Abstract
The silent pandemic of antimicrobial resistance (AMR) is a global issue needing prompt attention. A comprehensive one-health approach across human and animal health, agriculture and the environment is needed to solve this, addressing overuse of antibacterials, and of course, optimising measures for preventing and controlling infection. We also need a robust pipeline of new antibacterials. However, the current pipeline is inadequate and several companies with new antibacterials have gone bankrupt due to low sales, leading to a ‘broken market’. To address this, the UK has completed a project using novel approaches to value assessment and reimbursement for two antibacterials. The new funding arrangements for these products commenced on 1st July 2022, delinking reimbursement from volume of sales; a so-called ‘pull incentive’, with payments based on the added value to the whole-health and social-care system, not just to individual patients. This article describes how the project was devised, developed, and progressed. The learning from this work might help other countries to adopt or adapt the approach to fit with their national systems, and collectively achieve a global incentive to reinvigorate the antibacterial pipeline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is an inadequate pipeline of new antibacterials due to a ‘market failure’ caused by low volumes of prescribing at market entry. This is due to appropriate stewardship, lack of adequate evaluation of benefits and low levels of reimbursement. The potential cost of inaction is high. |
An enhanced health technology assessment (HTA) can more fully capture the value of new antibacterials, and can inform reimbursement delinked from volume of sales, but is challenging and subject to significant uncertainty. |
To reinvigorate the antibacterial pipeline, countries outside the UK should consider novel evaluation and reimbursement mechanisms to encourage the antibacterial development pipeline. |
1 Background
Antimicrobial resistance (AMR) is a global health crisis, estimated to have caused at least 1.27 million premature deaths globally in 2019 [1]. Multi-drug-resistant bacteria can spread rapidly, in both hospital and community settings, resulting in severe infections such as pneumonia and sepsis, that can often be fatal [2]. The World Health Organisation (WHO) maintains a list of ‘priority pathogens’ for which new antibiotics are urgently needed due to multi-drug resistance [3] with the aim of encouraging research. Yet, the number of new antibiotics currently in clinical development remains inadequate. In 2020, there were only 41 antibiotics being studied in clinical trials [4], whereas in the same year, there were approximately 1784 products in immune-oncology Phase I–III trials alone [5].
The inadequate pipeline of new antibacterials is the result of a market entry failure where new antibacterials tend to be used as little as possible to prevent rapid emergence of resistance. As a result, manufacturers cannot recoup development costs quickly as sales of their drug will likely remain low in the first years after market entry. This market entry failure is an accepted phenomenon internationally at the G7, UN High Level Meeting. It is summarised neatly in Wellcome Trust-commissioned report by Boston Consulting Group [6]. The low volume of sales has meant that several manufacturers with antibacterial pipelines (Melinta, Achaogen, Entasis) have filed for bankruptcy or merged under extreme financial pressure, delivering a significant financial loss to their R&D investors [7]. Some manufacturers have decided not to pursue European marketing authorisations for new antibacterials or have chosen not to launch commercially in many high-income European countries despite a marketing authorisation from European Medicines Agency (EMA). Therefore, current market mechanisms for pricing and reimbursement of drugs are not sufficient to stimulate the development of new antibacterials, in contrast to oncology, for example, where profitability is quite significantly higher (reference Wellcome Trust BCG report).
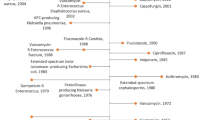
The O’Neill report, commissioned by the UK government and published in 2016, called for “push” incentives to fund early development of new antibacterials, and for “pull” incentives at market entry that delink payments to manufacturers from volume of sales, allowing appropriate stewardship alongside reimbursement to incentivise the development of antibacterials [8]. In response to these recommendations, the UK government set up an AMR working group comprising the Department of Health and Social Care and the British pharmaceutical industry. A subcommittee was established that explored ways in which to develop new reimbursement mechanisms that would help reinvigorate the antibacterial pipeline. This paper describes the resulting UK project to test a new model for evaluating and purchasing new antibacterials, which was initiated in 2019 and completed with delinked payments for the two products, based on the novel evaluation, commencing on 1st July 2022 (see Fig. 1).
