Abstract
Lower urinary tract symptoms (LUTS) commonly occur as a consequence of benign prostatic hyperplasia (BPH), also known as prostate enlargement. Treatments for this can involve electrosurgical removal of a section of the prostate via transurethral resection of the prostate (TURP), Holmium laser enucleation of the prostate (HoLEP), or prostatic urethral lift using the UroLift system. The UroLift system implants to pull excess prostatic tissue away so that it does not narrow or block the urethra. In this way, the device is designed to relieve symptoms of urinary outflow obstruction without cutting or removing tissue. National guidance recommending the use of UroLift in the UK NHS was first issued in 2015 by the National Institute for Health and Care Excellence (NICE MTG26). We now report on the process to update the economic evaluation of UroLift, leading to updated NICE guidance published in May 2021 (NICE MTG58). The conclusions of the available clinical evidence were mixed and suggested that whilst UroLift improves symptoms over time, this improvement is smaller than that of TURP for symptom severity (IPSS) and urological outcomes. However, UroLift appears to be superior to Rezum for symptom severity and measures of erectile dysfunction and ejaculatory dysfunction. The updated economic model estimated that using UroLift as a day-case procedure for people with prostate of volume 30–80 mL creates a saving of £981 per person compared with bipolar TURP, £1242 compared with monopolar TURP, and £1230 compared with HoLEP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since the publication of NICE MTG26, a larger body of clinical evidence has emerged, with 5-year follow-up, and with direct comparisons with TURP and other surgical procedures. The clinical benefits of UroLift are sustained; it is not as efficacious as TURP but is recommended by NICE as a less invasive option with fewer complications for people of age over 50 years with prostate volume of 30–80 mL. |
The cost saving arising from UroLift is also sustained, under most circumstances. UroLift as a day-case procedure remains cost saving relative to TURP and HoLEP. Cost savings are uncertain when UroLift is used for treating an obstructive median lobe. |
Transurethral water vapour therapy using Rezum has emerged as a comparator therapy to UroLift. It is uncertain whether UroLift is cost saving compared to Rezum. |
1 Introduction
The aim of medical technologies guidance (MTG) issued by the UK National Institute for Health and Care Excellence (NICE) is to support adoption of clinically effective and cost-saving technologies in the UK National Health Service (NHS). This paper summarises Cedar’s assessment report update [1] and how it was used to inform NICE MTG58: ‘The UroLift system for treating lower urinary tract symptoms of benign prostatic hyperplasia’ [2]. This is an update of the original guidance MTG26 [3]. Cedar is a healthcare technology research centre formed through collaboration between Cardiff and Vale University Health Board and Cardiff University. This paper is part of a series that report the development of NICE MTG. NICE produces guidance on new or innovative medical devices or diagnostics, Medical Technologies Guidance (MTG). The aim of this paper is to provide an insight into the development of updated recommendations for the use of the UroLift system.
1.1 Background to the Technology and Application
Prostatic urethral lift using the UroLift system is an endoscopic treatment for men with lower urinary tract symptoms (LUTS) due to benign prostatic hypertrophy (BPH). The UroLift system comprises a single-use pistol-gripped endoscopic delivery device (probe), used to deliver an implant. Each UroLift implant consists of two ‘anchors’ connected by a nonabsorbable suture. The surgeon inserts the probe into the urethra until it reaches the prostatic urethra. A fine needle at the end of the probe deploys the suture through a lateral lobe of the prostate and the capsular anchor fixes the suture outside of the prostate. The needle is withdrawn, tensioning the suture, which is then secured in the urethra with the urethral anchor. The result is that the lobe is pulled away from the area of obstruction, thus opening the urethra. This is repeated on the other lateral lobe of the prostate. A procedure uses an average of 3.5 implants and can be done with the patient under local or general anaesthetic and as an in-patient, out-patient or day-case procedure. There have been no changes to the technology since NICE MTG26 and UroLift has a current CE mark.
