r/r B-ALL infused with commercial tisagenlecleucel was 72% [39]. The Center for International Blood and Marrow Transplant Research (CIBMTR) registry of patients with r/r B-ALL reported a 3-year OS rate of 58.7% among 578 patients infused with commercial tisagenlecleucel, but the safety outcomes were more favorable than in ELIANA [40]. This finding is notable given that the real-world CIBMTR population is broader and more diverse than that of ELIANA, which excluded patients under the age of 3 years or with < 5% bone marrow blasts.
In a recent (2023) public summary by MSAC for the review of tisagenlecleucel in r/r ALL [41], released after the conduct of the present SLR, MSAC considered that the economic model had overestimated the benefits of tisagenlecleucel and underestimated the value of blinatumomab. The statement was partly due to the fact that data used for tisagenlecleucel and blinatumomab in the model submitted to MSAC were directly derived from the respective clinical trials without accounting for cross-trial differences in patient populations. Conversely, MAIC of tisagenlecleucel versus comparators was used in the Wakase CEA and our updated analysis. In addition, the statement was in the context of the comparison between tisagenlecleucel and blinatumomab. However, based on the OS rate in infused patients, the 30-month OS rate per 5-year ELIANA data (65.3%; November 2022 data cutoff) was higher than that reported in the MSAC document based on prior data cuts of ELIANA and ENSIGN data (55.11% and 59.64%, respectively, from ELIANA December 2017 and ENSIGN September 2022 data cutoffs); this is consistent with our findings in the present study that the value of tisagenlecleucel was likely underestimated when using the prior data cut. MSAC also considered that some of the benefit of tisagenlecleucel could be influenced by the use of subsequent treatments, particularly HSCT. However, subsequent HSCT was considered as part of the treatment strategy in clinical practice for some patients receiving tisagenlecleucel and was allowed in ELIANA. Therefore, assessments for tisagenlecleucel should reflect both the benefit as well as costs and disutilities caused by subsequent HSCT, which was the case in the Wakase et al. CEA, our updated analysis, and the MSAC submission. The real-world evidence from the CIBMTR registry suggests that tisagenlecleucel is also effective without subsequent HSCT [42]. The use of subsequent HSCT after tisagenlecleucel in current clinical practice is driven partially by efficacy and partially by existing practice habit and belief, which may change in the future.
These results demonstrate the importance of long-term data and the challenge of projecting outcomes with limited observed data for novel therapies like CAR-T, where both manufacturing and real-world clinical practices will evolve. Further, our findings underscore the need to evaluate the appropriate timing to derive value-based pricing for novel therapies, as the results from CEAs based on early trial data could be uncertain.
Nevertheless, coverage and reimbursement decisions for one-time, potentially curative therapy require balancing the needs of diverse stakeholders. These include patients’ urgent need for a potentially life-saving therapy, payers’ needs for robust evidence to quantify the treatment’s value, and the manufacturer’s need to preserve the value and sustainable innovation of novel medical technologies. Irrespective of the selected reimbursement approaches, it is important for policy makers to promptly establish interim reimbursement policies to ensure that patients have timely access to new treatments while allowing long-term data and real-world evidence on both efficacy and safety to be continuously collected. Periodic re-assessment could help ensure that the scarce resources are used wisely to cover treatments that provide value to society. As new data emerge, reimbursement decisions can be revised accordingly to better reflect the demonstrated value of treatments. Additionally, reassessments could encourage more detailed safety and efficacy monitoring post-approval, which could be of great benefit to patients and to the development of future therapies. By experimenting with flexible approaches such as outcome-based contracts, we can mitigate uncertainties when long-term data are not available while shortening the gap between the time of regulatory approval and patients’ receipt of treatments.
The results of this study are subject to several limitations. First, the impact of long-term efficacy data and the real-world infusion rate of tisagenlecleucel was only assessed using the model by Wakase et al., as that was the only model available to the authors. Similar or potentially larger impacts could be expected on other models, as the OS estimates in Wakase et al. were closer to the values observed in the long-term ELIANA data whereas other studies tended to be more conservative. Second, the current studies found that prior CEAs made conservative assumptions for tisagenlecleucel, although this does not imply that conservative assumptions were used in CEAs of other novel therapies. Third, this study utilized conventional cost-effectiveness thresholds when a case can be made for higher thresholds for rare, health-catastrophic conditions such as r/r ALL [43].
