Abstract
Background
Larotrectinib is the first tumour-agnostic therapy that has been approved by the European Medicines Agency. Tumour-agnostic therapies are indicated for a multitude of tumour types. The economic models supporting reimbursement submissions of tumour-agnostic therapies are complex because of the multitude of indications per model.
Objective
The objective of this paper was to evaluate the cost effectiveness of larotrectinib compared with standard of care in patients with cancer with tropomyosin receptor kinase fusion-positive tumour types in the Netherlands.
Methods
A previously constructed cost-effectiveness model with a partitioned survival approach was adapted to the Dutch setting, simulating costs and effects of treatment in patients with tropomyosin receptor kinase fusion-positive cancer. The cost-effectiveness model conducts a naïve comparison of larotrectinib to a weighted comparator standard-of-care arm. Dutch specific resource use and costs were implemented and inflated to reflect 2019 euros. The analysis includes a lifetime horizon and a societal perspective.
Results
Larotrectinib versus Dutch standard of care resulted in 5.61 incremental (QALYs) and €232,260 incremental costs, leading to an incremental cost-effectivenes ratio of €41,424/QALY. The probabilistic sensitivity analysis reveals a 88% chance of larotrectinib being cost effective compared with the pooled comparator standard-of-care arm at the applicable €80,000/QALY willingness-to-pay threshold in the Netherlands.
Conclusions
The incremental cost-effectivenes ratio was well below the applicable threshold for diseases with a high burden of disease in the Netherlands (€80,000). At this threshold, larotrectinib was estimated to be a cost-effective treatment for patients with tropomyosin receptor kinase fusion-positive cancer compared with current standard of care in the Netherlands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first ever cost-effectiveness analysis of a tumour-agnostic therapy to be conducted for the Netherlands. The analysis was performed from a full societal perspective, including indirect medical costs, productivity costs and costs for informal care. |
Tumour-agnostic indications require modelling across multiple tumour localisations, each with their own parameters, assumptions and uncertainties. This paper discusses the complexities of modelling cost effectiveness for tumour-agnostic therapies. |
Larotrectinib was estimated to be a cost-effective treatment for patients with tropomyosin receptor kinase fusion-positive cancer compared with current standard of care in the Netherlands. |
1 Introduction
Larotrectinib is the first tumour-agnostic therapy that has been approved by the European Medicines Agency (EMA). Larotrectinib is registered as a monotherapy for the treatment of adult and paediatric patients with solid tumours that display a neurotrophic tyrosine receptor kinase (NTRK) gene fusion, who have a disease that is locally advanced or metastatic, or where surgical resection is likely to result in severe morbidity and who have no satisfactory treatment options [1]. NTRK gene fusions have been shown to be oncogenic drivers [2, 3], and are responsible for tumour growth, regardless of cancer type. The different cancer types are heterogeneous apart from one important similarity: the NTRK gene fusion. The target of action for larotrectinib is the tyrosine receptor kinase (TRK) family of proteins including TRKA, TRKB and TRKC, which are encoded by the NTRK1, NTRK2 and NTRK3 genes, respectively. Larotrectinib was studied in several basket trials [1]. A basket trial’s population consists of patients with the same genomic mutation or biomarker who all receive the same treatment. Basket trials generally do not include a comparator arm.
Tumour-agnostic therapies bring forward a new promising approach to treat cancer. However, challenges exist in terms of how these therapies are assessed for effectiveness, cost effectiveness and, subsequently, reimbursement. Tumour-agnostic drugs are indicated for a multitude of cancer types, provided they express the mutation. Historically speaking, oncological medication has always been assessed on a cancer-specific basis and not based on the underlying mutation occurring in almost all cancer types. Moreover, although in some rare cancers the incidence of NTRK fusions is high, in common cancers, the incidence is very low (0.5%), meaning that clinical evidence informing reimbursement decisions for tumour-agnostic drugs is based on studies with small sample sizes, usually without a control group, and the patient population across the different tumour localisations and lines of therapy is heterogenous. This makes it difficult to assess whether the drug will provide value for money against standard of care (SoC), as a directly comparable SoC currently does not exist. Namely, current treatment is still cancer specific and not pan-agnostic solely based on an underlying mutation. [4] Moreover, the economic models supporting reimbursement submissions are complex, facing challenges in terms of, for example, model structure, choice for comparator(s) and clinical inputs.
The aim of this study was to assess the cost effectiveness of larotrectinib in the Netherlands in the registered indication, from a societal perspective [5]. Furthermore, a description is given of key reimbursement challenges for tumour-agnostic therapies in general, and larotrectinib in particular. This is the first cost-effectiveness model (CEM) evaluating a tumour-agnostic indication from a societal perspective, using a weighted combination of different cancer types in the comparator arm. These include breast, colorectal, melanoma, non-small cell lung cancer (NSCLC), pancreas, primary central nervous system (CNS), salivary gland, small cell lung cancer (SCLC), thyroid cancer, and one location gathering all paediatric tumours. Various assumptions were necessary to estimate the cost effectiveness, as is described in this paper.