1.2 Decision Problem (Scope)
The NICE Scope defined the decision problem as:
-
Population—Adults with LUTS caused by BPH, aged 45 years or over, with prostate volumes ≤ 100 mL
-
Indication—Prostatic urethral lift using the UroLift system
-
Comparators:
-
Monopolar or bipolar transurethral resection of the prostate (TURP)
-
Holmium laser enucleation of the prostate (HoLEP)
-
Transurethral water vapour therapy using Rezum (Boston Scientific)
-
-
Outcomes—Length of hospital stay, changes in ejaculatory or sexual function, need for and duration of post-operative catheterisation, symptoms of BPH, quality of life (QoL) and procedure time.
2 Cedar’s Review of the Evidence
The company submitted to NICE the available clinical and cost evidence for use of the UroLift system, alongside a de novo cost model. Cedar’s assessment report update provided NICE with an independent appraisal of this evidence [1].
2.1 Review of Clinical Effectiveness Evidence
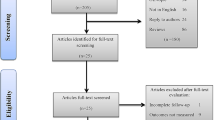
The company did not update their search strategy for the purpose of the guidance update; however, NICE conducted literature searches, based on the searches conducted for the original assessment report. These were completed on 31 July 2019. Please see Ray et al. [4] for critique of the original company search strategies. The External Assessment Centre (EAC) conducted an additional update search for this update from July 2019 to 14 July 2020. The EAC identified 427 references, 129 adverse event reports and two ongoing trials.
The EAC identified 12 publications (from 10 studies) for full-text review, 11 of which were not selected by the company, but were relevant to the scope. There were two randomised controlled trials and eight nonrandomised studies.
2.1.1 Critical Appraisal of Studies
Most of the included studies were comparative studies of moderate or high quality, with relevant patient samples and/or blinded assessment of outcomes. The two publications reporting results from the LIFT randomised controlled trial [5, 6] had a low risk of bias with both patients and assessors blinded to procedure and outcome. However, there was a risk of bias with the two publications from the BPH6 randomised controlled trial [7, 8] as the study was not blinded and did not state whether analysis was by intention-to-treat.
Some non-randomised crossover and comparative studies [9,10,11] were of moderate quality. The main limitations were small (< 20) sample sizes [12,13,14], limited reporting of eligibility criteria [15, 16], and unclear reporting of outcome measures [13]. None of these studies reported blinding [9,10,11,12,13,14,15,16]. Two studies had good quality overall [12, 14].
2.1.2 Clinical Results
The current guidance update provides the first data comparing UroLift directly with TURP or Rezum. Results from the clinical evidence are mixed and do not show that UroLift is superior when compared to TURP for urological, QoL or symptom-severity outcomes. Nevertheless, UroLift provides significant symptomatic improvements. The best available evidence comes from the LIFT randomised controlled trial [5, 6], which found that UroLift provided significant improvements sustained at 5 years as follows:
-
Reduction in Benign Prostate Hyperplasia Impact Index (BPHII) from baseline (6.92) to 5-year follow-up (3.51, p < 0.0001).
-
Reduction in International Prostate Symptom Score (IPSS) from baseline (22.32) to 5-year follow-up (14.47, p < 0.0001) [6].
-
Increase in maximum urine flow rate (Qmax) from baseline (7.88) to 5-year follow-up (11.08, p < 0.0001).
UroLift appears to be superior to Rezum for erectile dysfunction and symptom severity outcomes [11]: Sexual Health Inventory for Men (SHIM) scores were significantly higher (better) in the UroLift group (14.8) versus Rezum (9.2), p = 0.02. The same study found that scores on the Male Sexual Health Questionnaire-Ejaculatory Dysfunction (MSHQ-EjD) scale were significantly higher (better) in the UroLift group (12.2) versus Rezum (9.2), p = 0.04. There is also limited evidence that changes in MSHQ-EjD scores over time were significantly better for UroLift patients when compared to TURP [7, 8] and Rezum procedures [11].
The evidence generally demonstrates that UroLift brings long-lasting improvements in symptom severity, urological outcomes and QoL measures. Further details of the clinical efficacy outcomes are provided in the Online Supplementary Materials (OSM) S1.
2.1.3 Review of Safety Outcomes
Table 1 reports the adverse events for included studies. The reporting was inconsistent between studies with some reporting full details using the Clavien-Dindo classification and some not reporting adverse events. For studies that reported adverse events but not using Clavien-Dindo Classification, the EAC compared the events listed to those within the Clavien-Dindo classification to arrive at the correct Grade [17, 18].