2 Market Challenges for New Antibacterials in the UK
There are three main marketing challenges faced by developers of new antibacterials. First, currently available antibacterials are generally relatively cheap, and the majority of those used in clinical practice are generics. New antimicrobials will be competing with alternatives, which have much lower prices. This poses a challenge, particularly because we lack the rapid diagnostics to identify in a timely manner which patients require the new drug—information that could be used to justify a higher price for the new drug. Without that information, the comparator drug is almost always an inexpensive generic, adding to the difficulty of illustrating cost effectiveness.
Second, antimicrobials tend to be prescribed for a short period of time to treat an infection, as opposed to treatments for chronic conditions that are typically prescribed for long periods of time, meaning low sales volume. Third, new antimicrobials are usually held back, or stewarded, after arrival to market to reduce the chance of early emergence of resistance. The traditional pharmaceutical model of recouping investment through volume of prescribing in the first few years after market entry therefore does not work for new antimicrobials.
Antibacterials have not been subjected to health technology assessment (HTA) processes in the UK, with the exception of inhaled powder formulations of colistin and tobramycin, which have been recommended by the National Institute for Health and Care Excellence (NICE) [9]. In the UK context it has generally been considered that new antibacterials will find their place in the clinical pathway without HTA, and data on new antibacterials tend to be non-inferiority data, one of several factors making HTA of antibacterials challenging. Most studies of novel antibacterials are non-inferiority trials against standard care, which make it difficult to assess the added value of the new drug [10]. In addition, unmet need tends to be greatest for severe infections due to multi-drug-resistant organisms. Performing randomised trials in this patient population is challenging, both from a practical point of recruiting patients but also ethically as one cannot compare a new drug to an existing agent to which the pathogen is not sensitive (same reference as previous). As a result, drugs are unlikely to be licensed in the area where the unmet need is greatest, because of the lack of data on efficacy required for licensing. Furthermore, new antibacterials tend to have a marketing authorisation that covers a wide range of infections where the drug is efficacious, but it is difficult to build health economic models for such heterogeneous populations, which poses a challenge for HTA.
3 Recognising the Full Value of Antibacterials
Standard approaches to HTA focus on establishing the expected health benefits and costs of a new drug for each patient eligible for treatment. Antibacterials might generate value of relevance to health systems beyond the benefit to treated patients, which relates to the emergence of AMR. Collectively, these benefits have recently been referred to using the acronym “STEDI”, which stands for spectrum, transmission, enablement, diversity, and insurance value (Table 1). For example, having antibacterials that are effective against multi-drug–resistant pathogens will not only allow health systems to continue to treat a range of infections effectively, but will enable a range of other procedures—such as operations and chemotherapy—to continue to take place even if AMR increases. Furthermore, having a wider range of antibacterials available reduces pressure on the use of existing antibacterials and could also slow down emerging resistance against those existing antibacterials. The way in which AMR develops is difficult to predict and therefore we need a range of effective treatment options to deal with outbreaks of AMR in the future.
A report commissioned by NICE and the UK Department of Health and Social Care (DHSC), published in 2018 assessed how current HTA methodology might need to be adjusted to assess the value of new antibacterials [12]. The report concluded that it is challenging, but not impossible, to capture the full value of new antibacterials. There is high uncertainty about how antibacterial usage might change over time, which is required to model ways in which resistance to new and existing antibacterials might develop and change. Furthermore, there are evidential challenges around the use of non-inferiority trials and a lack of trials in multi-drug-resistant infections. As a result, there is a need to rely on pre-clinical and non-clinical data including in vitro susceptibility studies (in which a bacterial sample from a patient is cultured along with increasing concentrations of the antibacterial to determine how well the antibacterial slows growth) and pharmacokinetic and pharmacodynamic data, which is not routinely done in HTA. Reducing the uncertainty in these datasets may require using other non-traditional evidence sources such as structured expert elicitation.
4 Models for the Evaluation and Purchase of Antibacterials
In January 2019, the UK Secretary of State for Health announced that the UK would start a project to develop and test novel methods to assess the full value of new antibacterials, and a new mechanism of reimbursement that delinks manufacturer income from sales volumes. The project formally launched in July 2019 [14,15,16,17,18,19].
The project selected 2 antibacterials to test the new methodology and purchase arrangements: one existing antibacterial and one new-to-market antibacterial. The project was jointly led by NICE and NHS England and NHS Improvement (NHSE&I), and the project team also had members from the Department of Health and Social Care (DHSC).
