Abstract
Background
We aimed to investigate the cost effectiveness of lorlatinib, a third-generation anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor (TKI), used first-line in Sweden to treat patients with ALK-positive (ALK+) non-small cell lung cancer (NSCLC). In January 2022, the European Medicines Agency (EMA) extended its approval of lorlatinib to include adult patients with ALK+ NSCLC not previously treated with an ALK inhibitor. Extended first-line approval was based on results from CROWN, a phase III randomized trial that enlisted 296 patients randomized 1:1 to receive lorlatinib or crizotinib. Our analysis compared lorlatinib against the first-generation ALK-TKI crizotinib, and second-generation ALK TKIs alectinib and brigatinib.
Methods
A partitioned survival model with four health states [pre-progression, non-intracranial (non-central nervous system [CNS]) progression, CNS progression, and death] was constructed. The progressed disease state (which is typically modelled in cost-effectiveness analyses of oncology treatments) was explicitly separated into non-CNS and CNS progression as brain metastases, which are common in NSCLC, and can have a large impact on patient prognosis and health-related quality of life. Treatment effectiveness estimates in the lorlatinib and crizotinib arms of the model were derived from CROWN data, while indirect relative effectiveness estimates for alectinib and brigatinib were informed using network meta-analysis (NMA). Utility data were derived from the CROWN study in the base case, and cost-effectiveness results were compared when applying UK and Swedish value sets. Costs were obtained from Swedish national data. Deterministic and probabilistic sensitivity analyses were conducted to test model robustness.
Results
Fully incremental analysis identified crizotinib as the least costly and least effective treatment. Brigatinib was extendedly dominated by alectinib and, subsequently, alectinib was extendedly dominated by lorlatinib. Lorlatinib was associated with an incremental cost-effectiveness ratio (ICER) of Swedish Krona (SEK) 613,032 per quality-adjusted life-year (QALY) gained compared with crizotinib. Probabilistic results were generally consistent with deterministic results, and one-way sensitivity identified NMA HRs, alectinib and brigatinib treatment duration, and the CNS-progressed utility multiplier as key model drivers.
Conclusions
The ICER of SEK613,032 for lorlatinib versus crizotinib falls below the typical willingness-to-pay threshold per QALY gained for high-severity diseases in Sweden (approximately SEK1,000,000). Furthermore, as brigatinib and alectinib were extendedly dominated in the incremental analysis, the results of our study indicate that lorlatinib may be considered a cost-effective treatment option for first-line patients with ALK+ NSCLC in Sweden when compared with crizotinib, alectinib, and brigatinib. Longer-term follow-up data for endpoints informing treatment effectiveness for all first-line treatments would help to reduce uncertainty in the findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our analysis, based on CROWN trial data and network meta-analysis (NMA), suggests first-line treatment with lorlatinib is likely to be a cost-effective option for patients affected by anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer. |
Treating with lorlatinib instead of first- and second-generation ALK tyrosine kinase inhibitors leads to substantial gains in quality-adjusted life-years. |
The results are robust but the cost effectiveness of lorlatinib is most sensitive to the utility multiplier for the CNS-progressed health state, relative efficacy derived from the NMA, and the alectinib and brigatinib treatment duration. |
1 Introduction
Non-small cell lung cancer (NSCLC) is an epithelial lung cancer responsible for 80–85% of all lung cancers [1]. In Sweden, 4657 new cases were diagnosed in 2018 alone [2]. NSCLC can be asymptomatic and is typically diagnosed after metastasizing to other organs, particularly the central nervous system (CNS). The prognosis is poor as patients typically develop resistance to traditional therapy [3, 4].
Anaplastic lymphoma kinase (ALK) is a key oncogenic driver in NSCLC [5]. In Sweden, estimates suggest the prevalence of newly diagnosed ALK mutation-positive (ALK+) NSCLC is 60–80 patients eligible to start treatment per year [6,7,8]. At diagnosis, studies show up to 40% of people with ALK+ metastatic NSCLC already have brain metastases [9,10,11].
In Sweden, guidelines for treating ALK+ NSCLC are based on European Society for Medical Oncology (ESMO) recommendations [8, 12]. The latest national lung cancer treatment programme recommends that patients with ALK rearrangement should be treated with an ALK tyrosine kinase inhibitor (ALK-TKI). For clinicians, the first-choice ALK-TKIs are alectinib and brigatinib; alternatives are crizotinib and ceritinib, however ceritinib has not been evaluated by Tandvårds- och läkemedelsförmånsverket (TLV, the Dental and Pharmaceutical benefits Agency).
In Sweden, 2021 data from the National Board of Health and Welfare, National Prescribed Drug Register, show 108 patients were initiated on first-line ALK-TKI treatment. The breakdown was 35 patients initiated on crizotinib, 62 on alectinib, 6 on ceritinib and 5 on lorlatinib; no patients were initiated on brigatinib [13].
