Abstract
Objectives
Combinations of on-patent therapies (CTs) are increasingly common in oncology. They cause challenges for funding and affordability, and hence patient access, especially when constituent therapies are owned by different manufacturers. The aim of our study was to develop policy proposals for the assessment, pricing, and funding of CTs and identify which might be relevant in different European countries.
Methods
Following a review of available literature, seven hypothetical policy proposals were developed and subsequently assessed through 19 semi-structured interviews with health policy, pricing, technology assessment and legal experts in seven European countries to identify those most likely to gain traction.
Results
Experts saw a need for agreed approaches within a country to manage affordability and funding challenges for CTs. Changes to health technology assessment (HTA) and funding models were considered unlikely, but other policy proposals were seen as mostly useful, with country-specific adaptations. Bilateral discussions between manufacturers and payers were deemed important, and less challenging and protracted than arbitrated dialogue between manufacturers. Usage-specific pricing, possibly using weighted average prices, was considered a prerequisite for the financial management of CTs.
Conclusions
There is a growing need to ensure that CTs are affordable to health systems. It would appear that there is no one set of policies that is appropriate for all countries in Europe, so countries wishing to ensure that patients have (or continue to have) access to CTs of value to them must explore and implement the policies that are best suited to their general approach to funding health care and to the assessment and reimbursement of medicines.

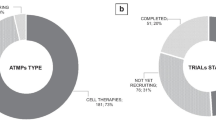
(Source: authors’ compilation, based on the EU Clinical Trials Register, https://www.clinicaltrialsregister.eu/. Analysis performed in January, 2022)
Similar content being viewed by others
Notes
The authors are consciously using the term 'usage-specific pricing' rather than 'indication-based pricing'. The main reason is that the baseline therapy may remain in use as monotherapy and/or become a baseline for other add-on therapies in exactly the same indication. The proposed way forward is therefore to make prices specific not to the indication, but rather to the specific usage (i.e., a specific combination or monotherapy).
Germany was not included in the scope of the analysis underlying this manuscript.
References
World Health Organisation (WHO). Global health estimates 2019: disease burden by cause, age, sex, by country and by region, 2000–2019. Geneva: World Health Organization; 2020. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates.
Jemal A, Torre L, Soerjomataram I, Bray F (Eds). The Cancer Atlas, 3rd edn. American Cancer Society; 2019. https://canceratlas.cancer.org/wp-content/uploads/2019/10/ACS_CA3_Book.pdf. Accessed 16 Feb 2022.
Humphrey RW, Brockway-Lunardi LM, Bonk DT, et al. Opportunities and challenges in the development of experimental drug combinations for cancer. J Natl Cancer Inst. 2011;103(16):1222–6. https://doi.org/10.1093/jnci/djr246.
Latimer NR, Towse A, Henshall C. Not cost-effective at zero price: valuing and paying for combination therapies in cancer. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):331–3. https://doi.org/10.1080/14737167.2021.1879644.
Persson U, Norlin JM. Multi-indication and combination pricing and reimbursement of pharmaceuticals: opportunities for improved health care through faster uptake of new innovations. Appl Health Econ Health Policy. 2018;16(2):157–65. https://doi.org/10.1007/s40258-018-0377-7.
European Federation of Pharmaceutical Industries Associations. EFPIA pipeline review 2021 update. Project report. 2021. https://www.efpia.eu/media/602564/iqvia_efpia_pipeline-review_final.pdf. Accessed 17 Mar 2022.
Briggs AH, Doyle A, Schneider J, et al. An attribution of value framework for combination therapies. Report by the value attribution working group. 2021. https://www.takeda.com/49a844/siteassets/en-gb/home/what-we-do/combination-treatments/a-value-attribution-framework-for-combination-therapies-takeda-whitepaper.pdf. Accessed 21 Sept 2021.
Dankó D, Blay JY, Garrison LP. Challenges in the value assessment, pricing and funding of targeted combination therapies in oncology. Health Policy. 2019;123(12):1230–6. https://doi.org/10.1016/j.healthpol.2019.07.009.
Garrison LP Jr, Towse A. A strategy to support efficient development and use of innovations in personalized medicine and precision medicine. J Manag Care Spec Pharm. 2019;25(10):1082–7. https://doi.org/10.18553/jmcp.2019.25.10.1082.
Towse A, Lothgren M., Steuten L, Bruce A. Why we need a new outcomes-based value attribution framework for combination regimens in oncology. Office of Health Economic; 2021. https://www.ohe.org/publications/why-we-need-new-outcomes-based-value-attribution-framework-combination-regimens. Accessed 21 Sept 2021.