5 Selection of the Antimicrobials Included in the Project: Competitive Tendering Process
Given the competitive nature of selecting two products for contracts with NHSE&I, the UK project needed to comply with public contracts regulations 2015 legislation (PCR2015). The competitive dialogue process (competitive dialogue (CD) is a public sector procurement tendering procedure whereby you enter into dialogue with suppliers until you find a solution that meets the needs of your organisation. It is ideal for complex and high-risk solutions where there are gaps in requirements, outcomes, contract or commercial arrangements) was felt to be the model best suited to this project and the invitation to participate in the tendering process was advertised in June 2020 in the official journal of the EU. Interested manufacturers then went through a qualification and selection process, which resulted in the selection of 2 branded antibacterial products. Companies were free to propose their product or products once it (they) met the qualification criteria.
After the qualification stage, which assessed whether the manufacturers were eligible to participate, a dialogue phase took place to ensure that all companies fully understood the project’s processes and requirements, including the product selection criteria, evaluation process, methods for the adapted HTA, approach to the commercial discussion, and components and requirements of the final payment contracts. This was also an opportunity to discuss the benefits and likely clinical use of the antibacterial products that had qualified for consideration. Companies were invited to submit questions between the formal meetings. Once the dialogue process was completed the companies were invited to submit their final tender applications, which were assessed by an expert panel comprising representatives from NICE, NHSE&I, the UK government’s Advisory Committee on Antimicrobial Prescribing, Resistance and Healthcare Associated Infection (APRHAI), Public Health England (PHE), The British Society for Antimicrobial Chemotherapy (BSAC) and members of the clinical community. Each member of the panel independently scored each company submission against the selection criteria, and then met to ensure consistency of scoring and make the final selection of the two products to participate in the project.
The selection criteria had three components: clinical, non-clinical and cost [14,15,16,17,18,19]. With only two slots available in the project, one for an existing antibacterial and one for a new-to-market antibacterial, it was important to focus on antibacterials active in the clinical areas with the highest unmet need. Therefore, the scoring system was weighted so that the most important decider in a product being selected was the clinical value of the product to the NHS. Unmet need was defined as activity against priority pathogens identified by the WHO [3], including carbapenem-resistant Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacteriaceae; performance against key determinants of antimicrobial resistance; the severity of clinical setting; and specific areas of unmet need in the UK setting. The UK English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) report [20] highlighted increasing numbers of bloodstream infections due to resistant Gram-negative bacteria, and the WHO pipeline report [21] also highlighted the poor antimicrobial pipeline in the area of resistant Gram-negative infections, with particular emphasis on a dearth of products active against metallo-beta-lactamase-producing organisms. The key starting point therefore was to select two products active against resistant Gram-negative infection.
Other selection criteria included the degree of novelty of the product, surety of supply, antimicrobial stewardship and manufacturing practices, antimicrobial surveillance, and cost. Cost was mandated to be included as part of the PCR2015 regulations in the following way; companies could bid at or below the £10 million/product/annum cap at the point of application. If a company bid below the £10 million cap there was a modest points gain from that in the scoring system.
Using this competitive tendering process, by December 2020 the products selected for evaluation were cefiderocol (manufactured by Shionogi) as the new-to-market product and ceftazidime-avibactam (manufactured by Pfizer) as the existing product. Documents explaining the qualification and selection process, including full details of the scoring criteria, evaluation framework, commercial framework, and a draft payment contract, are available from the NICE website [22]. The specific selection criteria and scoring system are detailed in ESM Appendix 1.
6 HTA Evaluation & NICE Committee Review
The project used an adapted HTA, which aimed to estimate the total value of the antibacterial for its entire lifecycle to the NHS in England. Health economic modelling, expert elicitation, and qualitative evidence were all used to estimate the long-term value of the antibacterial.