Lorlatinib is a third-generation TKI that targets ALK and receptor tyrosine kinase c-ros oncogene 1 (ROS1). In 2019, the European Medicines Agency (EMA) approved lorlatinib to treat adult patients with ALK+ metastatic NSCLC with disease that progressed on either crizotinib and at least one other ALK inhibitor for metastatic disease, or alectinib or ceritinib as the first ALK inhibitor therapy for metastatic disease [14]. In January 2022, the EMA extended the indication to enable first-line lorlatinib to treat adult patients with ALK+ NSCLC previously not treated with an ALK inhibitor [14]. Following the EMA’s initial decision, in Sweden in September 2019, healthcare regulator TLV decided to reimburse lorlatinib at second-line or later (after treatment with alectinib, ceritinib or crizotinib) based on benefit of treatment on progressive NSCLC, a condition leading to reduced quality of life and short life expectancy. TLV estimated the cost per quality-adjusted life-year (QALY) gained was Swedish Krona (SEK) 730,000–980,000 for lorlatinib compared with platinum-based chemotherapy at second-line or later. TLV concluded the cost was reasonable based on the benefit of treatment and the severity of disease [15]. Following the EMA’s extension decision, TLV decided in March 2022 to also reimburse lorlatinib as a first-line treatment. The first-line TLV decision was not based on a cost-utility model; it stated that since lorlatinib is at least as effective as the most relevant comparator alectinib, and since the treatment costs are similar, lorlatinib is a cost-effective treatment alternative [16].
The EMA based its lorlatinib extended first-line approval on CROWN [17], a phase III randomized trial that enlisted 296 patients with previously untreated advanced ALK+ NSCLC (randomized 1:1 to receive lorlatinib or crizotinib). Patients in CROWN were randomized by ethnic group (Asian/non-Asian) and, notably, the presence of brain metastases at baseline (yes/no).
We developed an economic model to assess the cost effectiveness of lorlatinib as a first-line treatment for patients with advanced ALK+ NSCLC in Sweden, versus existing treatment options—crizotinib, alectinib and brigatinib. The model considers whether the additional QALYs gained are worth the additional cost of lorlatinib compared with these existing treatments at willingness-to-pay thresholds used in Sweden.
2 Methods
2.1 Model Structure
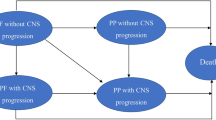
The cost-effectiveness model was created in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and uses a partitioned survival approach. There are four health states: progression-free, non-CNS-progressed disease, CNS-progressed disease, and death (Fig. 1). This structure was chosen over alternative approaches as it reflects the progressive natural history of NSCLC, allowing pre- and post-progression differences in health-related quality of life (HRQoL) and costs to be captured. Furthermore, partitioned survival models have been accepted in first- and later-line ALK+ NSCLC by both TLV and the National Institute for Health and Care Excellence (NICE) [15, 16, 18,19,20,21,22]. To use an alternative structure such as a Markov model would require additional outcomes (including time to progression and post-progression survival), which were highly immature in the CROWN trial and were not available for comparator treatments alectinib and brigatinib. Therefore, use of a Markov approach would add complexity and increase uncertainty and would be unlikely to produce a robust result.
Model structure diagram. Patients enter the model in the progression-free state and receive lorlatinib or a comparator therapy, either crizotinib, alectinib or brigatinib. Patients in the progression-free state may remain progression-free, their cancer may progress with or without CNS involvement, or they may die. Patients with non-CNS-progressed disease may remain in their current state, experience CNS progression, or die. CNS-progressed patients can remain alive with CNS-progressed disease or die. Death is an absorbing state. The three alive health states—progression-free, non-CNS-progressed, and CNS progressed, are further divided into on-treatment and off-treatment to reflect costs and HRQoL more accurately. CNS central nervous system, HRQoL health-related quality of life
By explicitly capturing non-CNS-progressed disease and CNS-progressed disease, the model reflects the substantial impact of intracranial progression on patient prognosis (further influencing HRQoL and resource use). This four-state model structure was used in recent NICE technology appraisals in first-line ALK-positive NSCLC in the UK for alectinib and brigatinib (TA536 and TA670) [19, 20]. Similarly, CNS-progression costs were considered in the TLV evaluation of alectinib [18].
A 30-years lifetime horizon was considered in the base-case analysis. A 30-days cycle length was considered appropriate to adequately capture transitions and reflect changes in health. A half-cycle correction was applied to costs (except for those known to occur at the start of a cycle) and outcomes. In line with Swedish health technology assessment guidance, a 3% time-preference discount rate was applied to costs and outcomes and a societal perspective was used in the base-case analysis [23].
2.2 Patient Population
The CROWN study used to inform this analysis included patients with locally advanced or metastatic ALK+ NSCLC who had received no previous systemic treatment for metastatic disease. Adjuvant/neoadjuvant NSCLC treatment was only allowed if completed more than 12 months before randomization [17]. In the intention-to-treat (ITT) population, patients were randomized, with 149 receiving lorlatinib and 147 receiving crizotinib. The baseline age of the cohort was 57.4 years, 59.1% of patients were female, and 26.4% of patients had brain metastases at baseline.
2.3 Efficacy and Safety
To inform efficacy in the lorlatinib and crizotinib arms of the model, parametric survival curves (exponential, generalized gamma, gompertz, log-logistic, log-normal, Weibull and gamma) were fit to time-to-event endpoints from CROWN. Extrapolation of overall survival (OS), intracranial progression-free survival (CNS-PFS), PFS and time-on-treatment (ToT) data for lorlatinib and crizotinib were performed to estimate outcomes beyond the observed duration of the clinical trial, as observed data were not complete at the data cut-off used to inform these analyses (20 March 2020).