The severity of adverse events for this device is low with most events being Grade I or II. However, one study reported Grade IIIb events: severe bleeding and secondary treatment. No severe (above a Grade IIIb) events were reported.
2.2 Economic Evidence
The company did not submit any new economic evidence for this review. However, the EAC identified one published cost-effectiveness model [19], one cost equivalence [20] and one related review [21] that included cost information from the main literature search for the topic. All were set in the USA, and so were not of direct relevance to inform the current guidance review.
A detailed evaluation of the economic evidence considered for NICE MTG26 [3] has been previously described [4].
2.2.1 The Manufacturer’s de novo Economic Model
The manufacturer submitted an updated economic model based on that of NICE MTG26 [3], with a decision tree design. The arms of the decision tree included UroLift, mTURP, bTURP, HoLEP and Rezum as competing treatment alternatives. Rezum was a new addition in this iteration of the model. In the UroLift arm, treatment of an obstructive median prostate lobe was included as an optional, new intervention. The model took the perspective of NHS and social care services and the time horizon was extended to 5 years to reflect the length of follow-up in the LIFT randomised controlled trial [6]. There was no discount rate applied in the manufacturer’s model.
Each treatment arm had branches for success and failure, based on probabilities derived from clinical evidence. Following first treatment failure there were two possibilities, either a second procedure, or urinary incontinence as an adverse outcome that incurred a cost for management with pads and medicines for the remaining time horizon of the model.
Clinical parameters for the Rezum treatment were taken from a randomised controlled trial [22] that was included in NICE MTG49 [23]. Clinical parameters for UroLift were based on 5-year data from the LIFT randomised controlled trial [6]. Clinical parameters for mTURP, bTURP and HoLEP were based on a published Health Technology Assessment [24], which provided inputs for NICE MTG26 [3], MTG29 [25] and MTG49 [23]. Table 2 shows the clinical parameters.
There were substantial changes in options for repeat procedures since NICE MTG26 [3]. Table 3 shows the proportion of patients who underwent each repeat procedure in the model, including changes made since NICE MTG26 [3]. For example, bTURP is now more commonly performed than mTURP due to its improved safety profile [26].
Where first treatment was UroLift, approximately 32% of repeat procedures used UroLift and the remainder used TURP. This was accepted for MTG49 [23] and was considered appropriate for use in this model. The data are from the 5-year follow-up point in the LIFT study [6]. Repeat procedures were modelled with the same success probabilities as initial procedures.
The unit costs in the model were mainly based on the previous iteration of the guidance: NICE MTG26 [3] and related, subsequently published guidance: NICE MTG29 [25] and NICE MTG49 [23].
The updated model assumed that different follow-up arrangements applied to each of the surgical procedures. Following UroLift, a 20-min telephone consultation with a nurse was applied, costing £15.70, whereas mTURP and bTURP were modelled with an outpatient appointment with a consultant costing £94, and Rezum was modelled with a consultant appointment plus a trial without catheter, costing £238. Capital costs for reusable systems (e.g. electrosurgical units) were removed from the model, with negligible effect on the results. The manufacturer’s updated model removed some costs that were included for NICE MTG26 [3], namely pre-procedure tests and consultations and fluid consumables. These had been equal for all procedures, so their removal did not bias the model’s results.
2.2.2 Manufacturer’s Base-Case Results
The manufacturer’s updated base-case analysis found that compared to providing UroLift on an outpatient basis, the other treatment options incurred additional, incremental (per procedure) costs as follows: UroLift (day case): £24, Rezum: £66, bTURP: £1057, mTURP: £1148 and HoLEP: £1303. UroLift as day-case surgery incurred lower costs than in NICE MTG 26 [3, 4].
Compared to the prior guidance, the increased saving delivered by UroLift was driven by use of fewer UroLift implants (3.5 vs. 4), reduced theatre time for UroLift procedures (14 vs. 30 min) and use of nurse-led telephone follow-up. These modifications were supported by patient tracker data submitted by the company. Other important drivers reducing the cost of UroLift were addition of a trial without catheter in the Rezum arm, greater use of bTURP with therefore increased consumables cost, and increased impact of lasting incontinence for the remainder of the model’s 5-year time horizon. Also important as a driver of cost was use of an assumed reduced length of hospital stay (since NICE MTG26 [3]) for UroLift from 0.5 to 0.125 days.