The evaluation was co-ordinated by NICE, using a process that closely mimics NICE’s standard technology appraisal process for pharmaceuticals. However, the methods and outcome of the evaluation are modified to account for the unique situation of antibacterials [14, 15]. Under the existing technology appraisal approach, NICE’s independent committee uses an incremental cost-effectiveness ratio (ICER) to determine whether the drug provides value for money to the NHS, based on a price proposed by the manufacturer. The committee give a positive or negative recommendation on whether the drug should be used in the NHS. By contrast, the output of this adapted HTA was population incremental net health benefits (INHB) of the antibacterial, expressed in quality-adjusted life-years (QALYs), with the price of the drug set to £0 in the analysis. The QALY values were determined at a patient level, then extrapolated to the wider population estimated to appropriately receive the treatment. The QALY calculations included consideration of emergence of resistance to the two agents, but was unable to assess potential downside of ‘bystander resistance’ due to their broad spectrum due to a lack of any evidence to support this potential impact, expected usage, and where possible the STEDI additional attributes of value.
The NICE committee reviewed the HTA reports, assessing whether population estimates and other parameters of value were fully captured by the HTA. The committee agreed on what they considered to be the most plausible estimate of INHB, which represents the total potential value of the drug to the NHS. However, the committee considered that the INHB was underestimated by the HTA due to under-sizing of the potential population appropriate to receive the two products, and what was felt to be an underestimate of the enablement, diversity and insurance benefits brought to the health system by the two products.
The NICE committee reports, published as final guidance on the NICE website [16, 17], formed the starting point of the commercial discussions between NHSE&I and each manufacturer, in which the total value of the antibacterial was translated into an annual payment that will be made to the manufacturer.
The reimbursement of the products in this project is via a delinked payment contract. Each manufacturer was offered, and signed, a contract for an initial 3-year period, with the option of extending for up to 10 years, during which they will receive a fixed, annual payment for the use of their product within the NHS, which will be made irrespective of the amount of antimicrobial that is actually used. The NICE committee concluded on the total value of each antimicrobial product over its lifetime, expressed as INHB in QALYs, and also advised that no less than 60 % of that value should be rewarded during the 10-year contract period. The INHB in QALYs over a 20-year lifespan allocated to each product (8880 QALYs for ceftazidime-avibactam and 16,200 QALYs for cefiderocol), if one used a standard cost per QALY of £20,000, would easily justify the maximum payment of £10 million per product per annum.
6.1 Maximum Contract Value
As part of the tender process, a maximum contract value had to be defined. In deriving the maximum payment threshold, the UK team took account of the available literature on the level of global sales that would be needed for antibacterials to become attractive investment propositions and considered £10 million per annum for each antibacterial to be a reasonable “fair share” for England. The ‘fair share’ was calculated based on published estimates of what would be a reasonable ‘pull incentive’ given development costs, then the UK share of the global market was estimated to be 2–3%. The figure of £10 million/product/annum is consistent with this. If G20 countries rolled out payments based on their percentage share of the global market, this would act as a significant global pull incentive, even without low- and middle-income countries (LMICs) making a contribution, and facilitates companies working with organisations such as the Global Antibiotic Research and Development Partnership (GARDP) to facilitate access of their products to LMICs. Facilitating access for LMICs is also a component of the AMR benchmarking carried out by the Access to Medicines Foundation at regular intervals.
Based on the current NHS England spend data on antibacterials, the project team are confident that £10 million per annum for each antibacterial represents a significantly higher level of reimbursement than companies would receive from NHS England under the current arrangements. Various commentators (representing funders of antibacterial research and development, industry, health system, clinical and academic community) at the stakeholder webinars held at multiple points during the UK project (to share progress and seek feedback) have also emphasised the important ‘signal’ sent to funders of research and development, academics and companies involved in antibacterial research, and health systems by this ‘novel’ approach taken by the UK. The ‘signal’ is felt to be greater than the monetary amounts awarded by this project to the two companies, i.e., maximum of £10 million/product per year. The maximum payment threshold (and all other terms in the procurement documents) apply specifically to the current project. Learnings from the project, including the value estimates from the HTA, will inform future arrangements for the valuation and purchase of antibacterials.