Parametric curves were fit separately to the lorlatinib and crizotinib arms, as it is not necessary to rely on the proportional hazards assumption and fit joint models when patient-level data are available. For lorlatinib and crizotinib, Weibull curves were selected for OS and exponential curves selected across all other endpoints (further information including justification for this choice is provided in electronic supplementary Appendix 1). Curve selection was based on the plausibility of long-term extrapolations and ensuring consistency of curve shape across treatment arms and correlated endpoints (for example, ToT and PFS) where possible, as well as recent precedent in first-line ALK+ NSCLC submissions to the TLV and NICE (TA670 and TA536) [18,19,20]. Precedent was considered here given that all treatments considered are ALK-TKIs and have the same mechanism of action. We therefore expect that the shape of curves should be similar for all treatment arms and may be aligned with that accepted in previous analyses of comparator treatments. Although consideration was given to the statistical goodness-of-fit of models to the observed data, less weight was placed on statistical fit due to the immaturity of the CROWN data at cut-off.
To compare against relevant treatments outside of CROWN (alectinib and brigatinib), a systematic review of the literature and subsequent network meta-analysis (NMA) of randomized controlled trials was conducted. The NMA produced hazard ratios (HRs) versus baseline (crizotinib) as the treatment effect estimate. We applied the HRs to OS and PFS extrapolations to predict outcomes for alectinib and brigatinib. Within the analysis, a network for the CNS-PFS and ToT endpoints could not be formed due to a lack of published data in comparator trials. Consequently, alternative approaches were used to estimate CNS-PFS and ToT for treatments outside of CROWN. For all endpoints, the hazard of survival was capped based on expected general population survival data.
In the NMA, a network was formed between lorlatinib, brigatinib and alectinib through the CROWN (lorlatinib vs. crizotinib), ALEX (alectinib vs. crizotinib) and ALTA-1L (brigatinib vs. crizotinib) studies. In ALEX, 303 patients aged ≥18 years with untreated, stage IIIB/IV ALK+ NSCLC with an Eastern Cooperative Oncology Group (ECOG) performance score of 0–2 were randomized to receive alectinib 600 mg twice daily or crizotinib 250 mg twice daily [9]. In ALTA-1L, 275 patients with advanced ALK-positive NSCLC who had not previously received ALK inhibitors were randomized 1:1 to brigatinib 180 mg once daily (with a 7-days lead-in period at 90 mg) or crizotinib 250 mg twice daily [24].
Three of the studies identified in the systematic literature review were conducted solely in Asian countries (ALESIA, PROFILE 1029 and J-ALEX) [25,26,27], whereas other identified studies were conducted globally. These three additional studies were not included in the network informing the base-case analysis, as they may not be considered generalizable to Sweden. However, the impact of including studies conducted solely in Asian countries was explored in a scenario analysis.
NMA results for all OS networks considered in the analysis—remove all-Asian studies (base case) and ITT (scenario analysis)—were adjusted for crossover, which was permitted in the ALTA-1L trial. As the OS results may be affected by patients crossing from one treatment arm to another after progression, the crossover-adjusted NMA HRs provide a fairer comparison across all trials.
NMA results for the PFS endpoint indicate that alectinib (HR 0.50, 95% credible interval [CrI] 0.36–70) and brigatinib (HR 0.49, 95% CrI 0.35–0.68) reduce the continuous probability of experiencing progression or death compared with crizotinib. Similarly, for the OS endpoint, point estimate NMA results were favourable for alectinib (HR 0.69, 95% CrI 0.47–1.01) and brigatinib (HR 0.87, 95% CrI 0.41–1.85) compared with crizotinib. For both OS and PFS, NMA results were consistent in the ITT scenario (which included studies conducted in all-Asian countries).
As it was not possible to form a network for CNS-PFS, an endpoint rarely reported in clinical trial publications, we tested two alternative assumptions for estimating alectinib and brigatinib. First, we assumed the output of the PFS NMA (HRs vs. crizotinib) was applicable to CNS-PFS. In a scenario analysis, HRs from a network of intracranial time to progression (CNS-TTP) were used as a proxy for CNS-PFS. The CNS-TTP as a proxy for the CNS-PFS scenario was not selected in the base-case model, as, unlike in PFS, deaths are not considered events in TTP endpoints.
As Kaplan–Meier data for ToT were not reported for treatments outside of CROWN, ToT curves were estimated by fitting an exponential distribution to unadjusted median reported treatment duration for alectinib (median ToT, 28.10 months) [28] and brigatinib (median ToT, 24.30 months) [20]. This approach is consistent with the approach accepted in the brigatinib NICE appraisal [20]. As the alectinib EMA license states treatment should be continued until disease progression or unacceptable toxicity, and the brigatinib EMA license indicates that treatment should continue as long as clinical benefit is observed [29, 30], a scenario analysis is explored assuming ToT is equal to PFS for treatments outside of CROWN. Similarly, a scenario analysis assuming ToT is equal to PFS for all treatments (including lorlatinib and crizotinib) is presented.
A summary of survival curves used to derive health state occupancy over time in the cost-effectiveness model, derived from the CROWN patient-level data analyses and NMA, is presented in electronic supplementary Appendix 2.
