Abstract
Objective
To assess the range of strategies analysed in European cost-effectiveness analyses (CEAs) of colorectal cancer (CRC) screening with respect to the screening intervals, age ranges and test cut-offs used to define positivity, to examine how this might influence what strategies are found to be optimal, and compare them with the current screening policies with a focus on the screening interval.
Methods
We searched PubMed, Web of Science and Scopus for peer-reviewed, model-based CEAs of CRC screening. We included studies on average-risk European populations using the guaiac faecal occult blood test (gFOBT) or faecal immunochemical test (FIT). We adapted Drummond’s ten-point checklist to appraise study quality.
Results
We included 39 studies that met the inclusion criteria. Biennial screening was the most frequently used interval which was analysed in 37 studies. Annual screening was assessed in 13 studies, all of which found it optimally cost-effective. Despite this, 25 of 26 European stool-based programmes use biennial screening. Many CEAs did not vary the age range, but the 14 that did generally found broader ranges optimal. Only 11 studies considered alternative FIT cut-offs, 9 of which found lower cut-offs superior. Conflicts between current policy and CEA evidence are less clear regarding age ranges and cut-offs.
Conclusions
The existing CEA evidence indicates that the widely adopted biennial frequency of stool-based testing in Europe is suboptimal. It is likely that many more lives could be saved throughout Europe if programmes could be offered with more intensive annual screening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Most colorectal cancer (CRC) screening programmes in Europe employ biennial screening using stool-based tests. |
This review assessed the screening intensities analysed by European CEAs of stool-based CRC screening, and while many only assessed biennial screening all of those that also considered annual screening found it to be optimally cost-effective. |
Our findings indicate that many European CEAs of CRC screening have not included a sufficient range of screening strategies in their analyses and imply that current European CRC screening is likely of suboptimal intensity and that many more lives could be saved if programmes could offer annual screening. |
1 Introduction
Colorectal cancer (CRC) is a major public health problem in Europe. It is the most common cancer in terms of incidence and second in terms of mortality, with 520,000 new cases and 245,000 deaths annually [1], and an estimated economic burden of ~€19 billion [2]. CRC screening can reduce mortality by detecting disease at an earlier, more treatable stage [3]. Stool-based screening modalities are non-invasive tests such as the guaiac faecal occult blood test (gFOBT), faecal immunochemical test (FIT) and faecal DNA test. Imaging tests are invasive and employ either endoscopic approaches such as colonoscopy, sigmoidoscopy or non-endoscopic techniques such as computed tomography colonoscopy, magnetic resonance colonoscopy or colon capsule endoscopy [4].
In 2003, the Council of the European Union (EU) recommended population-based CRC screening between ages 50–74 years using gFOBT [5]. Many European countries have since initiated or expanded population-based CRC screening programmes [4]. Programmes have typically used gFOBT or FIT as the primary screening test and then employ colonoscopy to triage primary screen positives. FIT has superior test sensitivity to gFOBT and the advantage of the ability to quantitatively adjust the sensitivity and specificity trade-off by varying the FIT cut-off threshold for test positivity [6]. Thus, many programmes have switched from gFOBT to FIT [7].
Like many other screening programmes, the intensity of CRC screening can be varied by adjusting the screening start age, stop age and screening interval. CRC screening is cost-effective when offered at an appropriate intensity [6]. The relevance of multiple possible alternative intervention intensities has implications for the identification of optimally cost-effective strategies that have been long recognised within cost-effectiveness analysis (CEA) [8]. Problems can arise if CEAs include insufficient strategies. A study may conclude a given strategy is cost-effective but may have failed to consider other relevant strategies, including those that are potentially more effective and cost-effective [6]. Thus, when interpreting cost-effectiveness estimates of CRC screening it is important to consider whether analyses included sufficient variation in screening age range, screening intervals and FIT cut-offs to provide the most useful policy guidance.
The issue of whether sufficient strategies have been included within a CEA has to be considered within the context of the objective of the analysis. While most CEAs may have a broad objective of attempting to find the optimally cost-effective strategy from among all possible strategies, others may have narrower objectives such as assessing the cost-effectiveness of moving from an old test to a new test while holding all other aspects of the screening schedule constant. In the latter case, the exclusion of a range of strategies from a CEA might not be considered problematic given the specific study objectives.
The primary objective of our review is to assess the range of screening intensities considered in European CEAs regarding the screening age ranges, screening intervals and FIT cut-offs, and how this might influence what strategies are found to be optimally cost-effective. In particular, we aim to assess how many CEAs included annual screening and to examine if it is the optimal interval in those studies that have included it. Secondly, we aim to assess the intensity of current European CRC screening policies in the context of the current cost-effectiveness evidence regarding optimal screening strategies. We are not aware of any prior such examination of the CEA evidence of CRC screening regarding the optimally cost-effective choice and European screening policies.
2 Methods
Our review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. The protocol is registered on PROSPERO (CRD42021283988), an international register of prospective systematic reviews.
2.1 Search Strategy
We conducted a systematic search of the PubMed, Web of Science and Scopus databases on 9 September 2022. The search strings are provided in the Supplementary Appendix 1. We also manually reviewed the reference lists of seven relevant systematic reviews on CEAs of CRC screening [10,11,12,13,14,15,16]. Characteristics of the European screening programmes were identified from recent academic publications and official websites for the national screening programmes. If the information were not available from these sources, we contacted local expert sources via email.