7 Discussion
This ground-breaking UK project of ways to pay for antibacterials based on a more holistic value assessment with payments delinked from volume of sales will not solve the antibacterial pipeline issue on its own, as the UK accounts for only 2–3% of the global pharmaceutical market. Other countries, including the USA, Sweden and Germany have acknowledged that reimbursement for antibacterials needs to change if we are to have a robust and sustainable pipeline of new antibacterials. The PASTEUR Act re-introduced in 2021 in the US Congress [23], if passed by the US legislature, will also deliver payments for novel antibacterials delinked from volume of sales. Sweden has implemented a revenue guarantee for 5 antimicrobials active against priority 1 pathogens on the WHO priority pathogen list, to ensure these products are available in Sweden [24]. Germany and France have both developed an adjusted reimbursement system for antibacterials allowing larger payment to companies [25]. This UK project will inform the future approach to reimbursement of antibacterials in the UK, but countries other than the USA, Sweden and Germany need to consider ‘pull’ incentives if a more global incentive is to be achieved [26].
It is worth reflecting on some key challenges with the project. The first key step was getting political, health system and industry support for the UK approach. There were initial misconceptions that the project set out to reward big pharma. Significant work was required to explain the ‘broken market’ for new antibacterials and the basis for this (low sales due to stewardship, short duration of usage, low price due to often mainly generic comparators, and inadequate assessment of the value of new antibacterials). The second challenge was to understand how to adjust HTA methodology to more adequately assess the additional attributes of value brought to the health system by new antibacterials, which took 18 months of work by academic experts in health economics. The third challenge was to develop a set of selection criteria and a robust and transparent scoring system to choose two antibacterials for this new approach. The fourth challenge was the complexity of the novel HTA, which took 12 months, and reflected the enormous range of clinical scenarios and pathogens relevant to the two antibacterials under evaluation, variability in standard care in terms of comparators, uncertainty around likely levels of usage and emergence of resistance to new and existing antibacterials. There were also significant difficulties in understanding how to assess the STEDI additional attributes of value and quantify these where possible.
The key enablers, which ultimately facilitated the success of this project, were as follows; The Lord O’Neill report on AMR, positive UK Government response to the O’Neill report [8], advocacy by the former chief medical officer (CMO) of England Professor Dame Sally Davies, who is the current UK AMR Envoy, a very active UK Department of Health and Social Care (DHSC) Global AMR team, early engagement with and support from the Association of the British Pharmaceutical Industry (ABPI) and individual industry companies, international encouragement and support (including from WHO, UN among others).
Throughout the project, the project team has engaged with national and international stakeholders to ensure wide awareness of the aims and progress of this project. This has been facilitated through several stakeholder webinars and numerous engagement meetings with relevant individuals, groups, organisations, and governments. This is a crucial time for an international collaborative attempt to fix the broken antibacterial market and reinvigorate the antibacterial pipeline. The UK alone cannot fix this, and we encourage other countries to find ways to give some certainty to antibacterial developers for income after market entry if the ‘broken market’ is to be fixed.
Currently, the antibacterial pipeline lacks novel products, with particular gaps around resistant Gram-negative infection [27]. Ideally, the international community need to define collectively the target product profiles that will be prioritised for novel assessment and reimbursement approaches going forward. This will help investors, developers, regulators, health system payers and governments to prioritise their approach to AMR in general and will facilitate development of novel reimbursement mechanisms to incentivise the antibacterial pipeline. A recent European initiative with in-depth interviews with multi-country stakeholders [28, 29] emphasised that countries broadly support the concept of pull incentives for antibacterials, but they are uncertain of how to value new antibacterials, and they are unclear about how to prioritise new antibacterials suitable for a ‘subscription’ or ‘pull incentive’ approach. There could also be other therapeutic areas where this approach is justifiable.
There has been some criticism by Glover et al of the idea of reimbursement for antibacterials delinked from volume of sales [30]. The authors emphasise the need to ‘avoid any risk of moving from an incentive scheme for new antibiotics towards a broader taxpayer funded grant state for big multinational drug companies’. The UK project is not aiming to fund the development of new antibacterials, but rather aims to pay manufacturers who successfully bring a product to market for the value their product brings to the NHS. We agree that simply giving the pharmaceutical industry a taxpayer-funded grant or subsidy will not resolve the problems. Instead, the UK project looked to address the current inadequate antibacterial pipeline resulting from ‘market entry failure’ (contributed to by low usage in the years following launch and relative inability of current HTA-based systems to fully evaluate antibacterial products) by delivering a novel evaluation, which in turn informed reimbursement delinked from volume of sales. In addition, most large pharmaceutical companies have moved away from antibacterial development, and most companies currently in the antibacterial development space are small-to-medium enterprises (SMEs) with significant financial insecurity [31,32,33]. This, and the bankruptcies of several antibacterial-producing companies in recent years, suggest that pull incentives are not golden tickets for wealthy companies. Glover et al argue that antibacterial development should be publicly funded. Abandoning private enterprise and relying exclusively on a public funding system is unnecessary at present and could be dangerous—why abandon the highly expert networks of global drug discovery and development and replace it with an entirely untested system with radically different incentives [34]?