Unadjusted safety data were taken from the relevant clinical trials for each treatment (electronic supplementary Appendix 3), to capture the impact of adverse events (AE) on costs and HRQoL.
2.4 Health-Related Quality of Life
The primary health outcome in the cost-effectiveness analysis is the QALY, a composite measure of quantity and quality of life generated by weighting LYs with a utility value representing HRQoL.
In the UK, NICE prefers the use of utility values calculated from the three-level EQ-5D (EQ-5D-3L) value set [31]. EQ-5D-5L data from CROWN were mapped to the EQ-5D-3L value set using the van Hout et al. [32] crosswalk algorithm. Although in Sweden the British value set is preferred by the TLV, the impact of applying the 5L Swedish value set [33] is tested in scenario analysis.
To capture the impact of CNS progression on HRQoL, a utility multiplier derived from Roughley et al. for CNS progression versus non-CNS progression was applied [34]. Roughley et al. considered patients with stage IV NSCLC and evaluated them for the impact of brain metastases versus other metastatic sites. They found the EQ-5D utility value associated with brain metastases was 0.52 compared with 0.69 for contralateral lung metastases, resulting in a multiplier of 75.36%. This approach was accepted by NICE in the recent appraisal of brigatinib for first-line ALK+ NSCLC [20]. AE disutility values are not considered in the base-case analysis; it is assumed that health state utility values derived from the trial capture the impact of any AEs. An age-related utility adjustment over time is applied using UK general population values as reported by Ara and Brazier [35], to account for a natural decline in HRQoL due to age.
Table 1 summarizes utility values applied to the base-case analysis and scenario analysis; CROWN utility values derived using the Swedish value set, utility values sourced from ALTA-1L and ALEX were tested in scenario analysis (as reported in NICE TA670 and TA536) [19, 20].
2.5 Resource Use and Costs
The analysis considered the following cost categories: drug costs, subsequent treatment costs, resource use costs, end-of-life care costs, AE management costs, and societal costs. ALK testing costs were not considered as all first-line treatments require confirmed ALK+ status; therefore, the costs of ALK testing would be equal for all treatment arms and would not impact the ICERs. A 2021 cost year was used for the analysis as this reflects the latest available costs at the time of the analysis. There were no changes in drug costs between 2021 and 2022. Any changes in other costs are expected to have a negligible impact on the results.
The model included drug list prices per pack for lorlatinib (SEK50,458.70; 30 × 100 mg tablets), crizotinib (SEK44,434.63; 60 × 250 mg tablets), alectinib (SEK47,655.97; 224 × 150 mg capsules), and brigatinib (SEK47,653.62; 28 × 180 mg tablets) [36]. A brigatinib starter pack (7 tablets × 90 mg and 21 tablets × 180 mg) was also included (SEK47,653.62) for the first 28-days treatment cycle. We assumed oral administration costs were implicitly captured by the difference in pharmacy purchasing prices and pharmacy selling prices. Total acquisition costs were calculated based on pack prices (assuming whole packs are dispensed and therefore including wastage); the recommended dosing schedules for lorlatinib (100 mg once daily), crizotinib (250 mg twice daily), alectinib (600 mg twice daily) and brigatinib (starter pack followed by 180 mg once daily); and the proportion of patients on-treatment each cycle [37,38,39]. Considering relative dose intensity (electronic supplementary Appendix 4) resulted in per 30-days model cycle costs of lorlatinib (SEK47,986.01), crizotinib (SEK43,612.59), alectinib (SEK49,936.65), and brigatinib (SEK47,358.34).
Subsequent treatment costs, which were applied to newly progressed patients using the proportion of PFS events that were progressions (90%), were estimated using the distribution of subsequent treatments received in the relevant clinical trials for each first-line treatment, list prices, and median second-line treatment durations reported in the literature. Administration costs of SEK5878 were included for subsequent treatments administered via intravenous infusion [40]. Subsequent treatment distributions, unit costs and durations, and the resulting per cycle costs applied following each first-line treatment are provided in electronic supplementary Appendix 5.
Resource use costs were stratified by health state but were assumed to be equal across ALK TKIs. Aggregate resource use costs were applied each model cycle to patients in the pre-progression (SEK1774) and post-progression (SEK3620) states. We assumed these aggregate costs reflect medical appointments (general practitioners, nurses and specialists), computerized tomography scans, x-rays and radiotherapy. The resource use costs associated with experiencing CNS progression were applied as a one-off lump sum to the proportion of patients entering the CNS-progressed state each cycle (SEK98,280). This is in accordance with the previous TLV appraisal of alectinib [18] and is appropriate since it captures the additional costs that are related to the event of CNS progression. Health state costs were based on values previously accepted by the TLV in the appraisal of alectinib for ALK+ NSCLC, uplifted to a 2021 cost year [18]. The proportion of patients experiencing one-off CNS-PFS costs in each state was estimated using the proportion of CNS-PFS events observed in the CROWN study that were progressions (68%). Due to the uncertainty introduced by uplifting the published Swedish costs, the impact of using a micro-costing approach to healthcare resource use was explored in a scenario analysis. In the micro-costing approach, Swedish unit costs for individual resources (reported in electronic supplementary Appendix 6) are applied to the frequency of physician visits and tests and procedures, as reported in prior NICE appraisals in ALK+ NSCLC in the UK [19].