2.2 Eligibility Criteria
We included peer-reviewed CEAs of CRC screening in average-risk populations in the 27 EU member states and the UK (henceforth collectively referred to as Europe). The inclusion criteria were (i) model-based CEA simulations of CRC screening using stool-based tests, (ii) conducted in average-risk populations and (iii) set in Europe. The exclusion criteria were studies (i) of stool-based tests other than FIT or gFOBT such as stool DNA testing, as these are not yet widely used in population based screening programmes; (ii) that did not report quality-adjusted life-years (QALYs) or life-years gained (LYG) or equivalents such as life-years saved; (iii) that did not report costs; (iv) not of a screening intervention; (v) not written in English and (vii) reviews, conference abstracts, editorials and notes.
2.3 Study Selection
The titles and abstracts of studies from the database searches and other sources were recorded in Microsoft Excel (version 2108) and duplicates were removed. Records were independently screened by two reviewers (R.P. and Y.L.). A third reviewer (J.O.M.) was consulted in case of disagreement and was resolved through discussion. Full text of studies that met the inclusion criteria were exported to the Mendeley reference management software (version 2.57) and examined in full.
2.4 Data Extraction
Two reviewers (R.P. and Y.L.) individually extracted the study publication year, authors, country setting, study objectives, details of the simulated strategies such as screening start and stop ages, intervals between screens, test modality, costs, estimated QALYs or LYG, reported incremental cost-effectiveness ratio (ICER) and cost-effectiveness threshold (if stated). A third reviewer (J.O.M.) checked a random 20% of the extracts for verification.
Regarding each study’s objectives, we extracted the stated study aim from each manuscript and categorised it according to whether it corresponded with the typical broad objective within CEA of attempting to find the optimally cost-effective strategy or if it stated a narrower aim of attempting to appraise a particular policy or a change in a limited number of policy elements. For example, Babela et al. stated its aim was to evaluate the cost-effectiveness of biennial and annual strategy among the Slovak population aged 50–75 years. Thus, we considered this as a narrow objective [17]. By comparison, Whyte et al. stated its aim was to determine the optimal strategy in terms of screening age range, screening frequency and FIT threshold to inform policy making, which we therefore considered as a broad objective [18].
2.5 Determining the Optimally Cost-Effective Strategy
We interpret the optimally cost-effective strategy in the conventional way as being the most effective strategy with an ICER within the cost-effectiveness threshold. As not all studies report a threshold, we used the threshold reported in other studies from the same setting as a proxy or if available, an explicit CEA threshold specific to the country. When other studies from the same setting that reported different thresholds were available, we used the highest as the proxy threshold. We also conducted a sensitivity analysis in which instead of using the highest threshold we found, we applied the lowest. In our base-case analysis we assess all studies on the assumption that a CEA should consider as many relevant strategies as practical to identify optimally cost-effective policies. As a secondary analysis, we exclude those studies identified as having a narrower study aim.
2.6 Assessment of Quality of Studies
A quality assessment checklist was developed based on Drummond’s ten-item checklist [19], with adjustments corresponding to our study aims. The adjusted checklist is attached in Supplementary Appendix 2. The adjusted Drummond checklist was used to assess the quality of the reviewed studies. We use the same ten topic areas as the original Drummond checklist but omitted 9 of the 33 specific subquestions as they did not apply to the studies considered here. Each study was graded on the ten topic areas, with a 1 and 0.5 being awarded where the study fully or partially satisfied the checklist questions, respectively, and 0 where the questions were not satisfied. Grades were totalled to generate a summary score. R.P., Y.L., E.M. and J.O.M. assessed the studies based on the quality assessment checklist.
3 Results
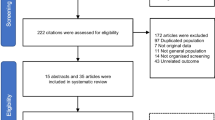
Our search identified 984 studies yielding 430 unique studies after removing duplicates. Following title and abstract screening, 382 studies were excluded. We assessed the full texts of the remaining 56 studies and 7 additional studies identified from manual reference checking. A total of 39 studies were ultimately included [6, 17, 18, 20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. A PRISMA diagram is shown in Fig. 1.
As detailed in Table 1, CEAs of stool-based tests were simulated in 14 different countries; France, the Netherlands and the UK were the most frequent source countries with nine, seven and seven studies respectively. Slovakia is the only country within the sample from Central and Eastern Europe as defined by the Organisation of Economic Cooperation and Development [57]. Most studies (n = 33, 85%) were conducted after 2010. Of the 39 studies, 37 studies (95%) were published after the European Council’s 2003 recommendations. Of the 39 studies, 11 had narrow analysis objectives while the remaining 28 corresponded to the broad CEA objective of seeking optimally cost-effective strategies (Supplementary Appendix 3). Of those studies that had narrow objective, seven focused on screening tests alone or in combination with age range and cut-offs [21, 24, 38,39,40, 46, 47], two focused on assessing specific policies [25, 34], one on a change to the screening interval [17] and one on changes to the age range and cut-off [50].
Of the 39 CEAs, 22 (56%) exclusively analysed stool-based tests while 17 (44%) also assessed image-based primary screening modalities. Of those that exclusively simulated stool-based tests, 12 assessed FIT only, 3 assessed gFOBT only, 6 assessed both FIT and gFOBT, and 1 assessed a biomarker test. Nearly all studies assessed strategies in which recipients receive the same modality over the entire screening programme. One exception was set in Germany, in which younger screenees start with stool-based testing but later switch to image-based testing [36].
While most studies included some variation of screening strategies regarding the screening age range, interval or FIT cut-off, the level of variation was limited. There were 25 (64%) studies that only simulated five strategies or fewer. Nine out of those 25 studies were from the set with narrow objectives.