2 Methods
2.1 Model Structure
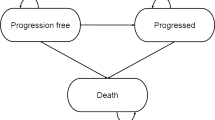
A global economic model was adapted to the Dutch setting to estimate lifetime outcomes associated with larotrectinib treatment or with Dutch SoC in the population of interest [6, 7]. The economic model is a cohort state-transition model with a partitioned survival approach. This technique is commonly used in late-stage/metastatic oncology modelling, and is appropriate for capturing progressive chronic conditions that are described with clinical outcomes requiring an ongoing time-dependent risk, such as progression and death. The model includes three health states: progression-free survival (PFS), progressive disease and death. In the intervention arm, patients progress through health states based on outputs from the single-arm larotrectinib basket trials [1]. For modelling survival in the weighted comparator SoC arm, efficacy inputs from naïve comparisons based on a targeted review of the literature (i.e. a non-systematic search in PubMed in 2019) for each of the ten comparators that are included as SoC (i.e. nine adult tumour locations and one location gathering all paediatric tumours) were considered. These were then weighted based on the distribution of patients across the tumour locations (Table 1), to form one weighted comparator arm (Fig. 1). The model uses a 7-day cycle length (1 week), capturing the varying treatment patterns and differences in survival of the numerous comparators that are included within the model (SoC treatment specific to each tumour location). Health outcomes and costs are accrued and summed for each arm of the economic model.
Both the larotrectinib and SoC arms of the model follow the same health states. However, health states are stratified by tumour site to account for differences in conventional SoC across tumour sites in the SoC arm. This means that the intervention arm is based on efficacy inputs from the pooled analysis of the larotrectinib clinical trial programme and cost elements associated with larotrectinib treatment and the SoC arm is based on cost and efficacy inputs per tumour localisation.
The model estimates the cost effectiveness over a lifetime period. A time horizon of 80 years was implemented in order to ensure enough weekly cycles (i.e. 4159 weekly cycles) to accommodate at least 99% of patients modelled in each treatment arm to eventually transition into the ‘death’ health state. This approach is considered appropriate, given that larotrectinib is associated with reduced mortality and expected long-term survivors and the model deals with paediatric patients who could remain in the model over a long time period. These data cannot be acquired directly from the clinical studies [1]; hence, a combination of clinical data and model extrapolations were required. For more information on this extrapolation method, see Appendix 2 of the Electronic Supplementary Material (ESM).
2.2 Study Population and Comparators
The patient population in the economic model reflects the registered EMA indication: adult and paediatric patients with solid tumours that display a NTRK gene fusion, who have a disease that is locally advanced or metastatic or where surgical resection is likely to result in severe morbidity and who have no satisfactory treatment options [1]. For the larotrectinib arm, this is the pooled analysis of two analysis sets: the analysis sets in solid tumours excluding primary CNS tumours (n = 93), describing non-CNS primary tumours, and the analysis set in solid tumours including primary CNS tumours (n = 9), describing CNS primary tumours. Together these analysis sets make up n = 102 patients. This is the same pooled data on which EMA authorisation was based. The baseline characteristics of these patients are presented in Appendix 1 of the ESM. The mean age was 5 years for children and 53 years for adults. Overall, 53% of patients were male (based on the clinical trial population). Furthermore, 19.6% had locally advanced disease and 75.5% had metastatic disease. In addition, 46% had an Eastern Cooperative Oncology Group performance status of 0, 43% had an Eastern Cooperative Oncology Group performance status of 1 and 11% had an Eastern Cooperative Oncology Group performance status of 3. Patients enrolled in the larotrectinib clinical trial programme were heavily pre-treated (79.5% of patients receiving one or more prior systemic therapies and 32% of patients receiving more than three prior systemic therapies). Approximately 20% of patients were enrolled who had not responded to previous therapies but did not qualify for conventional therapy. For example, where the patient’s disease stage or severity (i.e. risk of amputation) would have rendered approved therapies ineffective [1].
In the model, larotrectinib is compared with a weighted SoC arm consisting of various tumour localisations (colorectal, NSCLC, melanoma, primary CNS, thyroid, SCLC, breast, pancreas, salivary gland and paediatric). Note that the clinical trial programme included more than 15 different cancer types. However, cancer types from the clinical trial programme of larotrectinib, of which fewer than three patients are expected per year in the Netherlands, are excluded from the CEM. The excluded tumour localisations comprise cholangio-carcinoma, soft-tissue sarcomas (including bone sarcomas; in adults), appendix cancer and gastrointestinal stromal tumour. Although the weighting according to the tumour localisations from the larotrectinib clinical trial programme was not fully in accordance with the weighting found in Dutch clinical practice, it is used in the base-case analysis, as it reflects the weighting that informs the clinical efficacy and safety of larotrectinib (Table 1). A scenario analysis has been included in which the weighting of the SoC arm is based on Dutch clinical practice. The rate of TRK fusion-positive tumours per cancer type was based on a systematic literature review [8].