The cost-effectiveness analysis captured grade 3 or higher treatment-emergent AEs (all-causality) occurring in 5% or more of patients in the lorlatinib or crizotinib arms of CROWN, the alectinib arm of ALEX, or the brigatinib arm of ALTA-1L. To avoid biasing in favour of treatments with a shorter trial follow-up, annual AE rates were calculated using average treatment duration and total patient numbers. AE unit costs were sourced from standard Swedish sources (reported in electronic supplementary Appendix 3). Annual per patient AE costs applied in the model were SEK1506 for lorlatinib, SEK1735 for crizotinib, SEK435 for alectinib, and SEK626 for brigatinib.
Societal costs were included in the model as productivity gains for patients in the progression-free health state. Productivity gains were calculated using the average labour force participation rate in Sweden (75.6%) [41], the proportion of work hours lost (8%) [42] based on a chart review of second-line ALK+ NSCLC patients treated with crizotinib, and the average monthly salary in Sweden, including compulsory employer contributions (SEK47,442.62) [43, 44].
3 Analysis
The primary outcomes of the analysis were lifetime costs, LYs and QALYs. Outcomes were presented in disaggregated form by model health state and cost category. Cost-effectiveness results were calculated as the ICER (cost per QALY gained). Pairwise ICERs were calculated for lorlatinib versus each comparator. Furthermore, in fully incremental analysis, all treatments were compared simultaneously; treatments were ranked in order of least to most expensive before ICERs were calculated, treatments with a higher ICER than the next most effective treatment were excluded from the analysis as extendedly dominated, and ICERs were recalculated.
Sensitivity analyses were conducted to test the robustness of model results to parametric uncertainty. In one-way sensitivity analyses (OWSAs), key model drivers were identified by varying inputs in turn at their lower and upper bound and recording model results. In probabilistic sensitivity analysis, all uncertain inputs were simultaneously, randomly sampled from an assigned probability distribution (rather than using a point estimate per deterministic analysis) and costs and outcomes were recorded over 5000 probabilistic iterations. Probability distributions were chosen based on the characteristics of the parameters. Beta distributions were used for parameters bounded between 0 and 1, CODA samples from the NMA were used for the HRs, multivariate normal distributions were used for correlated parameters such as survival curve parameters, Dirichlet distributions were used for multinomial distributions, and normal distributions were used for all other parameters.
Various scenario analyses were conducted to explore methodological and structural uncertainty, including testing alternative discount rates, time horizons, NMA subgroups, utility value sources, and resource use costing approaches.
3.1 Validation
Model validation was carried out throughout development. Internal validity and technical accuracy were checked routinely by the model developers and by an independent health economist not involved in the development, using a comprehensive checklist that included all elements discussed in the Assessment of the Validation Status of Health-Economic decision models (AdViSHE) validation-assessment tool. Errors identified during the quality control checks were addressed in the final economic model [45].
4 Results
Table 2 presents deterministic pairwise results for lorlatinib against crizotinib, alectinib, and brigatinib, respectively. The fully incremental analysis shows crizotinib is the least costly and least effective treatment (generating 3.35 QALYs at a total cost of SEK1,293,552). Brigatinib is extendedly dominated having a higher ICER compared with crizotinib (SEK1,165,967) than alectinib compared with brigatinib (SEK550,574), and alectinib produces more QALYs than both crizotinib and brigatinib. Alectinib has an ICER of SEK901,147 (incremental costs SEK1,222,557, incremental QALYs 1.36) compared with crizotinib. However, lorlatinib has an ICER of SEK291,680 compared with alectinib (incremental costs SEK354,784, incremental QALYs 1.22). Alectinib is therefore extendedly dominated by lorlatinib. Lorlatinib is associated with an ICER of SEK613,032 per QALY gained compared with crizotinib. The cost-effectiveness frontier is presented in Fig. 2.
OWSA identified the utility values reported by Roughley et al. for contralateral lung metastases and brain metastases; the alectinib and brigatinib OS HRs versus crizotinib; and the alectinib and brigatinib median reported treatment durations as the parameters with the greatest influence on cost-effectiveness results. The OWSA results are presented in electronic supplementary Appendix 7. Probabilistic results were generally consistent with deterministic results, with average costs and QALYs over 5000 iterations indicating that brigatinib and alectinib are extendedly dominated and lorlatinib is associated with a probabilistic ICER of SEK654,908 versus crizotinib. Figure 3 presents the cost-effectiveness acceptability curve for all included treatments. Probabilistic results indicate that lorlatinib is most likely to be the cost-effective treatment option at willingness-to-pay thresholds of approximately SEK600,000 and above. Table 3 presents results of the scenario analysis. Lorlatinib remains the treatment of choice in all scenarios based on the willingness-to-pay threshold in Sweden (approximately SEK1,000,000) [46]
When conducting the analysis from a payer perspective, ICERs for lorlatinib versus crizotinib, alectinib, and brigatinib increased by SEK37,480, SEK47,797 and SEK 31,367, respectively. When testing utility values derived from CROWN using the Swedish value set, ICERs for lorlatinib versus all other comparators decreased; conversely, applying utility values derived from ALEX and ALTA-1L (reported in prior NICE appraisals) increased ICERs for lorlatinib versus comparators. All pairwise ICERs in scenario analysis for lorlatinib versus crizotinib, alectinib, and brigatinib remained below a willingness-to-pay threshold of SEK1,000,000 per QALY gained, suggesting model findings were robust to methodological uncertainties.