Figure 2 illustrates the screening intervals considered by the reviewed studies. Regarding the screening interval, 14 studies (36%) examined more than one screening interval. Biennial screening was the most frequently simulated interval which was assessed in 37 (95%) studies, of which 23 (59%) considered no other interval, assessing only biennial screening. The 11 studies addressing narrow objective are shown with grey markers in Fig. 2, 8 of which only considered biennial screening. Whilst annual screening was assessed in only 13 (33%) studies, the majority of these (n = 12) also considered other intervals.
Figure 2 uses a white diamond to highlight the intervals found to be optimally cost-effective in those studies which considered multiple intervals. All 13 studies that assessed annual screening intervals found it to be optimally cost-effective. That is, annual screening was the most effective strategy with an ICER within the CEA threshold in all cases. Naturally, that annual screening was always found to be optimally cost-effective does not imply that all strategies featuring annual screening are cost-effective. Four studies that varied the screening age range and FIT cut-offs found some strategies featuring annual screening with ICERs above the threshold [6, 28, 31, 53].
Regarding the age ranges simulated, there was wide variation in the ranges examined by the studies, as depicted in Fig. 3. Of the 39 studies, 27 (69%) did not consider alternative age ranges. The 11 studies with narrow objectives are depicted with the white hatching, 9 of which only considered a single age range. Ages 50–74 years was the most commonly analysed range, which was assessed in 11 (28%) studies followed by 50–75 years used in 9 (23%). Only 7 (18%) included start ages below 50 and 12 (31%) analysed stop ages above 75 years. Figure 3 uses white diamonds to indicate the start and stop age of those age ranges found to be optimal within those studies that did consider alternative age ranges. All such studies found starting and stopping between 45–50 and 74–80 years, respectively, to be optimal. In the sensitivity analysis using the lowest CEA threshold where more than one is available, we found a policy change in one study. In the study, the start age of 50 years was found optimal instead of 45 years [28].
The FIT cut-off was reported in 26 of the 34 studies (77%) examining FIT-based strategies. Figure 4 presents the FIT cut-offs considered. The most common cut-offs used were 20 and 10 µg Hb/g of faeces with 18 and 10 studies, respectively (53% and 29%). Multiple cut-offs were examined in 11 (28%) studies. Of these, nine found lower cut-offs dominated, the exceptions being studies from the UK and France [18, 40]. There were eight studies with narrow objectives with FIT cut-offs to report. These are shown in Fig. 4 with the grey markers. Of these eight, five considered one cut-off alone.
3.1 Endoscopy Capacity Constraints
The potential for endoscopy capacity to constrain the feasibility of screening strategies has been recognised by several studies. Colonoscopy capacity is mentioned as an important consideration by 20 studies (48%) [6, 18, 20,21,22, 25, 28, 34, 37, 40, 42, 45, 48,49,50,51,52,53,54,55]. Of these, four studies actively considered strategies under capacity constraints within their analyses [6, 18, 50, 51]. Those studies found annual screening was not optimally cost-effective in constrained analyses but was optimal in analyses without capacity constraints.
3.2 European CRC Screening Provision
Table 2 summarises aspects of CRC screening in the European nations considered. Population-based screening is implemented in 22 of 28 European countries at national and regional levels. Cyprus and Estonia have pilot programmes. The countries without population-based programmes are Romania, Bulgaria, Greece and Latvia.
Most countries use stool-based primary testing, except Poland and the Austrian region of Vorarlberg which both use primary colonoscopy. All countries using stool-based testing employ FIT, except Croatia where gFOBT test is used. Colonoscopy is used as triage in all countries with stool-based primary testing.
Among the 26 countries with stool-based screening including pilot programmes, 23 screen biennially. The three exceptions are the Austrian region of Burgenland, which screens annually, and Germany and the Czech Republic, which reduce screening frequency with age. Germany screens annually between ages 50–54 years and then switches to biennial screening. Similarly, the Czech programme screens annually between 50–54 years and participants are then offered biennial FIT or 10 yearly colonoscopy.
Screening start and stop ages also vary. Vorarlberg (in Austria) starts at 40 years; 14 countries start at 50 years and 7 programmes start at 60 years. Estonia, Finland, Ireland, Spain and Sweden had the narrowest population age range covered of 60–69 years. Sex-specific start ages apply in Germany at 50 and 55 years for men and women, respectively.
There is considerable variation in the FIT cut-off used across the EU. All countries use defined FIT thresholds except Malta, which uses a range of 16–20 µg Hb/g. Germany and Lithuania use different cut-offs depending on the test manufacturer [66, 68]. Programmes using higher cut-offs are Ireland and Netherlands with 45 and 47 µg Hb/g, respectively, and the constituent countries of the UK, where 80–150 µg Hb/g is used. Programmes using lower cut-offs are Germany and Lithuania. The most common cut-off is 20 µg Hb/g which is used in six programmes. Sex-specific cut-offs are used in Finland and Sweden.
3.3 Comparison of Policy to CEA Evidence
The most apparent overall observation when comparing the CEA estimates with European screening policies is that although studies that compared annual and biennial clearly indicate annual screening is optimally cost-effective when using FIT, many screening programmes use biennial screening in practice, which is both less effective and less cost-effective. Studies indicating the superiority of annual screening have been published in the setting of the Netherlands, France, Denmark and Austria. In practice, while Burgenland (Austria) uses annual screening, it has done so since 2002, long before the Austrian CEA was published [35]. In the Netherlands, several Dutch CEAs were conducted prior to the implementation of organised screening in 2014 [28, 49, 50, 53]. All of those that assessed annual screening found it cost-effective, yet the programme has employed biennial screening since its introduction, likely reflecting colonoscopy capacity constraints. Similarly, in France, while eight of nine CEAs only considered biennial screening [23, 24, 26, 27, 33, 38,39,40], one study did include annual screening and found it to be cost-effective [32]. Despite this, France has used biennial screening since 2002.