Evidence presented for the weighted comparator arm reflects Dutch SoC in the same line of treatment as the expected positioning of larotrectinib in the treatment algorithm per tumour localisation based on expert opinion (through an advisory board and expert interviews with eight participants). Comparative treatment was chosen based on current guidelines and expert opinion and the expected location of larotrectinib within the treatment algorithm of the tumour localisationFootnote 1. These comparators are shown in Table 2 and were validated by clinical experts. The clinical inputs for the comparator arm were sourced from the literature and implemented in the model using the Kaplan–Meier survival curves and digitisation software (Plot Digitizer version 2.1). For a full description of this method, see Appendix 2 of the ESM.
2.3 Model Inputs
Treatment costs and effects were evaluated using the societal perspective, as requested by the Dutch National Health Care Institute [5]. Dutch health-related quality of life (HRQoL) inputs and costs (e.g. indirect medical costs, productivity costs and costs for informal care) were specific to the Dutch setting. Costs and effects were discounted by 4% and 1.5%, respectively. Furthermore, an expected value of perfect information (EVPI) analysis was implemented. Model inputs were validated by Dutch clinical experts in oncology treatment.
2.3.1 Clinical
For the intervention arm (larotrectinib), the clinical inputs of interest are sourced from the clinical studies in the clinical trial programme: LOXO-TRK-14001, SCOUT and NAVIGATE trials [1]. See Appendix 1 of the ESM for an overview of these studies, the number of patients, and the tumour types included. To populate the CEM, parametric curves were fitted to the clinical data from the clinical study. For larotrectinib, the Weibull function was chosen as the most appropriate fit for both PFS and overall survival (OS). The tables supporting this decision are presented in Appendix 2 of the ESM (in addition to the Akaike Information Criteria). Clinical plausibility was considered as well. It was decided to set by default PFS and OS for larotrectinib to the Weibull function, in order to reflect clinical plausibility and allow a change in hazard with ageing. The comparison of larotrectinib is made against a comparator arm, which consolidates the efficacy inputs for each of the tumour locations. The PFS and OS curves specific for each tumour location were fitted and their parameters were fed into the model. The resulting curves were then weighted following the representation of each of the same tumour locations in the larotrectinib clinical study programme (Table 1). Together they make up one comparator arm, weighted for the various tumour locations. The extrapolated PFS and OS curves for the comparator arm are given in Appendix 2 of the ESM, as well as an overview of efficacy data fed into the model and the data sources.
2.3.2 Utilities
HRQoL is modelled based on the EQ-5D-5L data from the larotrectinib trial. The Dutch tariff was applied to the utility values (Table 3) [23]. Note that the HRQoL of the children in the SCOUT larotrectinib trial was assessed by means of the Paediatric Quality of Life Inventory. In the absence of a dataset to map the Paediatric Quality of Life Inventory to the Dutch EQ-5D tariffs, the Dutch utility values applied in the health economic model are only based on the EQ-5D-5L data collected in the NAVIGATE trial of the adult population.
The CEM also considers the HRQoL impact of adverse events by means of applying disutilities to the included grade 3 or grade 4 adverse events (AEs). As is typical for Dutch economic evaluations, it was expected that AEs graded below 3 or 4 are captured by the utilities associated with the health states. The disutilities for each grade 3 or 4 adverse event are provided in Table 4 below. To capture the full impact of the AEs, disutilities are applied to the full modelled cohort within the first cycle for each arm based on the event rates from the relevant clinical trials. The HRQoL impact of AEs are applied in the first cycle of the model, which is a simplistic approach applied due to missing or inconsistent evidence available for the comparators regarding the time to resolution or reversal of AEs.
2.3.3 Costs
To model costs and resource use, the following sources were used: Google Scholar, PubMed, previous Dutch reimbursement submissions, Zorginstituut Nederland costing manual, Nederlandse Zorgautoriteit online tariff application, or previous National Institute for Health and Care Excellence submissions. Drug costs in the Netherlands were retrieved from medicijnkosten.nl (VAT excluded). Data applied in previous Zorginstituut Nederland submissions were used unless new Dutch specific data had been released since the date of the relevant Zorginstituut Nederland submissions. Costs were determined for the year 2019 by using the consumer price index available from Statline. Cost components include drug acquisition costs, drug administration costs, healthcare resource utilisation costs, end-of-life costs, indirect medical costs, AE costs, travel costs, productivity costs and informal care costs. These are discussed in detail in Appendix 3 of the ESM.