Davis S. Assessing technologies that are not cost-effective at a zero price. London: National Institute for Health and Care Excellence (NICE); 2014.
Organisation for Economic Co-operation and Development. Addressing challenges in access to oncology medicines. Analytical report. organisation for economic co-operation and development; 2020. https://www.oecd.org/health/health-systems/Addressing-Challenges-in-Access-to-Oncology-Medicines-Analytical-Report.pdf. Accessed 20 Sept 2021.
Podkonjak T, Taylor H, Taylor A et al. Voluntary arbitration framework for combination therapies a proposed process by the voluntary arbitration working group. 2021. https://www.takeda.com/4a81d5/siteassets/en-gb/home/what-we-do/combination-treatments/voluntaryarbitrationframeworkforcombinationtherapies_takedawhitepaper_september2021.pdf. Accessed 21 Sept 2021.
Lawlor R, Wilsdon T, Darquennes E, et al. Accelerating patient access to oncology medicines with multiple indications in Europe. J Mark Access Health Policy. 2021;9(1):1964791. https://doi.org/10.1080/20016689.2021.1964791.
Mestre-Ferrandiz J, Zozaya N, Alcalá B, Hidalgo-Vega Á. Multi-indication pricing: nice in theory but can it work in practice? Pharmacoeconomics. 2018;36(12):1407–20. https://doi.org/10.1007/s40273-018-0716-4.
National Institute of Health and Care Excellence. Developing NICE guidelines—the manual. Last updated: 18 January 2022. https://www.nice.org.uk/process/pmg20/resources/developing-nice-guidelines-the-manual-pdf-72286708700869. Accessed 28 Feb 2022.
Migliore A. Towards a regulation of HTA in Europe: the proposal from the European Commission. Expert Rev Med Devices. 2019;16(1):1–2. https://doi.org/10.1080/17434440.2019.1557047.
The European Parliament and Council of the European Union. Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on health technology assessment and amending Directive 2011/24/EU. 2021. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R2282&from=EN. Accessed 17 Mar 2022.
Acknowledgements
Editorial assistance was provided by Julia Heagerty associated with Obsidian Healthcare Group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This paper builds on independent analysis funded by Novartis Pharma AG, Basel, Switzerland. All authors had complete freedom in conducting the study, compiled all data in the study themselves and take full responsibility for the content of the publication.
Conflict of interest
CH reports consulting fees, support to travel to meetings, and honoraria for conference presentations on HTA from various not-for-profit, consultancies, and companies including University of Warwick, ICER, Bellberry, OHE, CRA, Ideas and Solutions, Pfizer, Janssen, Abbott, Medtronic, Roche. DD reports a research grant from Amgen Switzerland GmbH, related to previous scientific publication on combination therapies, and consulting fees from MSD, Sanofi, Janssen, J&J for consulting projects in oncology. PI received honorarium from Swiss Health Quality Association for lectures and he is a member of the Steering Board of Swisstransplant. GP reports consulting fees from MSD, Janssen, Takeda, and Astra Zeneca for participation at scientific boards and honorarium from GSK for a press conference. LV reports honorarium and consulting fees from Novartis Pharma AG for participation at various oncology-related projects. NW reports payments from AstraZeneca, Bayer, Daiichi Sankyo, Incyte, Janssen, MSD, Novartis, Pierre Fabre and Oasmia for participation in advisory boards and educational activities outside the scope of the present manuscript; and fees from Roche/Genentech for participation in independent data monitoring committee. For the remaining authors, no conflicts of interest were declared in relation to the subject of this article.
Ethics approval
Not applicable.
Consent to participate
Informed consent was obtained from all interviewees.
Consent for publication
Not applicable (identifying information is not shared in this article).
Availability of data and material
Search strategies used for the literature review are available in the supplementary file. The discussion guide used in the interviews is available from the corresponding author upon request.
Code availability
Not applicable.
Author contributions
CH and DD conceived and designed the analysis, collected the data, and wrote the manuscript. All other authors participated in the expert discussion of the analysis, provided their inputs to the conclusions, and participated in the preparation, review and editing of the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Henshall, C.H., Dankó, D., Barham, L. et al. Review and Assessment of Policy Options for Improving Access to Combination Therapies in Oncology in Europe. Appl Health Econ Health Policy 21, 537–546 (2023). https://doi.org/10.1007/s40258-023-00795-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-023-00795-8