Regarding screening age ranges, CEA estimates suggest start ages of 50 years or lower to be optimal. In practice, 19 of 33 programmes feature screening start ages of 50 years or below. Six programmes use a screening start age of 60 years, despite no CEA evidence which indicates it to be optimal. Ten programmes are using a stop age of 70 years or below contrary to the CEA evidence of a stop age between 74 and 80 years being optimal.
Regarding the FIT cut-off, while most simulation estimates indicate 10 µg Hb/g to be optimal, only Burgenland uses such a low threshold. Germany and Lithuania both feature cut-offs as low as 4 µg Hb/g, but not on a programme-wide basis as there is variation in the cut-offs used within these systems. All Dutch CEA estimates report 20 µg Hb/g or lower to be optimal but the Dutch programme uses a higher cut-off of 47 µg Hb/g.
3.4 Quality Appraisal of the Studies Included in the Review
The quality appraisal checklist findings are reported in the Supplementary Appendix 2. Of the 39 studies, 2 achieved positive answers to all 10 checklist questions, and the remaining studies recorded 7.5/10 or higher overall. Principal areas of weakness were the comprehensive description of the competing alternatives and presentation and the description of the study results. Studies generally performed well on the remaining items.
4 Discussion
Our review assessed the screening intensities analysed by European CEAs of stool-based CRC screening and compared them with policy provision in Europe. We found that nearly all CEAs examined biennial screening, while only a minority considered annual screening. Every study that assessed both intervals found annual screening to be both more effective and cost-effective. Despite this, most European screening programmes currently use biennial screening. This indicates the widely adopted biennial screening interval in Europe is likely of suboptimal intensity. That many studies omitted what appears to be the optimal screening interval from their analyses may have led policy makers across Europe to adopt an insufficiently intensive screening frequency.
Although in some cases the choice of only simulating biennial screening can be explained by a narrow study objective, this does not explain why CEA analysts have simply chosen to simulate biennial screening rather than consider broader options is unclear in all cases. The 2003 European Council recommendation to adopt colorectal screening did not specify a particular interval [5]. The three European randomised controlled trials (RCTs) of gFOBT published at that time had all examined a biennial interval [77,78,79]. It is possible that health economists simply reflected the trialled intervals in their analyses. Despite this, a US trial completed in 1992 included both annual and biennial screening and found the former more efficacious [80]. Indeed, North American RCTs and CEAs assessing stool-based testing have mostly analysed annual screening [14, 15]. Furthermore, annual testing is typically recommended by North American guidelines when stool-based testing is employed [81].
Existing CEA methods guidance strongly encourages modellers to simulate as broad a range of strategies as possible to correctly identify optimally cost-effective strategies [8]. Indeed, it has long been recognised that considering a broad range of strategies is of particular relevance to CEAs of cancer screening [8]. Furthermore, one of the motivating rationales of simulation modelling is that it permits the comparison of additional strategies not assessed in trials [82]. Moreover, it is noteworthy that the two earliest studies within our review by Gyrd-Hansen from 1998 examined a range of intervals including annual screening [30, 31]. In summary, the omission of annual screening by many studies is despite relevant trial evidence, well-established methods guidelines and one early simulation study indicating annual screening is relevant for policy consideration.
Another possible explanation of why many modellers restricted their analysis to biennial screening could be tacit acceptance of colonoscopy constraints, not explicitly explored in their analyses. The Netherlands, UK, Finland and Ireland have faced colonoscopy capacity constraints and have been transparent in modifying aspects of screening provisions to operate within available capacity [18, 28, 45, 83]. It is possible that analysts only considered biennial intervals as they anticipated there would be insufficient endoscopy capacity for annual screening. We believe, however, it is good practice to simulate a broad range of strategies to correctly estimate the optimal strategy, with and without constraints. Such analyses have been published [6, 18], allowing modellers to demonstrate the shortcomings imposed by constraints and ensure policy makers are aware of the benefits of alleviating them.
The primary focus of this review has been the optimal interval, motivated in part by recent findings quantifying the potential QALY gains from an increase in colonoscopy capacity that would facilitate an increase in screening frequency [6]. That study conducted in Irish setting estimated gains in QALYs of 167% compared with the current policy if colonoscopy capacity was expanded to permit intensifying the screening interval as well as broadening the age range and lowering the FIT cut-off [6]. From the studies reviewed here it is quite clear that annual screening seems preferable. Analogous findings also apply regarding the screening age range and FIT cut-off, though the conclusions may be less definitive. CEAs that varied the screening age range generally find that broader age ranges are preferable. Similarly, many but not all studies that vary the FIT cut-off found lower cut-offs preferable. Accordingly, the conclusions regarding the need for strategy variation apply to age range and cut-off parameters also. The feasibility of optimising screening depends, in part, on available colonoscopy capacity. Moving from biennial to annual screening will increase colonoscopy demand, as will broadening the screening age range and reducing FIT cut-offs [28].
Our review shows that the simulation of restricted sets of policy alternatives is not merely a theoretical consideration but may have meaningful policy significance. That many studies have assessed an insufficient range of alternatives has resulted in incomplete cost-effectiveness evidence that may have misled decision-makers leading to inadvertently adopting supoptimal policies and failing to highlight how long-term capacity expansion should be prioritised in healthcare planning. If health systems can increase screening capacity to offer annual screening and populations chose to participate at that frequency, then it seems that many thousands of lives might be saved across Europe.