Please note that costs associated with testing for NTRK gene fusions were not included in the CEM for two reasons. First, NTRK gene fusions are tested in a next-generation sequencing (NGS) test, based on RNA analysis aimed at identifying mutations for which druggable targets exist or are under investigation. NGS-based tests are reimbursed in the Netherlands. As a result of a public debate that started before the introduction of larotrectinib, in July 2021, members of parliament adopted a motion that all patients diagnosed with metastasised cancer should be broadly tested on genetic mutations of the tumour. The increasing need for NGS testing is thus an autonomous trend and unrelated to the introduction of larotrectinib. Second, physicians’ rationale to request an NGS test is to investigate whether a patient might benefit from any targeted therapy, as NGS tests are designed to map many biomarkers at once, so that the treating physician can make an informed choice about which therapy offers the best opportunities for his/her patient. Thus, the molecular diagnostic costs to detect the NTRK gene fusion cannot be specifically attributed to treatment with larotrectinib. Overall, diagnostic tests (such as computed tomography scans or biopsies) are not included in the model for both the intervention and the comparator arm.
2.4 Sensitivity Analysis
Deterministic sensitivity analyses were performed to identify those parameters that exhibit a significant influence on the model results, through varying individual input values and capturing the model results for each new evaluation. The upper and lower estimates were determined based on their 95% confidence intervals (whenever known), based on assumptions (e.g. time horizon or discounting), or assuming a +15% or −15% of the base-case values. For an overview of the parameters used in the OWSA, see Appendix 5 of the ESM. In addition, several scenario analyses were performed. These are described in more detail in Appendix 5 of the ESM. Probabilistic sensitivity analyses (PSAs) were performed to assess the variation in results stemming from the uncertainty in each individual model parameter combined. This process was repeated for 1000 iterations. The burden of disease was calculated by the proportional shortfall method [31]. The calculated burden of disease was 0.95, corresponding with a cost-effectiveness threshold of €80,000 per quality-adjusted life-year (QALY) gained [31]. The details of the parameters and distribution used are provided in Appendix 5 of the ESM. Finally, an EVPI analysis at the population level was conducted, in line with Dutch guidelines for economic evaluations [5]. The results are provided in Appendix 5 of the ESM.
3 Results
3.1 Base-Case Analyses
An overview of the final survival curve plot is presented in Fig. 2 and the results of the base-case analyses are given in Table 5. The results indicate that there is a larger substantial gain in OS than in PFS. Prior research indicates that this occurs more often, see for example Hess et al. [32]. The outcomes of the CEM show that the incremental cost-effectiveness ratio (ICER) of larotrectinib versus comparators is €41,424. The incremental QALY gain is 5.61. The incremental costs are €232,260. Cost increases are primarily driven by higher treatment costs of larotrectinib.
3.2 Sensitivity Analyses
A deterministic sensitivity analysis was performed, Fig. 3 highlights the impact of the ‘OS Weibull shape (p) of the larotrectinib adults’ parameter, followed by the ‘OS Weibull scale (lambda) of the larotrectinib adults’ parameter. Apart from the first parameter, the impact of the other parameter variation is limited. The outcomes of the probabilistic sensitivity analyses (Fig. 4) showed that larotrectinib was cost effective in 88% of iterations, at a threshold of €80,000 per QALY gained.
4 Discussion and Conclusions
This paper reports on the cost effectiveness of larotrectinib, the first tumour-agnostic therapy approved by the EMA. It details a comparison within the Dutch context between the costs and effects of the alternative treatments using a partitioned survival model. Larotrectinib versus the pooled comparator SoC arm resulted in incremental effects of 5.61 QALYs and 7.48 incremental life-years, and incremental costs of €232,260, leading to an ICER of €41,424/QALY. The probabilistic sensitivity analysis indicates that larotrectinib is cost effective versus comparators in 88% of iterations. The gain in life-years and quality of life as seen in the cost-effectiveness analysis is considered very high for a last-in-line oncology treatment. In cost-effectiveness studies for orphan drugs, health gains are often high but ICERs are generally less favourable compared with the cost effectiveness of non-orphan drugs [33]. In this case, however, the ICER was well below the applicable threshold for diseases with a high burden of disease in the Netherlands (€80,000).
Note that given the lack of a comparator arm in the pivotal trials and the multiple comparators included in the weighted comparator SoC arm, multiple assumptions were necessary to compute the cost-effectiveness analysis. First, the most important assumption is the naïve comparison that is made to the pooled comparator SoC arm, which entailed extraction of PFS and OS information from published data in order to inform the efficacy of the weighted comparator SoC arm in the different tumour locations. Even though the selection of these sources was made with the aim of using a population as similar as possible to the population included in the larotrectinib clinical programme, no adjustment for baseline patient characteristics took place. It is recognised that this is a naïve comparison, which is subject to bias. The input parameters are heterogenous and from a wide range of sources. The uncertainty this adds is in part due to the novelty of the tumour-agnostic therapies and the fact that evaluation of these therapies according to a standard procedure is difficult. As the field of tumour-agnostic therapies is fast developing, we expect new approaches to be developed.