5 Conclusions
Using tisagenlecleucel as a case study, we discovered that most prior CEAs included conservative assumptions regarding its long-term efficacy yet found it cost effective for r/r ALL. The application of long-term clinical and real-world data of tisagenlecleucel’s efficacy and improved infusion rate increased the projected LYs and QALYs gained, further supporting that it is a cost-effective option for r/r ALL. Considering the unique nature of one-time, potentially curative therapies like CAR-T and the insights gained from this case study, it is important for payers to promptly establish flexible, value-based reimbursement policies to balance the need for timely access to novel treatments with uncertainty in initial valuations.
References
Frieden TR. Evidence for health decision making - beyond randomized, controlled trials. N Engl J Med. 2017;377(5):465–75.
Rodrigues M, Duran E, Eschgfaeller B, Kuzan D, Habucky K. Optimizing commercial manufacturing of tisagenlecleucel for patients in the US: a 4-year experiential journey. Blood. 2021;138(Supplement 1):1768.
Rodrigues M, Kuzan D, Yallouridis A, Saffar J-M, Eschgfaeller B. Commercial manufacturing experience of tisagenlecleucel in Europe: > 3 years journey. 48th annual meeting of the European Society for Blood and Marrow Transplantation (EBMT); March 19-23, 2022; Prague, Czech Republic.
Mullard A. FDA approves first CAR T therapy. Nat Rev Drug Discov. 2017;16(10):669.
United States Food and Drug Administration. Highlights of prescribing information: KYMRIAH (tisagenlecleucel) 2017. https://www.fda.gov/media/107296/download. Accessed 1 June 2023.
Jeha S, Gaynon PS, Razzouk BI, Franklin J, Kadota R, Shen V, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24(12):1917–23.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551–65.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48.
Miliotou AN, Papadopoulou LC. CAR T-cell therapy: a new era in cancer immunotherapy. Curr Pharm Biotechnol. 2018;19(1):5–18.
Shimabukuro-Vornhagen A, Böll B, Schellongowski P, Valade S, Metaxa V, Azoulay E, et al. Critical care management of chimeric antigen receptor T-cell therapy recipients. CA Cancer J Clin. 2022;72(1):78–93.
Shi X, Wu H. Recent advances in the prevention and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Eur J Inflamm. 2022. https://doi.org/10.1177/1721727X221078727.
Ghassemi S, Durgin JS, Nunez-Cruz S, Patel J, Leferovich J, Pinzone M, et al. Rapid manufacturing of non-activated potent CAR T cells. Nat Biomed Engin. 2022;6(2):118–28.
Wakase S, Teshima T, Zhang J, Ma Q, Watanabe Y, Yang H, et al. Cost-effectiveness analysis of tisagenlecleucel for the treatment of pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia in Japan. Transplant Cell Ther. 2021;27(3):241 (e1-e11).
Japan Center for Outcomes Research and Economic Evaluation for Health- National Institute of Public Health (C2H). Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council, v3.0. January 19, 2022. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. Accessed 8 Nov 2023.
Centre for Health Technology Evaluation- National Institute for Health and Care Excellence (NICE). Exploring the assessment and appraisal of regenerative medicines and cell therapy products 2016. https://www.nice.org.uk/media/default/about/what-we-do/science%20policy%20and%20research/regenerative-medicine-study-march-2016.pdf. Accessed 19 Feb 2024.
MacArthur AC, Spinelli JJ, Rogers PC, Goddard KJ, Abanto ZU, McBride ML. Mortality among 5-year survivors of cancer diagnosed during childhood or adolescence in British Columbia, Canada. Pediatr Blood Cancer. 2007;48(4):460–7.
Mody R, Li S, Dover DC, Sallan S, Leisenring W, Oeffinger KC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008;111(12):5515–23.
Gye A, Goodall S, De Abreu LR. Cost-effectiveness analysis of tisagenlecleucel versus blinatumomab in children and young adults with acute lymphoblastic leukemia: partitioned survival model to assess the impact of an outcome-based payment arrangement. Pharmacoeconomics. 2023;41(2):175–86.
Carey N, Leahy J, Trela-Larsen L, McCullagh L, Barry M. Tisagenlecleucel for relapsed/refractory acute lymphoblastic leukemia in the Irish healthcare setting: cost-effectiveness and value of information analysis. Int J Technol Assess Health Care. 2022;38(1): e56.