Our conclusions are naturally subject to caveats. Whether the simulation of a broader range of strategies results in more effective colorectal cancer screening is naturally contingent on factors beyond the control of CEA modellers. Even if CEA modellers assess a comprehensive range of strategies, it not necessarily the case that policy makers will implement optimally cost-effective strategies. A full consideration of the limits of CEA evidence on policy is beyond the scope of this manuscript but there are several points to consider. Policy makers may have priorities other than cost-effectiveness and we should not presume that decision makers seek to optimise cost-effectiveness. As mentioned above, colonoscopy capacity constraints may prevent policy makers from expanding screening services in the short to medium run, as might budget constraints. Indeed, our review finds four Dutch studies have indicated that annual screening would be optimal in the Netherlands [28, 37, 49, 50], yet only biennial screening is offered due to well-documented colonoscopy capacity constraints.
Even if policy makers do not or cannot offer the screening strategies that CEAs indicate to be optimal, we still believe it is important that CEAs simulate a broad range of strategies. The only way policy makers can appreciate the consequences of limiting the set of strategies under consideration or the impact of capacity constraints is with reference to simulation model estimates.
A further important caveat to our analysis is that the sample of studies from which there were CEAs to examine are drawn from Western Europe and thus predominately from wealthier European states. Moreover, the 13 studies within our sample that considered annual screening were from 7 wealthy European countries (Austria, Denmark, France, Finland, Ireland, the Netherlands and the UK). Furthermore, all five Dutch studies employed the same model. It is open to question whether annual screening would remain cost-effective when assessed among less wealthy countries, including those in Central and Eastern Europe.
While our review indicates that the likely optimal interval for FIT screening is annual, there is emerging evidence regarding risk-stratified screening based on age, sex, prior screening history, lifestyle and/or genetic information [84]. Thus, the policies that provide optimally cost-effective CRC prevention might not necessarily require universal annual FIT screening and associated colonoscopy capacity. It seems likely that further research will address the potential for such tailoring and will probably support new policy recommendations without necessarily requiring large increases in colonoscopy capacity. The relevance of considering a breadth of strategies identified by our review will apply equally to research considering stratified policies.
Our review differs from previous systematic reviews of CEAs on CRC screening as those studies did not focus on the methodological consideration of the adequacy of the range of strategies. We focused on a specific question of the CEA evidence regarding optimal intervals for stool-based testing in the context of European screening policies. Thus, our review is limited compared with prior reviews in terms of the screening modalities examined and the context of simulations [10,11,12,13,14,15,16].
Our findings are based on reviewing the outcomes of modelling studies. We appreciate the uncertainty of simulation analyses. There may be scope for further RCTs to reduce uncertainties regarding the benefits of intensified screening, especially as there has been only one RCT comparing the biennial and annual intervals [80]. The same applies regarding extending screening ages and lowering FIT cut-offs.
4.1 Limitations
Our study has several limitations. First, not all screening programmes clearly document screening strategy employed, especially the FIT cut-off used. Accordingly, we cannot be definitive about screening practices in all programmes. Second, we did not examine the model types or assumptions used in studies which might have played a role in the optimal strategy recommendations. Despite possible differences, all relevant studies consistently found annual screening to be more effective and cost-effective than biennial screening. Third, we limited our analysis to stool-based based tests and did not consider image-based screening modalities or a combination of stool and image-based modalities. Fourth, we limited our analysis to papers written in English.
5 Conclusions
The existing CEA evidence indicates that the widely adopted biennial stool-based testing in Europe is likely suboptimal. Every CEA that examined annual screening found it to be optimally cost-effective. It is the responsibility of modellers to include a wide range of strategies beyond those examined in trials or recommended within guidelines to correctly identify optimal strategies and best inform decision-makers. CRC screening strategies, especially the intervals in FIT based testing should be reconsidered. It is likely that many more lives could be saved throughout Europe if programmes can offer more intensive annual screening. The feasibility of offering such frequent screening depends however on the colonoscopy capacity within each health system.
References
Cardoso R, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–13. https://doi.org/10.1016/S1470-2045(21)00199-6.
Henderson RH, et al. The economic burden of colorectal cancer across Europe: a population-based cost-of-illness study. Lancet Gastroenterol Hepatol. 2021;6(9):709–22. https://doi.org/10.1016/S2468-1253(21)00147-3.
Jodal HC, et al. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: a systematic review and network meta-analysis. BMJ Open. 2019;9(10): e032773. https://doi.org/10.1136/bmjopen-2019-032773.
Schreuders EH, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–49. https://doi.org/10.1136/gutjnl-2014-309086.
European Council. Council recommendation of 2 December 2003 on cancer screening. 2003. http://data.europa.eu/eli/reco/2003/878/oj. Accessed 23 Mar 2022.
McFerran E, et al. Colorectal cancer screening within colonoscopy capacity constraints: can FIT-based programs save more lives by trading off more sensitive test cutoffs against longer screening intervals? MDM Policy Pract. 2022;7(1):23814683221097064. https://doi.org/10.1177/23814683221097064.
Meklin J, Syrjänen K, Eskelinen M. Colorectal cancer screening with traditional and new-generation fecal immunochemical tests: a critical review of fecal occult blood tests. Anticancer Res. 2020;40(2):575–81. https://doi.org/10.21873/anticanres.13987.
Gold MR, et al. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996.
Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. https://doi.org/10.1016/j.jclinepi.2009.06.006.
Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33(1):88–100. https://doi.org/10.1093/epirev/mxr004.
Ran T, et al. Cost-effectiveness of colorectal cancer screening strategies—a systematic review. Clin Gastroenterol Hepatol. 2019;17(10):1969-1981.e15. https://doi.org/10.1016/j.cgh.2019.01.014.
Mendivil J, et al. Economic evaluations of screening strategies for the early detection of colorectal cancer in the average-risk population: a systematic literature review. PLoS ONE. 2019;14(12): e0227251. https://doi.org/10.1371/journal.pone.0227251.