Second, the survival data of the patients in the larotrectinib trial were immature resulting in uncertainty. Post-progression survival in the larotrectinib arm was rather high compared with pre-progression, which might be explained by the fact that 14% of the patients in the larotrectinib clinical trial programme received treatment beyond progression as the treating physician was of the opinion that the patient continued to derive clinical benefit. Furthermore, approximately 22% of patients in the larotrectinib clinical trials received post-discontinuation therapy, with 4% receiving radiotherapy and 18% receiving pharmaceutical treatments [1].
Third, the choice for comparator treatment was validated in an advisory board in 2019, clinical practice and standard of care may have evolved since then. Another limitation is that the evidence base for larotrectinib is still evolving. For instance, an intra-patient comparison comparative analysis was published in 2020 [34]. This would have been another way to model the efficacy data; however, this evidence was not yet available at the time of conducting this cost-effectiveness analysis. The findings of the intra-patient comparison suggest that larotrectinib improves PFS for patients with TRK fusion cancer compared with prior therapy, with a median growth modulation index of 2.68 in 72 eligible patients and 47 patients (65%) who had a growth modulation index of ≥ 1.33 (the threshold of meaningful clinical activity) [34]. The findings of this intra-patient comparison are in line with the findings of our analysis, as both analyses indicate the added therapeutic value of larotrectinib.
Finally, the results of the clinical trials used for these analyses are based on low patient numbers without a comparator arm and had a short follow-up. Because of the low patient numbers, we decided to exclude tumour types of fewer than three patients per year, as including these patients was deemed to have little effect on the cost-effectiveness outcomes. It is important to continue to monitor these patients in practice to see if safety and efficacy as measures correspond to the information gathered in the clinical trial programme. Nevertheless, given the poor prognosis of patients and promising results of larotrectinib, it is important that the evaluation of promising tumour-agnostic therapies such as larotrectinib is organised [35].
In terms of modelling, there is considerable uncertainty in the analysis because the tumour-agnostic indication requires modelling across multiple tumour localisations, each with their own parameters, assumptions and uncertainties. Although necessary to be able to model the cost effectiveness of larotrectinib in these populations, these assumptions form an important limitation to the CEM at hand. In addition, the model does not include subsequent treatments for both the larotrectinib arm and the comparator arm. Although the impact of this modelling decision is expected to be minimal, this is still a limitation to this cost-effectiveness analysis. In the Netherlands, we do not expect considerable post-progression treatments, because of the registered indication in which patients are only eligible for larotrectinib in case of no other satisfactory treatment option. Therefore, it was modelled in the vast majority of tumour localisations as last-in-line treatment.
Finally, for the weighted comparator SoC arm, we do not specifically use TRK fusion-positive cancers. It is not yet completely understood whether the prognosis of TRK fusion-positive cancers differs from non-TRK fusion-positive cancers. Several analyses suggest that NTRK fusion-positive cancers have a similar or worse prognosis to that of matched patients who do not harbour these fusions, suggesting that differences in prognoses are not driving the higher effectiveness in the larotrectinib arm [36,37,38].
To our knowledge, this is the first paper that details a cost-effectiveness analysis for a tumour-agnostic indication from a societal perspective. The expectation is that several other tumour-agnostic therapies will enter the market in the next decade. Given that tumour-agnostic therapies are a novel phenomenon, there are certain challenges to health economic modelling of these therapies. Several scholars have written about these challenges for tumour-agnostic therapies (e.g. [39, 40]). One challenge is that the basket trials investigating tumour-agnostic therapies’ clinical effectiveness are usually small in sample size. This challenge is seen across orphan diseases, and it introduces uncertainty to the clinical data. Furthermore, these basket trials usually do not include a comparator arm. Because the clinical trials are usually single-arm trials, the models need to include a naïve comparison using external comparators from unrelated previously conducted studies. This introduces additional uncertainty to the data populating the models. Additionally, the ICER that is presented as an average ICER across indications may well vary per indication. However, because patient numbers are low and naïve comparisons must be made, a subgroup analysis is usually not possible. An additional challenge is that health economic models are preferably populated with local population-specific inputs from the country of interest. In the case of tumour-agnostic therapies across multiple indications, daily practice across these different indications may differ substantially per country. This makes it a very time-consuming effort to adapt health economic models to country-specific situations. Last, because testing strategies will likely differ per country and when compared to the testing strategy in the basket trial, there may be additional differences between the trial population and the population in the countries of interest.
The average response to larotrectinib when compared to regular oncology treatment poses an interesting perspective on this heterogeneity of patients across tumour localisations. Regardless of the treatment under assessment, tumour specific or pan-tumour, there is always uncertainty. In a standard cost-effectiveness analysis looking at one tumour localisation, heterogeneity still exists in the form of DNA/RNA mutations. In the case of a pan-tumour indication, this heterogeneity is reversed, i.e. not across DNA/RNA mutations but across tumour localisation. Given the improved response, it could be argued that the localisation type of heterogeneity is less relevant compared to the DNA/RNA type of heterogeneity. More research into this phenomenon is necessary.