2.2.3 Appraisal of Model Structure, Model Inputs and Changes Made by the External Assessment Centre (EAC)
The updated manufacturer’s model also used structural elements and parameters from models used for related prostate national guidance: NICE MTG29 [25] and NICE MTG49 [23]. These have been previously accepted for NICE guidance and were judged to be reasonable by the EAC. The EAC made a number of modifications to the manufacturer’s model including:
-
Correction of minor errors/inconsistencies
-
Update of all costs to Year 2019 values
-
Further alignment where required, to NICE MTG29 [25] and NICE MTG49 [23]
-
Removal of repeat procedures following HoLEP as initial procedure; expert clinical advice stated that this rarely occurred
-
Application of a discount rate of 3.5% per annum to all costs
-
Changes to probability of success for each competing treatment (defined as an improvement of > 10% in IPSS score within 12 months) and complication rates for UroLift and comparator procedures (Table 2), based on updated published sources
-
Changes to numerous unit costs where the EAC deemed necessary for improved accuracy.
Table 4 shows the changes made by the EAC to the cost inputs.
2.2.4 Effects of Base-Case Changes Made by the EAC
Discussion between the EAC and NICE concluded that the reference treatment for the economic analysis should be UroLift provided on a day-case basis. The EAC’s updated base-case analysis found that compared to providing UroLift on a day-case basis, the other treatment options incurred additional, incremental (per procedure) costs as follows: UroLift (outpatient): £-24, Rezum: £96, bTURP: £981, mTURP: £1242 and HoLEP: £1230. Therefore, like the manufacturer’s base case, UroLift retained its small cost saving (Table 5), with UroLift offered as an outpatient procedure being the cheapest of all options.
2.2.5 Sensitivity Analysis
One-way sensitivity analysis The manufacturer provided, in its updated model, one-way sensitivity analyses to accompany its base case, varying one parameter at a time to explore the impact of each parameter on the model’s results. Parameters included the number of UroLift implants used and their unit cost, use of additional implants for obstructive median lobe UroLift procedures, and the incidence of urinary tract infection following Rezum treatment. The EAC repeated the same sensitivity analyses, but applying them to its modified base case (Table 5). The results are shown in Table 6. A total of 11 parameters were varied. Of these, there were eight variables that had potential to change the model’s result from UroLift being cost saving, to UroLift incurring a cost relative to comparator interventions. Rezum was the comparator most likely to emerge as the cheaper therapy if threshold values were exceeded (Table 6).
Additional scenarios presented by the EAC To explore areas of uncertainty arising from assumptions in the model, the EAC modelled additional scenarios as follows.
The updated model assumed that hospital stay following treatment with Rezum was 0.5 days based on NICE MTG49 [23], where Rezum is a day-case procedure. The EAC added a scenario where length of stay following UroLift was equal to that following Rezum, since UroLift may also be performed as day-case surgery (i.e. a change from 0.125 to 0.5 days). The effect was that UroLift was no longer cost saving relative to Rezum.
To explore the impact of telephone-based follow-up introduced to the model for patients treated with UroLift, the EAC applied telephone-based follow-up to all treatment options in the model. The effect was to reduce the cost for all comparator interventions, to the extent that UroLift was no longer cost saving relative to Rezum.
The EAC explored the effect of applying to bTURP a reduced length of hospital stay of 0.5 days, reflecting day-case surgery, based on published evidence [27]. The effect was minimal, with UroLift remaining cost saving relative to all comparators.
The EAC noted that the updated model had an element of double counting of operating theatre staff costs: these were included as a distinct parameter but also formed part of aggregated procedural costs. The EAC removed the distinct staff costs for all procedures. The effect was to make all interventions cheaper, but UroLift remained cost saving relative to all comparators.
The updated model included a shorter theatre time per UroLift case compared to that used for NICE MTG26 [3]: 14 min versus 30 min, respectively. The EAC added a scenario using the original value of 30 min per UroLift case. The effect was that UroLift was no longer cost saving relative to Rezum.