Zhong G-C, et al. Efficacy and cost-effectiveness of fecal immunochemical test versus colonoscopy in colorectal cancer screening: a systematic review and meta-analysis. Gastrointest Endosc. 2020;91(3):684-697.e15. https://doi.org/10.1016/j.gie.2019.11.035.
Pignone M, et al. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2002;137(2):96–104. https://doi.org/10.1177/23814683221097064.
Patel SS, Kilgore ML. Cost effectiveness of colorectal cancer screening strategies. Cancer Control. 2015;22(2):248–58. https://doi.org/10.1177/107327481502200219.
Jeong KE, Cairns JA. Review of economic evidence in the prevention and early detection of colorectal cancer. Heal Econ Rev. 2013;3(1):1–10. https://doi.org/10.1186/2191-1991-3-20.
Babela R, et al. Cost-effectiveness of colorectal cancer screening in Slovakia. Eur J Cancer Prev. 2021;31(5):415–21. https://doi.org/10.1097/CEJ.0000000000000727.
Whyte S, et al. Optimizing the design of a repeated fecal immunochemical test bowel cancer screening programme with a limited endoscopy capacity from a health economic perspective. Value in Health. 2022;25(6):954–64. https://doi.org/10.1016/j.jval.2021.10.002.
Drummond MF, et al. Methods for the economic evaluation of health care programmes. New York: Oxford University Press; 2015.
Areia M, et al. Cost-utility analysis of colonoscopy or faecal immunochemical test for population-based organised colorectal cancer screening. United Eur Gastroenterol J. 2019;7(1):105–13. https://doi.org/10.1177/2050640618803196.
Aronsson M, et al. Cost-effectiveness of high-sensitivity faecal immunochemical test and colonoscopy screening for colorectal cancer. Br J Surg. 2017;104(8):1078–86. https://doi.org/10.1002/bjs.10536.
Arrospide A, et al. Cost-effectiveness and budget impact analyses of a colorectal cancer screening programme in a high adenoma prevalence scenario using MISCAN-Colon microsimulation model. BMC Cancer. 2018;18(1):1–11. https://doi.org/10.1186/s12885-018-4362-1.
Barré S, et al. Cost-effectiveness analysis of alternative colon cancer screening strategies in the context of the French national screening program. Ther Adv Gastroenterol. 2020;13:1756284820953364. https://doi.org/10.1177/1756284820953364.
Berchi C, et al. Cost-effectiveness analysis of two strategies for mass screening for colorectal cancer in France. Health Econ. 2004;13(3):227–38. https://doi.org/10.1002/hec.819.
Coretti S, et al. Economic evaluation of colorectal cancer screening programs: Affordability for the health service. J Med Screen. 2020;27(4):186–93. https://doi.org/10.1177/0969141319898732.
Chauvin P, Josselin J-M, Heresbach D. Incremental net benefit and acceptability of alternative health policies: a case study of mass screening for colorectal cancer. Eur J Health Econ. 2012;13(3):237–50. https://doi.org/10.1007/s10198-011-0300-8.
Currais P, et al. Should colorectal cancer screening in Portugal start at the age of 45 years? A cost-utility analysis. GE Port J Gastroenterol. 2021;28(5):311–8. https://doi.org/10.1159/000513592.
Goede SL, et al. Cost-effectiveness of one versus two sample faecal immunochemical testing for colorectal cancer screening. Gut. 2013;62(5):727–34. https://doi.org/10.1136/gutjnl-2011-301917.
Greuter MJ, et al. The potential of imaging techniques as a screening tool for colorectal cancer: a cost-effectiveness analysis. Br J Radiol. 2016;89(1063):20150910. https://doi.org/10.1259/bjr.20150910.
Gyrd-Hansen D. Fecal occult blood tests: a cost-effectiveness analysis. Int J Technol Assess Health Care. 1998;14(2):290–301. https://doi.org/10.1017/s0266462300012265.
Gyrd-Hansen D, Søggard J, Kronborg O. Colorectal cancer screening: efficiency and effectiveness. Health Econ. 1998;7(1):9–20. https://doi.org/10.1002/(sici)1099-1050(199802)7:1%3c9::aid-hec304%3e3.0.co;2-h.
Hassan C, et al. Cost effectiveness and projected national impact of colorectal cancer screening in France. Endoscopy. 2011;43(09):780–93. https://doi.org/10.1055/s-0030-1256409.
Heresbach D, et al. Cost-effectiveness of colorectal cancer screening with computed tomography colonography or fecal blood tests. Eur J Gastroenterol Hepatol. 2010;22(11):1372–9. https://doi.org/10.1097/MEG.0b013e32833eaa71.
Idigoras I, et al. Evaluation of the colorectal cancer screening Programme in the Basque Country (Spain) and its effectiveness based on the Miscan-colon model. BMC Public Health. 2018;18(1):1–12. https://doi.org/10.1186/s12889-017-4639-3.
Jahn B, et al. Effectiveness, benefit harm and cost effectiveness of colorectal cancer screening in Austria. BMC Gastroenterol. 2019;19(1):1–13. https://doi.org/10.1186/s12876-019-1121-y.
Ladabaum U, et al. Cost-effectiveness of colorectal cancer screening in Germany: current endoscopic and fecal testing strategies versus plasma methylated Septin 9 DNA. Endosc Int Open. 2014;2(02):E96–104. https://doi.org/10.1055/s-0034-1377182.