Wang XJ, Wang YH, Ong MJC, Gkitzia C, Soh SY, Hwang WYK. Cost-effectiveness and budget impact analyses of tisagenlecleucel in pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia from the Singapore healthcare system perspective. Clinicoecon Outcomes Res. 2022;14:333–55.
Moradi-Lakeh M, Yaghoubi M, Seitz P, Javanbakht M, Brock E. Cost-effectiveness of tisagenlecleucel in paediatric acute lymphoblastic leukaemia (pALL) and adult diffuse large B-Cell lymphoma (DLBCL) in Switzerland. Adv Ther. 2021;38(6):3427–43.
Thielen FW, van Dongen-Leunis A, Arons AMM, Ladestein JR, Hoogerbrugge PM, Uyl-de Groot CA. Cost-effectiveness of anti-CD19 chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. A societal view. Eur J Haematol. 2020;105(2):203–15.
Ribera Santasusana JM, de Andres Saldana A, Garcia-Munoz N, Gostkorzewicz J, Martinez Llinas D, Diaz de Heredia C. Cost-effectiveness analysis of tisagenlecleucel in the treatment of relapsed or refractory B-cell acute lymphoblastic leukaemia in children and young adults in Spain. Clinicoecon Outcomes Res. 2020;12:253–64.
Furzer J, Gupta S, Nathan PC, Schechter T, Pole JD, Krueger J, et al. Cost-effectiveness of tisagenlecleucel vs standard care in high-risk relapsed pediatric acute lymphoblastic leukemia in Canada. JAMA Oncol. 2020;6(3):393–401.
Sarkar RR, Gloude NJ, Schiff D, Murphy JD. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111(7):719–26.
Lin JK, Lerman BJ, Barnes JI, Boursiquot BC, Tan YJ, Robinson AQL, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36(32):3192–202.
Whittington MD, McQueen RB, Ollendorf DA, Kumar VM, Chapman RH, Tice JA, et al. Long-term survival and value of chimeric antigen receptor T-cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr. 2018;172(12):1161–8.
Canadian Agency for Drugs and Technologies in Health (CADTH). Tisagenlecleucel for acute lymphoblastic leukemia and diffuse large B-cell lymphoma: Economic review report. CADTH Optimal Use Report, vol. 8. no. 3e. 2019. https://www.cadth.ca/sites/default/files/pdf/car-t/op0538-tisagenlecleucel-economic-report-DLBCL-jan2019.pdf. Accessed 21 May 2023.
Institute for Clinical and Economic Review (ICER). Chimeric antigen receptor T-cell therapy for B- cell cancers: Effectiveness and value. Final evidence report. 2018. https://icer.org/wp-content/uploads/2020/10/ICER_CAR_T_Final_Evidence_Report_032318.pdf. Accessed 21 May 2023.
National Institute for Health and Care Excellence (NICE). Tisagenlecleucel for treating relapsed or refractory B-cell acute lymphoblastic leukaemia in people aged up to 25 years. Technology appraisal guidance. 2018. https://www.nice.org.uk/guidance/ta554/resources/tisagenlecleucel-for-treating-relapsed-or-refractory-bcell-acute-lymphoblastic-leukaemia-in-people-aged-up-to-25-years-pdf-82607021872837. Accessed 21 May 2023.
Scottish Medicines Consortium (SMC). Tisagenlecleucel 1.2 x 106 – 6 x 108 cells dispersion for infusion (Kymriah®). Novartis Pharmaceuticals UK Ltd. 2019. https://www.scottishmedicines.org.uk/media/4228/tisagenlecleucel-kymriah-final-feb-2019-amended-7319-for-website.pdf. Accessed 21 May 2023.
Center for Outcomes Research and Economic Evaluation for Health. C2H Evaluation Report: C2H1902. Tisagenlecleucel /B-ALL (Kymriah). https://c2h.niph.go.jp/results/C2H1902/C2H1902_Report_BALL_Eng.pdf. Accessed 19 Feb 2024.
Medical Services Advisory Committee. 1519 - Tisagenlecleucel (CTL019) for treatment of refractory CD19-positive leukaemia and lymphoma. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1519-public. Accessed 19 Feb 2024.