It is important that healthcare decision makers such as health technology assessment (HTA) bodies ensure that their decision framework takes into account these difficulties in order to meet the specific needs for tumour-agnostic therapies. This will hopefully ensure that uncertainties are dealt with properly and allow for new promising agents to arrive faster onto the market. The accelerated approval witnessed for larotrectinib at the US Food and Drug Administration and the conditional approval at the EMA level show that these therapies are seen as promising. However, as can be seen, clinical evidence supporting them remains challenging when compared with more typical assessments. For example, whereas both the EMA and US Food and Drug Administration have decided that a high response rate can be considered a proxy for efficacy, HTA bodies usually require endpoints such as survival and quality of life [4]. This means that, although medicines may have received EMA and US Food and Drug Administration approval, HTA bodies may find the available evidence insufficient to allow for reimbursement. Applying the standard HTA rules to tumour-agnostic therapies might mean local rejection. A pragmatic approach seems inevitable here. A potential solution might be coverage with evidence development, meaning that these therapies will be reimbursed despite the limited available evidence at the moment of entering the market. In the Netherlands, in the absence of an appropriate assessment framework for agnostic therapies, these therapies can momentarily only apply for conditional reimbursement. Larotrectinib is currently conditionally reimbursed in the Netherlands. The main conditions for reimbursement are: an indication committee to check the patient’s eligibility to larotrectinib, data collection of the use and outcomes of TRK inhibitors in daily practice and the concentration of treatment in a few appointed expert centres [41]. Other strategies to support these approvals may include post-authorisation monitoring, reflecting ‘real-world data’. These post-marketing data will be important in measuring the clinical benefit and safety of these new therapies observed in clinical practice. Furthermore, personalised reimbursement schemes in the form of a pay-for-performance structure might be a solution to the uncertainty associated with tumour agnostic therapies. However, here it is important to realise that evidence development on a local level may not always be feasible given the low patient numbers. Therefore, HTA bodies may want to consider developing a joint evidence development strategy together with the manufacturer and the (European) clinical experts.
In conclusion, this paper reports on the cost effectiveness of larotrectinib versus a pooled SoC comparator, showing that larotrectinib is cost effective versus the weighted comparators in 88% of iterations. Furthermore, this paper discusses challenges considering market access and reimbursement decisions for tumour-agnostic therapies. It articulates that patient access to these new drugs will depend on opportunities for post-authorisation evidence generation and a pragmatic approach by decision makers. Regulatory agencies need to consider the challenges for HTA bodies of tumour-agnostic therapies, to prepare for the inevitable uncertainty associated with the evidence from basket trials, lacking randomisation and pooling across heterogenous populations.
Notes
Please note that the choice for comparator treatment was validated in an advisory board in May of 2019. Clinical practice and standard of care may have evolved since then. This limitation is acknowledged in the discussion.
References
European Medicines Agency. Vitrakvi larotrectinib; 2019. https://www.ema.europa.eu/en/documents/product-information/vitrakvi-epar-product-information_en.pdf [Accessed 26 Sep 2019].
Yates LR, Seoane J, Le Tourneau C, et al. The European Society for Medical Oncology (ESMO) precision medicine glossary. Ann Oncol. 2018;29(1):30–5.
Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29(9):1895–902.
Cooper S, Bouvy JC, Baker L, et al. How should we assess the clinical and cost effectiveness of histology independent cancer drugs? BMJ. 2020;368:I6435.
Zorginstituut Nederland. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg (verdiepingsmodules); 2016. https://www.zorginstituutnederland.nl/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg [Accessed 5 Jan 2018].
NICE. Single technology appraisal. Larotrectinib for treating NTRK fusion-positive advanced solid tumours [ID1299]; 2020.
Briggs A, Wehler B, Gaultney JG, et al. Comparison of alternative methods to assess the cost-effectiveness of tumor-agnostic therapies: a triangulation approach using larotrectinib as a case study. Value Health. 2022;25(6):1002–9.
Forsythe A, Zhang W, Phillip Strauss U, et al. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther Adv Med Oncol. 2020;21(12): 1758835920975613.
Forsythe A, Zhang W, Strauss UP, et al. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther Adv Med Oncol. 2020;12:1–10.
Reck M, Rodriquez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Eulop A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumour proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–46.
Airoldi M, Pedani F, Succo G, Gabriele AM, Ragona R, Marchionatti S, et al. Phase II randomized trial comparing vinorelbine versus vinorelbine plus cisplatin in patients with recurrent salivary gland malignancies. Cancer. 2001;91(3):541–7.
Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):901–18.
Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706–13.
Socinski MA, Smit EF, Lorigan P, Konduri K, Reck M, Szczesna A, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-I patients with extensive-stage small-cell lung cancer. J Clin Oncol. 2009;27(28):4787–92.
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–23.
Mascarenhas L, Lyden ER, Breitfeld PP, Walterhouse DO, Donaldson SS, Paidas CN, et al. Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2010;28(30):4658–63.
Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–8.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–28.