Rex and Outterson have stated that we need to think of antibacterials as the ‘fire extinguishers’ of medicine [35]. The COVID-19 pandemic and subsequent vaccination processes have driven home the power of proactive preventive approaches to infectious disease. Globally, we need to all work together to find a new way to pay for antibacterials, not necessarily by identical mechanisms to the UK approach, but perhaps tailored to different health systems in order to achieve a global pull incentive to drive antibacterial innovation.
References
Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022;399:629–55.
WHO. WHO publishes list of bacteria for which new antibiotics are urgently needed. News release. 2017. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed 29 Nov 2021.
WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf.
PEW. Tracking the Global Pipeline of Antibiotics in Development, March 2021. Issue Br. 2021. https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2021/03/tracking-the-global-pipeline-of-antibiotics-in-development. Accessed 29 Nov 2021.
Upadhaya S, Yu A. Immuno-Oncology Landscape. Cancer Res. Inst. 2020. https://www.cancerresearch.org/scientists/immuno-oncology-landscape. Accessed 1 Apr 2022.
Wellcome Trust discussion paper. The growing crisis in antibiotic R&D. Opportunities for G20 member action to support sustainable innovation. February 2020. https://wellcome.org/sites/default/files/the-growing-crisis-for-antibiotic-r-and-d.pdf. Accessed Dec 2022.
Årdal C, Balasegaram M, Laxminarayan R, et al. Antibiotic development—economic, regulatory and societal challenges. Nat Rev Microbiol. 2020;18:267–74.
O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf.
National Institute for Health and Care Excellence (NICE). Colistimethate sodium and tobramycin dry powders for inhalation for treating pseudomonas lung infection in cystic fibrosis. 2013. https://www.nice.org.uk/guidance/ta276/chapter/1-Guidance.
Rex JH, Fernandez LH, Cohen IG, et al. Designing development programs for non-traditional antibacterial agents. Nat Commun. 2019;10:3416.
Karlsberg Schaffer S, West P, Towse A, et al. Assessing the Value of New Antibiotics: Additional Elements of Value for Health Technology Assessment Decisions. London; 2017
Rothery C, Woods B, Schmitt L, Claxton K, Palmer S, Sculpher M. Framework for value assessment of new antimicrobials. Policy Res Unit Econ Eval Heal Care Interv 2018;178. http://www.eepru.org.uk/wp-content/uploads/2017/11/eepru-report-amr-oct-2018-059.pdf.
Outterson K, Rex JH. Evaluating for-profit public benefit corporations as an additional structure for antibiotic development and commercialization. Transl Res. 2020;220:182–90.
National Institute for Health and Care Excellence (NICE). Models for the evaluation and purchase of antimicrobials. 2021. https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials. Accessed 29 Nov 2021.
NHS England and NHS Improvement (NHSE&I), National Institute for Health and Care Excellence (NICE). Annex 7 : health technology assessment process. 2021. https://www.nice.org.uk/Media/Default/About/what-we-do/Life-sciences/evaluation-framework.pdf. Accessed 1 Apr 2022.
National Institute for Health and Care Excellence (NICE). Ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections. 2022. https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials/ceftazidime-with-avibactam. Accessed 1 Apr 2022.
National Institute for Health and Care Excellence (NICE). Cefiderocol for treating severe aerobic Gram-negative bacterial infections. 2022. https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials/cefiderocol. Accessed 1 Apr 2022.
NHS England and NHS Improvement (NHSE&I), National Institute for Health and Care Excellence (NICE). Annex 3: Award questions and criteria. 2021. https://www.nice.org.uk/Media/Default/About/what-we-do/Life-sciences/award-questions-criteria-annex3.pdf. Accessed 1 Apr 2022.