5 Discussion
To our knowledge, this is the first cost-effectiveness study of lorlatinib for the first-line treatment of ALK+ NSCLC in Sweden. A cost-effectiveness study of lorlatinib as a first-line treatment of ALK+ NSCLC in the US was published by Li et al. [47]. This study was not deemed relevant to our analysis given that the US healthcare system is very different to that in Sweden. Furthermore, the study included only a comparison with crizotinib and did not include alectinib or brigatinib, both of which are relevant comparators in Sweden, and the model did not consider CNS progression, which has been shown to be an outcome of interest in ALK+ patients and has been considered in recent UK and Swedish HTA submissions [18,19,20].
The base case showed alectinib and brigatinib to be extendedly dominated in fully incremental analysis. Furthermore, the ICER of SEK613,032 for lorlatinib versus crizotinib falls below the typical willingness-to-pay threshold per QALY gained for high-severity diseases in Sweden (approximately SEK1,000,000) [46, 48]. The results of our study indicate that lorlatinib may be considered a cost-effective treatment option for first-line patients with ALK+ NSCLC in Sweden.
In the comparison with alectinib and brigatinib, it is unsurprising that HRs for OS versus crizotinib were identified as key model drivers, given that OS data are immature, and these HRs are used to estimate survival in the alectinib and brigatinib arms of the model and consequently derive LYs and QALYs. Furthermore, it is expected that treatment durations would be identified as key model drivers, as they are used to estimate ToT curves for comparators outside of CROWN, which in turn are used to estimate treatment acquisition costs. In all comparisons, it is similarly unsurprising that the values used to derive the CNS-progressed disease utility multiplier were identified as key drivers as these are highly uncertain parameters.
A key strength of the analysis lies in its use of available clinical trial data to inform safety, treatment effectiveness and HRQoL, and the latest relevant data sources for costs. Furthermore, the analysis leverages an NMA of randomized controlled trials sourced through a systematic review of the literature with a common reference treatment (crizotinib), rather than relying on naïve or unanchored comparisons.
Utility data informing the analysis were derived from the EQ-5D, the commonly used generic HRQoL instrument often preferred by health technology assessment bodies. The EQ-5D-5L was developed due to concerns that the EQ-5D-3L may not be sensitive enough to capture milder health problems and smaller changes in health status. Our study compares results when using both the EQ-5D-3L value set (preferred by NICE) and the EQ-5D-5L Swedish value set. Few studies have utilized the published Swedish 5-level value set to date, and to our knowledge, our study is the first to do so in the NSCLC setting.
Key assumptions were necessary to inform the analysis, particularly with respect to limitations in the trial data available to inform treatment effectiveness estimates in the model. In cost-effectiveness analysis, we estimate costs and outcomes for patients over a lifetime horizon; however, some data were immature at cut-off in the CROWN study. Consequently, we extrapolated outcomes beyond the trial period. The key modelling challenges therefore arose from the immaturity in the PFS, CNS-PFS and OS data, particularly for lorlatinib. At CROWN data cut-off, median PFS and CNS-PFS was reached only for patients in the crizotinib arm, while neither arm reached median survival for OS. Consequently, survival extrapolations (particularly in the lorlatinib arm of the model) were subject to a high degree of uncertainty. Although it is difficult to draw conclusions on the long-term outcomes for patients receiving lorlatinib based on the curve extrapolations, in the base analysis, the models that produce the most conservative available estimates of OS, CNS-PFS, and PFS in the lorlatinib arm were selected.
Although a network was formed to indirectly compare against treatments outside of CROWN for the OS and PFS endpoints, a network could not be formed for CNS-PFS due to little reported data. We therefore assumed the HRs for PFS were applicable to CNS-PFS. Although this approach was undertaken in the appraisal of brigatinib for ALK+ NSCLC by NICE in the UK, it is an assumption that generates uncertainty in the CNS-PFS estimates informing the model for treatments outside of CROWN.
To our knowledge, this study is the first to assess the cost effectiveness of lorlatinib at first-line for ALK+ NSCLC patients in Sweden. Longer-term follow-up data for endpoints informing treatment effectiveness for all therapies would help to reduce uncertainty in the findings.
6 Conclusions
Our analysis indicates that lorlatinib could be considered a cost-effective treatment option for first-line patients with ALK+ NSCLC in Sweden.
References
American Cancer Society. What is lung cancer? 2019. Updated 1 Oct 2019. Available at: https://www.cancer.org/cancer/lung-cancer/about/what-is.html. Accessed 4 Sep 2020.
Statista. Number of new cases of lung cancer in Sweden from 2010 to 2020. 2022. Available at: https://www.statista.com/statistics/891980/number-of-lung-cancer-cases-in-sweden/. Accessed 5 Apr 2022.
Hallberg B, Palmer RH. ALK and NSCLC: targeted therapy with ALK inhibitors. F1000 Med Rep. 2011;3:21. https://doi.org/10.3410/m3-21.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94. https://doi.org/10.4065/83.5.584.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6. https://doi.org/10.1038/nature05945.