Fidler MM, Reulen RC, Winter DL, Kelly J, Jenkinson HC, Skinner R, et al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ. 2016;354: i4351.
Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–42.
Hasegawa M, Komoto S, Shiroiwa T, Fukuda T. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020;23(1):43–51.
Medical Economics Division-Health Insurance Bureau, Ministry of Health Labour and Welfare. Full scale introduction of cost-effectiveness evaluations in Japan. Overview (2/20/2019). https://c2h.niph.go.jp/tools/system/overview_en.pdf. Accessed 15 May 2020.
Schultz LM, Baggott C, Prabhu S, Pacenta HL, Phillips CL, Rossoff J, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol. 2022;40(9):945–55.
John S, Heim M, Curran KJ, Hall EM, Keating AK, Baumeister SH, et al. Improved relapse-free survival (RFS) for pedriatric and young adult patients with relapsed or refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) and low or intermediate preinfusion disease burden treated with tisagenlecleucel: Results from the CIBMTR registry. Tandem Transplantation & Cellular Therapy Meeting of ASTCT and CIBMTR; February 15-19, 2023; Orlando, FL, US.
Medical Services Advisory Committee. Application No. 1748—Review of tisagenlecleucel (Kymriah®) for treatment of acute lymphoblastic leukaemia in paediatric and young adult patients (pALL) 2023. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/D900A0D953425184CA25895C0017A252/$File/1748%20Final%20PSD%20-%20July%202023%20(redacted).pdf. Accessed 19 Feb 2024.
John S, Heim M, Curran K, Hall E, Keating A, Baumeister S. Improved relapse-free survival (RFS) for pedriatric and young adult patients with relapsed or refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) and low or intermediate preinfusion disease burden treated with tisagenlecleucel: Results from the CIBMTR registry. Tandem Transplantation & Cellular Therapy Meeting of ASTCT and CIBMTR; February 15-19, 2023; Orlando, FL.
Garrison LP, Jackson T, Paul D, Kenston M. Value-based pricing for emerging gene therapies: the economic case for a higher cost-effectiveness threshold. J Manag Care Spec Pharm. 2019;25(7):793–9.
Acknowledgements
Medical writing assistance was provided by Shelley Batts, Ph.D., an independent contractor of Analysis Group, Inc., and funded by Novartis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Novartis.
Conflict of interest
Theodore Laetsch holds stock in Advanced Microbubbles; has consulting or advisory roles with Novartis, Aptitude Health, Jumo Health, Massive Bio, Medscape, AI Therapeutics, Jazz Pharmaceuticals, GentBio, Menarini, Pyramid Biosciences, Targeted Oncology, and Treeline Biosciences; and research funding from Lilly, Roche/Genentech, Taiho Oncology, Advanced Accelerator Applications/Novartis, Bristol-Myers Squibb, BioAtla, Hoffman-LaRoche, Pfizer, Bayer, and Turning Point Therapeutics. Louis Garrison reports having received consulting fees in the last 2 years for other research activities with a number of biopharmaceutical companies, including Novartis Gene Therapy, Pfizer, Astra-Zeneca, GSK, MSD, Eli Lilly, Genentech, Roche Molecular Systems, BioMarin, and UniQure. Jie Zhang is an employee of Novartis and owns stock/options. Hongbo Yang, Yanwen Xie, and Dudan Zhang are employees of Analysis Group, Inc., which has received consulting fees from Novartis.
Availability of data and material
The data supporting this study were derived from publicly available literature (CEAs) or are contained in the article. Individual patient data from ELIANA will not be shared due to patient privacy.
Ethics approval
This was systematic literature review and economic analysis using public or previously collected, anonymized data. Thus, no ethical review was required. The study was conducted in accordance with the 1964 Declaration of Helsinki and its amendments.
Consent to participate
N/A.
Consent to publish
N/A.
Code availability
The model described in this study will not be publicly shared due to confidential information.
Author contributions
All authors designed the study. HY, YX, and DZ performed the literature review and CEA analysis. All authors participated in revising the manuscript and have read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Laetsch, T., Zhang, J., Yang, H. et al. Evolving Evidence-Based Value Assessment of One-Time Therapies: Tisagenlecleucel as a Case Study. Appl Health Econ Health Policy (2024). https://doi.org/10.1007/s40258-024-00882-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s40258-024-00882-4