The EAC also explored raising the cost of treating urinary tract infections and applying a rate of 1% of urinary incontinence following treatment with UroLift or with Rezum, based on NICE MTG49 [23]. In both scenarios UroLift remained cost saving relative to all comparator treatments.
3 NICE Guidance
3.1 Development of Guidance
The NICE Medical Technologies Advisory Committee (MTAC) met in February 2021 and considered evidence from a range of sources, including the company’s submission, Cedar’s report and testimony from clinical experts. The committee made provisional recommendations that went to public consultation. During the consultation, NICE received 111 consultation comments from 14 consultees. The consultees included one company representative, nine healthcare professionals, three professional organisations and one comparator company. The comments related to anaesthetic use, procedure setting, prostate size, retreatment rates and comparison with the Rezum technology. Two comment themes led to amendments to the guidance recommendations. Consultees suggested that the evidence for using the UroLift System in men with prostate volume between 80 and 100 mL is limited. The committee agreed and amended recommendations to include the use of the UroLift System for treating lower urinary tract symptoms of benign prostatic hyperplasia in those with a prostate volume between 30 and 80 mL. The committee also received comments from consultees stating that the UroLift System is done as an outpatient procedure in a small number of NHS trusts. The committee updated the recommendations to additionally acknowledge the use of the UroLift System in an outpatient setting.
3.2 Recommendations
The recommendations in NICE MTG58 [2] are as follows:
“Evidence supports the case for adopting the UroLift System for treating lower urinary tract symptoms of benign prostatic hyperplasia. The UroLift System relieves lower urinary tract symptoms, avoids risk to sexual function, and improves quality of life.
The UroLift System is a minimally invasive procedure, which should be considered as an alternative to transurethral resection of the prostate (TURP) and Holmium laser enucleation of the prostate (HoLEP). It can be done as a day-case or outpatient procedure for people aged 50 years and older with a prostate volume between 30 and 80 mL.
Cost modelling shows that the UroLift System is likely to be cost saving compared with standard treatments, because of reduced length of stay and procedure time. Over 5 years, if done as a day‑case procedure, UroLift is estimated to save, per person:
-
£981 compared with bipolar TURP
-
£1242 compared with monopolar TURP
-
£1230 compared with HoLEP.
Cost savings are uncertain compared with transurethral water vapour therapy using Rezum and when UroLift is used for treating an obstructive median lobe.”
4 Key Challenges and Learning Points
There is a clinical evidence base of moderate to high quality and up to 5-year follow-up in support of UroLift as a treatment for people with LUTS, though few of the available studies were conducted in the UK and therefore direct applicability to the NHS setting is lacking.
There are published economic evaluations of UroLift used to treat men with LUTS but these have low applicability to the UK NHS and inconsistent findings. Therefore, an approach using a de novo economic model with a UK NHS and social care perspective was warranted. The economic model demonstrated that UroLift resulted in cost savings compared to HoLEP, bTURP and mTURP under most conditions. However, the economic model demonstrated uncertainty in the cost case for UroLift when compared to Rezum. The cost saving resulting from the use of UroLift is dependent upon some important assumptions. Two assumptions are based on unpublished NHS patient tracker data provided by the manufacturer:
-
UroLift theatre time is reduced from 30 min at the time of developing NICE MTG26 [3] to 14 min
-
UroLift procedures today use fewer implants than at the time of developing NICE MTG26 [3]: 3.5 versus 4 implants per procedure, respectively.
A further two assumptions have no evidence base:
-
Rezum has a longer length of hospital than stay (0.5 days, based on NICE MTG 49 [23]) than UroLift (0.125 days)
-
Patients who undergo UroLift procedures are followed up by telephone call with a nurse, whereas all other procedures require an outpatient visit with a consultant. In sensitivity analysis telephone follow-up was modelled for all comparators. UroLift remained cost saving versus all comparators with the exception of Rezum.