Socialstyrelsen National Board of Health and Welfare. Cancer registry, statistics and data. Available at: https://www.socialstyrelsen.se/statistik-och-data/register/cancerregistret/. Accessed 14 Jun 2022.
National Lung Cancer Register. 2022. Available at: https://statistik.incanet.se/Lunga/3
Regionalt Cancercentrum. Lungcancer Nationellt vårdprogram. Lungcancer Nationellt vårdprogram, 2022-05-10 Version 6.0. 2020. Available at: https://www.cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/vardprogram/nationellt-vardprogram-lungcancer.pdf. Accessed 13 Jun 2022.
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim D-W, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–38. https://doi.org/10.1056/NEJMoa1704795.
Soria J-C, Tan DS, Chiari R, Wu Y-L, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. The Lancet. 2017;389(10072):917–29.
Gainor JF, Tseng D, Yoda S, Dagogo-Jack I, Friboulet L, Lin JJ, et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol. 2017. https://doi.org/10.1200/po.17.00063.
ESMO. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Available at: https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-15SEPT2020.pdf. Accessed 13 Jun 2022.
National Prescribed Drug Register. Register of Medicinal Products. 2022. Available at: https://www.socialstyrelsen.se/statistik-och-data/register/lakemedelsregistret/. Accessed 13 Jun 2022.
European Medicines Agency (EMA). Summary of opinion (initial authorisation) Lorviqua (lorlatinib). 2019. Available at: https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-lorviqua_en.pdf. Accessed 13 Jun 2022.
Tandvårds-och läkemedelsförmånsverket (TLV). Lorviqua is included in the high-cost protection with limitation. 2019. Available at: https://www.tlv.se/beslut/beslut-lakemedel/begransad-subvention/arkiv/2019-09-30-lorviqua-ingar-i-hogkostnadsskyddet-med-begransning.html. Accessed 13 Jun 2022.
Tandvårds- och läkemedelsförmånsverket (TLV). Application within the pharmaceutical benefits. 2022. Available at: https://www.tlv.se/download/18.66b0fb1f17f96a8babdb5c46/1648480312859/bes220324_lorviqua_3992-2021.pdf. Accessed 13 Jun 2022.
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–29. https://doi.org/10.1056/NEJMoa2027187.
Tandvårds-och läkemedelsförmånsverket (TLV). Application within the pharmaceutical benefits. 2018. Available at: https://www.tlv.se/download/18.577a4290162f616250871891/1524726796321/bes180420_alecensa.pdf. Accessed 13 Jun 2022.
National Institute for Health and Care Excellence (NICE). Alectinib for untreated ALK-positive advanced non-small-cell lung cancer (TA536). 2018. Available at: https://www.nice.org.uk/guidance/ta536. Accessed 29 Mar 2021.
National Institute for Health and Care Excellence (NICE). Brigatinib for ALK-positive advanced non-small-cell lung cancer that has not been previously treated with an ALK inhibitor (TA670). 2021. Available at: https://www.nice.org.uk/guidance/ta670. Accessed 14 Jan 2021.
National Institute for Health and Care Excellence (NICE). Ceritinib for untreated ALK-positive non-small-cell lung cancer (TA500). 2018. Available at: https://www.nice.org.uk/guidance/ta500. Accessed 17 Jan 2023.
National Institute for Health and Care Excellence (NICE). Crizotinib for untreated anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer (TA406). 2016. Available at: https://www.nice.org.uk/guidance/ta406. Accessed 17 Jan 2023.
Tandvårds- och läkemedelsförmånsverket (TLV). Amendment to the Dental and Pharmaceutical Benefits Agency's general advice (TLVAR 2003:2) on economic evaluations. 2017. Available at: https://www.tlv.se/download/18.467926b615d084471ac3230c/1510316374332/TLVAR_2017_1.pdf. Accessed 13 Jun 2022.
Camidge DR, Kim HR, Ahn M-J, Yang JC-H, Han J-Y, Lee J-S, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. New Eng J Med. 2018;379(21):2027–39.
Zhou C, Kim S-W, Reungwetwattana T, Zhou J, Zhang Y, He J, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7(5):437–46.
Wu Y-L, Lu S, Lu Y, Zhou J, Shi Y-K, Sriuranpong V, et al. Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in east asian patients with ALK-Positive advanced non-small cell lung Cancer. J Thoracic Oncol. 2018;13(10):1539–48.
Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. The Lancet. 2017;390(10089):29–39.
Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31(8):1056–64. https://doi.org/10.1016/j.annonc.2020.04.478.
European Medicines Agency (EMA). Alecensa. 2021. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/alecensa. Accessed 13 Jun 2022.
European Medicines Agency (EMA). Alunbrig (Brigatinib). 2022. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/alunbrig. Accessed 14 Jun 2022.
National Institute for Health and Care Excellence (NICE). NICE health technology evaluations: the manual 2022. 2022. Available at: https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741
Van Hout B, Janssen M, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15.
Burström K, Teni FS, Gerdtham UG, Leidl R, Helgesson G, Rolfson O, et al. Experience-based Swedish TTO and VAS value sets for EQ-5D-5L health states. Pharmacoeconomics. 2020;38(8):839–56. https://doi.org/10.1007/s40273-020-00905-7.