Lansdorp-Vogelaar I, et al. Cost-effectiveness of high-performance biomarker tests vs fecal immunochemical test for noninvasive colorectal cancer screening. Clin Gastroenterol Hepatol. 2018;16(4):504-512.e11. https://doi.org/10.1016/j.cgh.2017.07.011.
Lejeune C, et al. Cost-effectiveness analysis of fecal occult blood screening for colorectal cancer. Int J Technol Assess Health Care. 2004;20(4):434–9. https://doi.org/10.1017/s0266462304001321.
Lejeune C, et al. Cost-effectiveness of screening for colorectal cancer in France using a guaiac test versus an immunochemical test. Int J Technol Assess Health Care. 2010;26(1):40–7. https://doi.org/10.1017/S026646230999078X.
Lejeune C, et al. The cost-effectiveness of immunochemical tests for colorectal cancer screening. Dig Liver Dis. 2014;46(1):76–81. https://doi.org/10.1016/j.dld.2013.07.018.
Macafee D, et al. Population screening for colorectal cancer: the implications of an ageing population. Br J Cancer. 2008;99(12):1991–2000. https://doi.org/10.1038/sj.bjc.6604788.
Murphy J, Halloran S, Gray A. Cost-effectiveness of the faecal immunochemical test at a range of positivity thresholds compared with the guaiac faecal occult blood test in the NHS Bowel Cancer Screening Programme in England. BMJ Open. 2017;7(10): e017186. https://doi.org/10.1136/bmjopen-2017-017186.
Pil L, et al. Cost-effectiveness and budget impact analysis of a population-based screening program for colorectal cancer. Eur J Intern Med. 2016;32:72–8. https://doi.org/10.1016/j.ejim.2016.03.031.
Senore C, et al. Cost-effectiveness of colorectal cancer screening programmes using sigmoidoscopy and immunochemical faecal occult blood test. J Med Screen. 2019;26(2):76–83. https://doi.org/10.1177/0969141318789710.
Sharp L, et al. Cost-effectiveness of population-based screening for colorectal cancer: a comparison of guaiac-based faecal occult blood testing, faecal immunochemical testing and flexible sigmoidoscopy. Br J Cancer. 2012;106(5):805–16. https://doi.org/10.1038/bjc.2011.580.
Sobhani I, et al. Cost-effectiveness of mass screening for colorectal cancer: choice of fecal occult blood test and screening strategy. Dis Colon Rectum. 2011;54(7):876–86. https://doi.org/10.1007/DCR.0b013e31820fd2bc.
van Rossum LG, et al. Colorectal cancer screening comparing no screening, immunochemical and guaiac fecal occult blood tests: a cost-effectiveness analysis. Int J Cancer. 2011;128(8):1908–17. https://doi.org/10.1002/ijc.25530.
Whyte S, Chilcott J, Halloran S. Reappraisal of the options for colorectal cancer screening in England. Colorectal Dis. 2012;14(9):e547–61. https://doi.org/10.1111/j.1463-1318.2012.03014.x.
Wilschut JA, et al. Cost-effectiveness analysis of a quantitative immunochemical test for colorectal cancer screening. Gastroenterology. 2011;141(5):1648-1655.e1. https://doi.org/10.1053/j.gastro.2011.07.020.
Wilschut JA, et al. Fecal occult blood testing when colonoscopy capacity is limited. J Natl Cancer Inst. 2011;103(23):1741–51. https://doi.org/10.1093/jnci/djr385.
Thomas C, et al. Should colorectal cancer screening start at different ages for men and women? Cost-effectiveness analysis for a resource-constrained service. Cancer Rep. 2021;4(4): e1344. https://doi.org/10.1002/cnr2.1344.
Heisser T, Hoffmeister M, Brenner H. Model based evaluation of long-term efficacy of existing and alternative colorectal cancer screening offers: a case study for Germany. Int J Cancer. 2022;150(9):1471–80. https://doi.org/10.1002/ijc.33894.
van der Meulen MP, et al. Do men and women need to be screened differently with fecal immunochemical testing? A cost-effectiveness analysisFIT screening stratified by gender. Cancer Epidemiol Biomark Prev. 2017;26(8):1328–36. https://doi.org/10.1158/1055-9965.EPI-16-0786.
Tappenden P, et al. Option appraisal of population-based colorectal cancer screening programmes in England. Gut. 2007;56(5):677–84. https://doi.org/10.1136/gut.2006.095109.
Heinävaara S, et al. Optimizing screening with faecal immunochemical test for both sexes-Cost-effectiveness analysis from Finland. Prev Med. 2022;157: 106990. https://doi.org/10.1016/j.ypmed.2022.106990.
Lee D, et al. Cost effectiveness of CT colonography for UK NHS colorectal cancer screening of asymptomatic adults aged 60–69 years. Appl Health Econ Health Policy. 2010;8(3):141–54. https://doi.org/10.2165/11535650-000000000-00000.
OECD. Glossary of statistical terms Central and Eastern European Countries (CEECS). 2022. https://stats.oecd.org/glossary/detail.asp?ID=303. Accessed 14 Nov 2022.
Rodríguez Barrios JM, et al. The use of cost per life year gained as a measurement of cost-effectiveness in Spain: a systematic review of recent publications. Eur J Health Econ. 2012;13:723–40.
Gyrd-Hansen D. Willingness to pay for a QALY. Health Econ. 2003;12(12):1049–60.
Gsur A, Baierl A, Brezina S. Colorectal cancer study of Austria (CORSA): a population-based multicenter study. Biology. 2021;10(8):722.
Tran TN, et al. Population-based data reveal factors associated with organised and non-organised colorectal cancer screening: an important step towards improving coverage. Int J Environ Res Public Health. 2021;18(16):8373.