5 Conclusions
The available clinical evidence suggests that UroLift improves LUTS but the magnitude of improvement is not as big as for TURP for several symptom and urological outcome measures. When compared to Rezum, however, UroLift resulted in bigger improvements for symptom severity and erectile dysfunction measures. The benefit for men with LUTS gained by UroLift appears to be sustained in the long term. In addition, the number of adverse events were reduced in UroLift patients when compared to TURP. UroLift has been shown to be suitable as a day-case treatment under local anaesthetic, resulting in reductions in catheterisation rates, catheterisation time and length of hospital stay.
The economic model estimated that UroLift resulted in a cost saving compared to mTURP, bTURP and HoLEP. The model estimated that UroLift resulted in a small cost saving compared to Rezum under most conditions, provided that important assumptions hold. Under some conditions the comparator treatment Rezum may be cost saving compared to UroLift.
References
Knight, L, Dale M, Morgan H, Morris R. (external assessment report). UroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia. 2021. https://www.nice.org.uk/guidance/mtg58/documents/supporting-documentation
NICE 2021, UroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia (MTG58). https://www.nice.org.uk/guidance/mtg58. Accessed 04 May 2021
NICE 2015, UroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia(MTG26). https://www.nice.org.uk/guidance/mtg26. Accessed 16 Sept 2016
Ray A, Morgan H, Wilkes A, Carter K, Carolan-Rees G. The Urolift system for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: a NICE medical technology guidance. Appl Health Econ Health Policy. 2016;14(5):515–26.
Roehrborn C, Rukstalis DB, Barkin J, Gange SN, Shore ND, Giddens JL, Bolton DM, Cowan BE, Cantwell AL, McVary KT, Te AE. Three year results of the prostatic urethral LIFT study. Can J Urol. 2015;22(3):7772–82.
Roehrborn CG, Barkin J, Gange SN, Shore ND, Giddens JL, Bolton DM, Cowan BE, Cantwell AL, McVary KT, Te AE, Gholami SS. Five year results of the prospective randomized controlled prostatic urethral LIFT study. Can J Urol. 2017;24(3):8802–13.
Sønksen J, Barber NJ, Speakman MJ, Berges R, Wetterauer U, Greene D, Sievert KD, Chapple CR, Montorsi F, Patterson JM, Fahrenkrug L. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68(4):643–52.
Gratzke C, Barber N, Speakman MJ, Berges R, Wetterauer U, Greene D, Sievert KD, Chapple CR, Patterson JM, Fahrenkrug L, Schoenthaler M. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH 6 prospective, multicentre, randomized study. BJU Int. 2017;119(5):767–75.
Rukstalis D, Rashid P, Bogache WK, Tutrone RF, Barkin J, Chin PT, Woo HH, Cantwell AL, Cowan BE, Bolton DM. 24-month durability after crossover to the prostatic urethral lift from randomised, blinded sham. BJU Int. 2016;118:14–22.
Rukstalis D, Grier D, Stroup SP, Tutrone R, deSouza E, Freedman S, David R, Kamientsky J, Eure G. Prostatic Urethral Lift (PUL) for obstructive median lobes: 12 month results of the MedLift Study. Prostate Cancer Prostatic Dis. 2019;22(3):411–9.
Tutrone RF, Schiff W. Early patient experience following treatment with the UroLift prostatic urethral lift and Rezum steam injection. Can J Urol. 2020;27(3):10214.
Bozkurt A, Karabakan M, Keskin E, Hirik E, Balci MC, Nuhoglu B. Prostatic urethral lift: a new minimally invasive treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urol Int. 2016;96(2):202–6.
Bardoli AD, Taylor WSJ, Mahmalji W. Can the UroLift prostatic implant device treat the symptoms of benign prostatic hypertrophy, avoid sexual dysfunction and reduce hospital TURP waiting times? A single centre, single surgeon experience and review of the literature. Aging Male. 2017;20(3):192–7.
Rubio CC, Costa YP, Escudero JJ, de Campo MR, Ibáñez JG, Biosca SM, Cataluña AM, Sierra KR, Arjona MF, Alcina EL. Minimally invasive treatment for lower urinary tract symptoms due to benign prostatic hyperplasia. Our initial experience with Urolift® under local anaesthesia and sedation. Actas Urológicas Españolas (English Edition). 2019;43(9):488–94.