Roughley A, Damonte E, Taylor-Stokes G, Rider A, Munk VC. Impact of brain metastases on quality of life and estimated life expectancy in patients with advanced non-small cell lung cancer. Value Health. 2014;17(7):A650. https://doi.org/10.1016/j.jval.2014.08.2364.
Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
Tandvårds- och läkemedelsförmånsverket (TLV). Search the database. Available at: https://www.tlv.se/beslut/sok-i-databasen.html. Accessed 13 Jun 2022.
Electronic Medicines Compendium (eMC). Summary of Product Characteristics: Alecensa. 2021. Updated 17 April 2020. Available at: https://www.medicines.org.uk/emc/product/2438/smpc#gref. Accessed 18 Mar 2021.
Electronic Medicines Compendium (eMC). Summary of Product Characteristics: Xalkori. 2021. Updated 9 October 2020. Available at: https://www.medicines.org.uk/emc/product/2857/smpc#gref. Accessed 18 Mar 2021.
Electronic Medicines Compendium (eMC). Summary of Product Characteristics: Lorviqua. 2021. Updated 28 September 2021. Available at: https://www.medicines.org.uk/emc/product/10632#gref. Accessed 8 Apr 2022.
Southern Healthcare Region. Regional prices and reimbursements for the Southern Health Care Region 2021. 2021. Available at: https://sodrasjukvardsregionen.se/download/regionala-priser-och-ersattningar-for-sodra-sjukvardsregionen-2021/. Accessed 13 Jun 2022.
Statistics Sweden (SCB). Basic tables LFS, aged 15-74, annual average according to international definition. Updated 25 May 2022. Available at: https://www.scb.se/hitta-statistik/statistik-efter-amne/arbetsmarknad/arbetskraftsundersokningar/arbetskraftsundersokningarna-aku/pong/tabell-och-diagram/icke-sasongrensade-data/grundtabeller-aku-1574-ar-ar/. Accessed 13 Jun 2022.
Nilsson FOL, Asanin ST, Masters ET, Iadeluca L, Almond C, Cooper M, et al. The cost-effectiveness of lorlatinib versus chemotherapy as a second- or third-line treatment in anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer in Sweden. Pharmacoeconomics. 2021;39(8):941–52. https://doi.org/10.1007/s40273-021-01015-8.
Skatteverket. Employer's contributions. Available at: https://www.skatteverket.se/foretagochorganisationer/arbetsgivare/arbetsgivaravgifterochskatteavdrag/arbetsgivaravgifter.4.233f91f71260075abe8800020817.html. Accessed 14 Jun 2022.
Statistics Sweden (SCB). Average monthly salary, SEK by sector, occupation (SSYK 2012), sex, level of education and year. 2021. Available at: https://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__AM__AM0110/. Accessed 13 Jun 2022.
Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61. https://doi.org/10.1007/s40273-015-0327-2.
Svensson M, Nilsson FO, Arnberg K. Reimbursement decisions for pharmaceuticals in Sweden: the impact of disease severity and cost effectiveness. Pharmacoeconomics. 2015;33(11):1229–36. https://doi.org/10.1007/s40273-015-0307-6.
Li S, Li J, Peng L, Li Y, Wan X. Cost-effectiveness of lorlatinib as a first-line therapy for untreated advanced anaplastic lymphoma kinase-positive non-small cell lung cancer. Front Oncol. 2021;11: 684073. https://doi.org/10.3389/fonc.2021.684073.
Svensson M, Nilsson F. The Swedish Dental and Pharmaceutical Benefits Agency’s willingness to pay for new drugs has been analyzed [in Swedish]. Lakartidningen. 2016;113:DX44.
Acknowledgements
The authors would like to thank Matthew Massello of Lumanity for his involvement in the development of the global cost-effectiveness model that was adapted to inform the analyses described in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Pfizer Inc.
Conflicts of Interest/Competing Interests
Fredrik O.L. Nilsson is an employee of Pfizer AB, Sweden, and owns stock in Pfizer Inc. At the time this study was conducted, Laura Iadeluca was an employee of Pfizer Inc, and owns stock in Pfizer Inc. Chrissy Lowry and Norma Beavers are employees of Lumanity, which was a paid consultant to Pfizer in connection with the development of this manuscript. At the time this study was conducted, Jaesh Naik was an employee of Lumanity, which was a paid consultant to Pfizer in connection with the development of this manuscript. The authors did not receive direct payment as a result of this work outside of their normal salary payments.
Availability of Data and Material
Data are contained within the article and its electronic supplementary material.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication from Patients/Participants
Not applicable.
Code Availability
Available upon request.
Authors’ Contributions
This manuscript was written and edited by Jaesh Naik, Norma Beavers, Fredrik O.L. Nilsson, Laura Iadeluca and Chrissy Lowry. The global cost-effectiveness model was developed by Jaesh Naik and Matthew Massello. Figures and tables were designed by Jaesh Naik and Chrissy Lowry. All authors approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Naik, J., Beavers, N., Nilsson, F.O.L. et al. Cost‑Effectiveness of Lorlatinib in First-Line Treatment of Adult Patients with Anaplastic Lymphoma Kinase (ALK)‑Positive Non‑Small‑Cell Lung Cancer in Sweden. Appl Health Econ Health Policy 21, 661–672 (2023). https://doi.org/10.1007/s40258-023-00807-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-023-00807-7