Lam ET, Ringel MD, Kloos RT, et al. Sorafenib bij het lokaal gevorderd of gemetastaseerd jodium-refractair gedifferentieerd schildkliercarcinoom; 2014. https://www.nvmo.org/bom/sorafenib-bij-het-lokaal-gevorderd-of-gemetastaseerd-jodium-refractair-gedifferentieerd-schildkliercarcinoom/?meta [Accessed 21 Jun 2022].
Kerst JM, Eskens FALM, Beerepoot LV. Lenvatinib bij het lokaal gevorderd of gemetastaseerd jodium-refractair gedifferentieerd schildkliercarcinoom; 2016. https://www.nvmo.org/bom/lenvatinib-bij-het-lokaal-gevorderd-of-gemetastaseerd-jodium-refractair-gedifferentieerd-schildkliercarcinoom/?meta [Accessed 21 Jun 2022].
Versteegh MM, et al. Dutch tariff for the five-level version of the EQ-5D. Value Health. 2016;19:343–52.
Zorginstituut Nederland. Pakketadvies osimertinib (Tagrisso®); 2018.
Retel VP, Steuten LMG, Geukes Foppen MH, Mewes JC, Lindenberg MA, Haanen J, et al. Early cost-effectiveness of tumor infiltrating lymphocytes (TIL) for second line treatment in advanced melanoma: a model-based economic evaluation. BMC Cancer. 2018;18(1):895.
Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84.
Institute for Clinical and Economic Review. Ovarian cancer. https://icer-review.org/meeting/ovarian-cancer/ [Accessed 21 Jun 2022].
Doyle N. Cancer survivorship: evolutionary concept analysis. J Adv Nurs. 2008;62(4):499–509.
Beusterien LM, Davies J, Leach M, Meiklejohn D, Grinspan JL, O’Toole A, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8(50):1–9.
Tabberer M, Stamuli E, Walker M, Summerhayes M, Lees M. PCN74 utilities associated with non-small cell lung cancer (NSCLC): a community study. Value Health. 2006;9(6):A298.
Versteegh MM. Severity adjusted probability of being cost-effective. Pharmacoeconomics. 2019;37(9):1155–63.
Hess LM, Brnabic A, Mason O, et al. Relationship between progression-free survival and overall survival in randomized clinical trials of targeted and biologic agents in oncology. J Cancer. 2019;10(16):3717–27.
Champers JD, Silver MC, Berklein FC, Cohen JT, Neumann PJ. Orphan drugs offer larger health gains but less favorable cost-effectiveness than non-orphan drugs. J Gen Intern Med. 2020;35(9):2629–36.
Italiano A, Nando S, Briggs A, et al. Larotrectinib versus prior therapies in tropomyosin receptor kinase fusion cancer: an intra-patient comparative analysis. Cancers (Basel). 2020;12(11):3246.
van Kempen L, van Wezel T, Morreau H, Cohen D, Timens W, Willems SM, Schuuring E. De rol van moleculaire diagnostiek in het identificeren van patiënten die baat hebben bij TRK-remmer-therapie. Nederlands Tijdschrift voor Oncologie. 2020;17:266–73.
Demetri GD, Peters S, Hibar DP, et al. Characteristics and outcomes of patients with NTRK fusion-positive (NTRK+) metastatic/locally advanced solid tumours receiving non-TRK inhibitor standard of care, and prognostic value of NTRK fusions in clinical practice. Ann Oncol. 2021;32(Suppl 5): S399.
Santi I, Vellekoop H, Huygens S, et al. Prognostic value of the NTRK fusion biomarker in the Netherlands. Ann Oncol. 2021;32(5):S401–21.
Bazhenova L, Lokker A, Snider J, et al. TRK-fusion cancer: patients characteristics and survival analysis in the real-world setting. Target Oncol. 2021;16(3):389–99.
Hierro C, Matos I, Martin-Liberal J, Ochoa de Olza M, Garralda E. Agnostic-histology approval of new drugs in oncology: are we already there? Clin Cancer Res. 2019;25:3210–9.
Yan L, Zhang W. Precision medicine becomes reality-tumour type-agnostic therapy. Cancer Commun (Lond). 2018;38(1):6.
Staatscourant Regeling van de Staatssecretaris van Volksgezondheid, Welzijn en sport, houdende wijziging Regeling zorgverzekering i.v.m. tijdelijke uitstroom sluis. 5 Sep 2021.
Zorginstituut Nederland. Medicijnkosten.nl. https://www.medicijnkosten.nl/ [Accessed 21 Jun 2022].
Mihaljovic J, Bax P, van Breugel E, Blommestein HM, Hoogendoorn M, Hospes W, et al. Microcosting study of rituximab subcutaneous injection versus intravenous infusion. Clin Ther. 2017;39(6):1221-32.e4.
Zorginstituut Nederland. Vergaderstuk Adviescommissie Pakket: dabrafenib in combinatie met trametinib (Tafinlar® + Mekinist®); 2019.