Eure G, Gange S, Walter P, Khan A, Chabert C, Mueller T, Cozzi P, Patel M, Freedman S, Chin P, Ochs S. Real-world evidence of prostatic urethral lift confirms pivotal clinical study results: 2-year outcomes of a retrospective multicenter study. J Endourol. 2019;33(7):576–84.
Sievert KD, Schonthaler M, Berges R, Toomey P, Drager D, Herlemann A, Miller F, Wetterauer U, Volkmer B, Gratzke C, Amend B. Minimally invasive prostatic urethral lift (PUL) efficacious in TURP candidates: a multicenter German evaluation after 2 years. World J Urol. 2019;37(7):1353–60.
Mamoulakis C, Efthimiou I, Kazoulis S, Christoulakis I, Sofras F. The modified Clavien classification system: a standardized platform for reporting complications in transurethral resection of the prostate. World J Urol. 2011;29(2):205–10.
Ouattara A, Paré AK, Kaboré AF, Kabré B, Bako A. Using Modified Clavien-Dindo’s Classification System for Reporting Postoperative Complications of Transvesical Prostatectomy at Souro Sanou University Teaching Hospital of Bobo-Dioulasso (Burkina-Faso). Int Arch Urol Complic. 2019;5:056.
Ulchaker JC, Martinson MS. Cost-effectiveness analysis of six therapies for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. CEOR. 2018;10:29.
DeWitt-Foy ME, Gill BC, Ulchaker JC. Cost comparison of benign prostatic hyperplasia treatment options. Curr Urol Rep. 2019;20(8):45.
Gill BC, Ulchaker JC. Costs of managing benign prostatic hyperplasia in the office and operating room. Curr Urol Rep. 2018;19(9):72.
McVary KT, Gange SN, Gittelman MC, Goldberg KA, Patel K, Shore ND, Levin RM, Rousseau M, Beahrs JR, Kaminetsky J, Cowan BE, Cantrill CH, Mynderse LA, Ulchaker JC, Larson TR, Dixon CM, Roehrborn CG. Minimally Invasive Prostate Convective Water Vapor Energy Ablation: a Multicenter, Randomized, Controlled Study for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. J Urol. 2016;195(5):1529–38. https://doi.org/10.1016/j.juro.2015.10.181 (Epub 2015 Nov 22 PMID: 26614889).
NICE Medical technologies guidance MTG49. Rezum for treating lower urinary tract symptoms secondary to benign prostatic hyperplasia. https://www.nice.org.uk/guidance/MTG49. Accessed 24 June 2020
Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al. Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement. Health Technol Assess 2008;12(35).
NICE Medical technologies guidance MTG29. GreenLight XPS for treating benign prostatic hyperplasia. https://www.nice.org.uk/guidance/mtg29. Accessed 14 June 2016
NICE Medical technologies guidance MTG53. The PLASMA system for transurethral resection and haemostasis of the prostate. https://www.nice.org.uk/guidance/mtg53. Accessed 06 Jan 2021
Lavan L, Kyriazis G, Mbiabjeu D, Gormley R, Hall S, Robinson R, Hodgson D. Day-case surgery is possible in the majority of men undergoing transurethral resection of the prostate—a report on over 1000 cases. J Clin Urol. 2018;11(6):403–8.
Acknowledgements
Acknowledgments are made to Helen Morgan (SURE, Cardiff University) and Simone Willis (SURE, Cardiff University) for conducting the literature searches for this article.
Author Contributions
LK, MD, AC, CP and RM contributed to the preparation of the manuscript. RM reviewed the full article, and can act as a guarantor for the overall content. This summary has not been externally reviewed by Applied Health Economics and Health Policy.
Funding
Cedar was funded by the NICE Medical Technologies Evaluation Programme for its work.
Conflict of interest
LK, MD, AC and RM are NHS employees and have no conflicts of interest. CP is an employee of NICE. This summary of the Medical Technologies Guidance was produced following the publication of the final guidance report.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Knight, L., Dale, M., Cleves, A. et al. UroLift for Treating Lower Urinary Tract Symptoms of Benign Prostatic Hyperplasia: A NICE Medical Technology Guidance Update. Appl Health Econ Health Policy 20, 669–680 (2022). https://doi.org/10.1007/s40258-022-00735-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-022-00735-y