Guo F, et al. Benefits of switching from guaiac-based faecal occult blood to faecal immunochemical testing: experience from the Wallonia-Brussels colorectal cancer screening programme. Br J Cancer. 2020;122(7):1109–17.
Ferlizza E, et al. The roadmap of colorectal cancer screening. Cancers. 2021;13(5):1101.
Suchanek S, Ngo O, Grega T, Majek O, Zavoral M. Colorectal cancer incidence and mortality reduction in the Czech Republic—effect of the national screening program? Gastrointest Endosc. 2019;89:AB376.
Sarkeala T, et al. Piloting gender-oriented colorectal cancer screening with a faecal immunochemical test: population-based registry study from Finland. BMJ Open. 2021;11(2): e046667.
Heisser T, et al. Age-specific sequence of colorectal cancer screening options in Germany: a model-based critical evaluation. PLoS Med. 2020;17(7): e1003194. https://doi.org/10.1371/journal.pmed.1003194.
Csanádi M, et al. Modeling costs and benefits of the organized colorectal cancer screening programme and its potential future improvements in Hungary. J Med Screen. 2021;28(3):268–76.
Poskus T, et al. Initial results of the national colorectal cancer screening program in Lithuania. Eur J Cancer Prev. 2015;24(2):76–80. https://doi.org/10.1097/CEJ.0000000000000096.
Kooyker AI, et al. The second round of the Dutch colorectal cancer screening program: Impact of an increased fecal immunochemical test cut-off level on yield of screening. Int J Cancer. 2020;147(4):1098–106.
Tepeš B, et al. Results of the FIT-based national colorectal cancer screening program in Slovenia. J Clin Gastroenterol. 2017;51(6):e52–9.
Ribbing Wilén H, Saraste D, Blom J. Gender-specific cut-off levels in colorectal cancer screening with fecal immunochemical test: a population-based study of colonoscopy findings and costs. J Med Screen. 2021;28(4):439–47.
Public Health England. Bowel cancer screening: programme overview. https://www.gov.uk/guidance/bowel-cancer-screening-programme-overview.
Cancer Research UK. For health professionals Together we will beat cancer Key things to know about FIT England version. 2021. https://www.cancerresearchuk.org/sites/default/files/england_key_differences_infographic_2021.pdf. Accessed 8 Dec 2022.
Cancer Research UK. Key things to know about FIT Wales. 2022. https://www.cancerresearchuk.org/sites/default/files/screening_vs._symptomatic_fit_infographic_-_wales.pdf. Accessed 8 Dec 2022.
Clark G, et al. Transition to quantitative faecal immunochemical testing from guaiac faecal occult blood testing in a fully rolled-out population-based national bowel screening programme. Gut. 2021;70(1):106–13.
Cancer Research UK. For health professionals in Northern Ireland Together we will beat cancer Key things to know about FIT. 2022. https://www.cancerresearchuk.org/sites/default/files/th_0031_ni_fit_resource_final_2021.pdf. Accessed 8 Dec 2022.
Kronborg O, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–71. https://doi.org/10.1016/S0140-6736(96)03430-7.
Hardcastle JD, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–7. https://doi.org/10.1016/S0140-6736(96)03386-7.
Kewenter J, et al. Results of screening, rescreening, and follow-up in a prospective randomized study for detection of colorectal cancer by fecal occult blood testing: results for 68,308 subjects. Scand J Gastroenterol. 1994;29(5):468–73. https://doi.org/10.3109/00365529409096840.
Mandel JS, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328(19):1365–71. https://doi.org/10.1056/NEJM199305133281901.
Bénard F, et al. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J Gastroenterol. 2018;24(1):124. https://doi.org/10.3748/wjg.v24.i1.124.
Knudsen AB, McMahon PM, Gazelle GS. Use of modeling to evaluate the cost-effectiveness of cancer screening programs. J Clin Oncol. 2007;25(2):203–8. https://doi.org/10.1200/JCO.2006.07.9202.
Toes-Zoutendijk E, et al. Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology. 2017;152(4):767-775.e2. https://doi.org/10.1053/j.gastro.2016.11.022.
Lansdorp-Vogelaar I, et al. Risk-stratified strategies in population screening for colorectal cancer. Int J Cancer. 2022;150(3):397–405. https://doi.org/10.1002/ijc.33784.
Acknowledgement
We would like to acknowledge the helpful feedback received from both the screening group at the Erasmus Medical Center's Department of Public Health and Professor Dorte Gyrd Hansen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding provided by the IReL Consortium. This work was supported by Ireland’s Health Research Board’s SPHeRE programme. EMF receives funding from Cancer Focus Northern Ireland and Health Data Research UK.
Conflict of interest
Authors declare no conflict of interest.
Availability of data
Data sharing is not applicable to this article as no datasets were generated or analysed during the current review.
Ethics approval
This study did not involve participation of human subjects; therefore, ethical approval was not required.
Informed consent
Not required.
Author contributions
Rajani Pokharel and James O’Mahony contributed to the study conception and design. Rajani Pokharel and Yishu Lin performed title/abstract screening, full text selection, data extraction. Rajani Pokharel, Yishu Lin, Ethna McFerran and James O’Mahony conducted the quality appraisal of the studies. The first draft of the manuscript was written by Rajani Pokharel and all authors contributed to the critical revision of the manuscript for intellectual content and approved the final draft submitted for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pokharel, R., Lin, YS., McFerran, E. et al. A Systematic Review of Cost-Effectiveness Analyses of Colorectal Cancer Screening in Europe: Have Studies Included Optimal Screening Intensities?. Appl Health Econ Health Policy 21, 701–717 (2023). https://doi.org/10.1007/s40258-023-00819-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-023-00819-3