NHS England and NHS Improvement (NHSE&I), National Institute for Health and Care Excellence (NICE). Invitation to submit final tender. 2021. https://www.nice.org.uk/Media/Default/About/what-we-do/Life-sciences/invitation-to-submit-final-tender.pdf. Accessed 1 Apr 2022.
Ashiru-Oredope D, Casale E, Ahmad A, Hoopkins S, Johnson A, Guy R. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR): Report 2019 to 2020. Public Heal Engl 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/936199/ESPAUR_Report_2019-20.pdf.
Antimicrobial Resistance Division, Partnership GC and. 2020 Antibacterial Agents in Clinical and Preclinical Development. 2020 https://www.who.int/publications/i/item/9789240021303.
National Institute for Health and Care Excellence (NICE). Models for the evaluation and purchase of antimicrobials - product selection documents. 2022. https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials. Accessed 1 Apr 2022.
Bennet M. Bennet, Young, Doyle, Ferguson Introduce PASTEUR Act to Fight Antimicrobial Resistance. Press Releases. 2021. https:www.bennet.senate.gov/public/index.cfm/2021/6/bennet-young-doyle-ferguson-introduce-pasteur-act-to-fight-antimicrobial-resistance. Accessed 29 Nov 2011.
Public Health Agency of Sweden. Availability of antibiotics. December 2020. https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/antibiotics-and-antimicrobial-resistance/availability-of-antibiotics/. Accessed Dec 2022.
Gotham D, Moja L, van der Heijden M, Paulin S, Smith I, Beyer P. Reimbursement models to tackle market failures for antimicrobials: approaches taken in France, Germany, Sweden, the United Kingdom, and the United States. Health Policy (New York). 2021;125:296–306.
G7 Germany (2022). G7 Health Ministers’ Communiqué. https://www.g7germany.de/resource/blob/974430/2042058/5651daa321517b089cdccfaffd1e37a1/2022-05-20-g7-health-ministers-communique-data.pdf
WHO. 2019 Antibacterial Agents in Clinical Development - an analysis of the antibacterial clinical development pipeline. 2019 file:///C:/Users/MWobbe/Downloads/9789240000193-eng.pdf.
Årdal C, Lacotte Y, Ploy MC. Financing Pull Mechanisms for Antibiotic-Related Innovation: Opportunities for Europe. Clin Infect Dis. 2020;71:1994–9.
Årdal C, Lacotte Y, Edwards S, Ploy MC. National facilitators and barriers to the implementation of incentives for antibiotic access and innovation. Antibiotics. 2021;10:1–9.
Glover RE, Manton J, Willcocks S, Stabler RA. Subscription model for antibiotic development. BMJ. 2019. https://doi.org/10.1136/bmj.l5364.
Theuretzbacher U, Gottwalt S, Beyer P, et al. Analysis of the clinical antibacterial and antituberculosis pipeline. Lancet Infect Dis. 2019;19:e40-50.
Harbarth S, Theuretzbacher U, Hackett J. Antibiotic research and development: business as usual? J Antimicrob Chemother. 2015;70:1604–7.
Theuretzbacher U, Outterson K, Engel A, Karlén A. The global preclinical antibacterial pipeline. Nat Rev Microbiol. 2020;18:275–85.
Outterson K, Rex JH. Evaluating for-profit public benefit corporations as an additional structure for antibiotic development and commercialization. Translational
Rex JH, Outterson K. Antibiotic reimbursement in a model delinked from sales: a benchmark-based worldwide approach. Lancet Infect Dis. 2016;16:500–5.
Acknowledgements
The UK project has been supported by the NHS England and NHS Improvement, the National Institute for Health and Care Excellence and the Department of Health and Social Care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributors
CL conceived the idea for the paper, CL wrote the first and final draft. JB, SC, DG, MP, NC and MW reviewed and edited the draft. All authors read and approved the final draft.
Conflict of interests/competing interest
We declare no competing interests.
Funding
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patient/participants)
Not applicable.
Availability of data and material
All project documents and reports available on NICE website.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Leonard, C., Crabb, N., Glover, D. et al. Can the UK ‘Netflix’ Payment Model Boost the Antibacterial Pipeline?. Appl Health Econ Health Policy 21, 365–372 (2023). https://doi.org/10.1007/s40258-022-00786-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-022-00786-1