Nederlandse Zorgautoriteit. NZa zorgproductapplicatie; 2019. https://zorgproducten.nza.nl/Home.aspx [Accessed 21 Jun 2022].
Uyl-de Groot CA, van Rooijen EM, Punt CJA, Pescott CP. Real-world cost-effectiveness of cetuximab in the third-line treatment of metastatic colorectal cancer based on patient chart review in the Netherlands. Health Econ Rev. 2018;8(1):13.
Zorginstituut Nederland. Pakketadvies pertuzumab (Perjeta®); 2016.
Zorginstituut Nederland. Everolimus (Afinitor®) bij gevorderde borstkanker; 2014.
Overheid.nl. Algemene Ouderdomswet; 2018.
Organisation for Economic Co-operation and Development (OECD). Employment data: Netherlands. https://data.oecd.org/jobs.htm#profile-Employment [Accessed 21 Jun 2022].
Centraal Bureau voor de Statistiek (CBS). Statline. https://opendata.cbs.nl/statline/#/CBS/nl/ [Accessed 21 Jun 2022].
van Baal PH, Wong A, Slobbe LJ, Polder JJ, Brouwer WB, de Wit GA. Standardizing the inclusion of indirect medical costs in economic evaluations. Pharmacoeconomics. 2011;29(3):175–87.
Zorginstituut Nederland. Pakketadvies pembrolizumab (Keytruda®); 2016.
van Kampen RJW, Ramaekers BLT, Lobbezoo DJA, de Boer M, Dercksen MW, van den Berkmortel F, et al. Real-world and trial-based cost-effectiveness analysis of bevacizumab in HER2-negative metastatic breast cancer patients: a study of the Southeast Netherlands Breast Cancer Consortium. Eur J Cancer. 2017;79:238–46.
National Institute for Health and Care Excellence (NICE). Technology appraisal guidance [TA405]: trifluridine-tipiracil for previously treated metastatic colorectal cancer; 2016. https://www.nice.org.uk/guidance/ta405 [Accessed 21 Jun 2022].
National Institute for Health and Care Excellence (NICE). Technology appraisal guidance [TA440]: pegylated liposomal irinotecan for treating pancreatic cancer after gemcitabine; 2017. https://www.nice.org.uk/guidance/ta440 [Accessed 21 Jun 2022].
National Institute for Health and Care Excellence (NICE). Technology appraisal guidance [TA535]: lenvatinib and sorafenib for treating differentiated thyroid cancer after radioactive iodine; 2018. https://www.nice.org.uk/guidance/ta535 [Accessed 21 Jun 2022].
National Institute for Health and Care Excellence (NICE). Technology appraisal guidance [TA268]: ipilimumab for previously treated advanced (unresectable or metastatic) melanoma; 2012. https://www.nice.org.uk/guidance/ta268 [Accessed 21 Jun 2022].
Liberato NL, Rognoni C, Rubrichi S, Quaglini S, Marchetti M, Gorlia T, et al. Adding docetaxel to cisplatin and fluorouracil in patients with unresectable head and neck cancer: a cost-utility analysis. Ann Oncol. 2012;23(7):1825–32.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12.
Zuluaga-Sanchez S, Hess LM, Wolowacz SE, D'Yachkova Y, Hawe E, Vickers AD, et al. Cost-effectiveness of olaratumab in combination with doxorubicin for patients with soft tissue sarcoma in the United States. Sarcoma (2018).
Delea TE, Amdahl J, Nakhaipour HR, Manson SC, Wang A, Fedor N, et al. Cost-effectiveness of pazopanib in advanced soft-tissue sarcoma in Canada. Curr Oncol. 2014;21(6):e748–59.
Zorginstituut Nederland. Pakketadvies axicabtagene ciloleucel (Yescarta®); 2019.
Acknowledgements
The authors thank Chantal van Gils and Claudine de Meijer for their strategic input. Furthermore, we thank all the clinical experts who participated in the advisory boards.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Bayer Netherlands B.V. provided funding to IQVIA, the Netherlands for the conduct of this study.
Conflict of interest
There are no conflicts of interest to report for all authors. Bayer Netherlands B.V. paid consultancy fees to IQVIA, the Netherlands to conduct the study on which this manuscript is based. The authors report no further conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analysed during the current study are not publicly available because of the protection of the intellectual property such as modelling techniques, but data are available from the corresponding author on reasonable request.
Code availability
See above.
Author contributions
RM, CA, MP and MK contributed to the study conception and design. Material preparation, data collection and analysis were performed by RM, CA and MP. The first draft of the manuscript was written by RM and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Michels, R.E., Arteaga, C.H., Peters, M.L. et al. Economic Evaluation of a Tumour-Agnostic Therapy: Dutch Economic Value of Larotrectinib in TRK Fusion-Positive Cancers. Appl Health Econ Health Policy 20, 717–729 (2022). https://doi.org/10.1007/s40258-022-00740-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-022-00740-1