Abstract
Background
A clinical–genetic function (Cardio inCode®) was generated using genetic variants associated with coronary heart disease (CHD), but not with classical CHD risk factors, to achieve a more precise estimation of the CHD risk of individuals by incorporating genetics into risk equations [Framingham and REGICOR (Registre Gironí del Cor)].
Objective
The objective of this study was to conduct an economic analysis of the CHD risk assessment with Cardio inCode®, which incorporates the patient’s genetic risk into the functions of REGICOR and Framingham, compared with the standard method (using only the functions).
Methods
A Markov model was developed with seven states of health (low CHD risk, moderate CHD risk, high CHD risk, CHD event, recurrent CHD, chronic CHD, and death). The reclassification of CHD risk derived from genetic information and transition probabilities between states was obtained from a validation study conducted in cohorts of REGICOR (Spain) and Framingham (USA). It was assumed that patients classified as at moderate risk by the standard method were the best candidates to test the risk reclassification with Cardio inCode®. The utilities and costs (€; year 2011 values) of Markov states were obtained from the literature and Spanish sources. The analysis was performed from the perspective of the Spanish National Health System, for a life expectancy of 82 years in Spain. An annual discount rate of 3.5 % for costs and benefits was applied.
Results
For a Cardio inCode® price of €400, the cost per QALY gained compared with the standard method [incremental cost-effectiveness ratio (ICER)] would be €12,969 and €21,385 in REGICOR and Framingham cohorts, respectively. The threshold price of Cardio inCode® to reach the ICER threshold generally accepted in Spain (€30,000/QALY) would range between €668 and €836. The greatest benefit occurred in the subgroup of patients with moderate–high risk, with a high-risk reclassification of 22.8 % and 12 % of patients and an ICER of €1,652/QALY and €5,884/QALY in the REGICOR and Framingham cohorts, respectively. Sensitivity analyses confirmed the stability of the study results.
Conclusions
Cardio inCode® is a cost-effective risk score option in CHD risk assessment compared with the standard method.
Similar content being viewed by others
• Cardio inCode® is a clinical–genetic function for coronary heart disease (CHD) risk assessment.
• For a Cardio inCode® price of €400 in Spain, the cost per QALY compared with the standard method would be €12,969 and €21,385 in REGICOR (Registre Gironí del Cor) and Framingham cohorts, respectively.
• The greatest benefit in Spain occurred in the subgroup of patients with moderate–high CHD risk, with a cost per QALY of €1,652 (REGICOR) and €5,884 (Framingham).
1 Introduction
Cardiovascular disease is the most common cause of death in Europe. Despite acquired knowledge, the demonstrated efficacy of preventive and therapeutic measures, as well as the availability of regional, national, and European guidelines and plans for the prevention of cardiovascular disease, the mortality due to cardiovascular disease continues to be high in Europe [1–4]. Cardiovascular disease is responsible for 31 % of all deaths in Spain, making it the main cause of death, with coronary heart disease (CHD) being the most frequent [5, 6].
Since the publication of the first European Guidelines on Prevention of CHD in 1994 [7] the assessment of CHD risk has been recommended as an essential screening tool in the management of patients in all guideline updates. The cardiovascular prevention approach of calculating the CHD risk has become an important criterion to establishing the intensity of preventive efforts [8]. Global CHD risk better describes the overall risk profile and is preferred to the assessment of risk factors separately. In Spain, the assessment of CHD risk is mainly performed using the original Framingham Risk Score [9], risk equations based on the Framingham Risk Score such as the REGICOR (Registre Gironí del Cor) Risk Score [10, 11], or the SCORE (Systematic COronary Risk Evaluation) function re-calibrated for Spain [12]. Although very useful for screening, the current equations have a modest sensitivity and specificity [13]. From a clinical perspective, the low precision of risk function prediction is illustrated by the fact that 53.6 % of cardiovascular events in a population aged 35–74 years occur in individuals classified as moderate CHD risk [14–16].
CHD is a disease of complex etiology involving genetic and environmental factors, as well as the interaction between them [13]. It is estimated that genetic factors explain between 40 and 55 % of the variability in the population for the onset of CHD [13, 14]. Several genome-wide association studies have been conducted over the past few years that have consistently identified genetic variants associated with CHD [17–23]. Some of these genetic variants are in turn associated with some classic CHD risk factors, although others are independent of the classical factors. The discovery of these genetic variants independently associated with CHD may enable the identification of new etiopathogenic mechanisms of the disease, as well as new therapeutic targets. Furthermore, these variants could be used as new biomarkers to improve the CHD risk prediction ability, or the reclassification of individuals at moderate risk, by providing additional information to that already included in the classic risk functions [24]. As a result, a more correct estimation of the risk, particularly in individuals with moderate risk, could have a significant impact on the total CHD risk, and on the effectiveness of population preventive strategies [15].
A clinical–genetic function (Cardio inCode®) was generated using genetic variants associated with CHD but not with classical CHD risk factors, to achieve a more precise estimation of the CHD risk of individuals by incorporating genetics into risk equations (Framingham and REGICOR). Cardio inCode® has been validated in studies that have used Framingham and REGICOR cohorts, following the recommendations of the American Heart Association [25]. These two cohorts have been used in order to validate Cardio inCode® in two populations with a different prevalence of CHD (Framingham, with an elevated prevalence, and REGICOR, with a low prevalence of CHD) [15]. The quantitative results of the validation study of Cardio inCode® are described in "Cardio inCode® Effectiveness".
The present study aims to perform a cost-effectiveness analysis of the evaluation of the risk of suffering a CHD event in Spain using Cardio inCode® compared with the standard method (applying the Framingham or REGICOR functions alone).
2 Methods
Cost-effectiveness analysis is a tool of special importance for making decisions in a National Health System (NHS). This type of analysis was performed in order to assess the efficiency of evaluating the risk of suffering a CHD event in Spain using Cardio inCode® and comparing it with the standard method (applying the Framingham or REGICOR functions alone).
2.1 Model
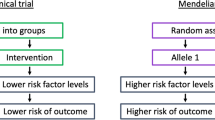
A Markov model [26] was prepared with the structure presented in Fig. 1, with a time horizon (duration of the simulation, for a joint life expectancy in men and women of 82 years) [27] of 28 years for the REGICOR cohort (start of the simulation at 54 years of age), and 26 years for the Framingham cohort (start of the simulation at 56 years of age), according to the validation study of the predictive ability of CHD risk estimation by Cardio inCode® in both patient cohorts [15]. Table 1 summarizes the seven health states used in the model, as well as their definitions. The primary variables used in the study were the costs, the utilities (patient perceived quality of life, expressed as QALYs) of the health states, the transition probabilities between them, and the survival expressed as life-years (LYs).
Markov economic model for the evaluation of Cardio inCode®, a clinical–genetic function for estimating the coronary heart disease risk by including the genetic risk, represented schematically as an influence diagram. Reclassification of the coronary heart disease risk in patients from the cohorts of REGICOR (Registre Gironí del Cor) and Framingham, all initially classified as moderate risk with the standard method [the usual method for estimating coronary heart disease risk, without a genetic risk score (Framingham or REGICOR functions)] [15]. Low risk: <5 and <10 %; moderate–low risk: 5–9.9 and 10–14.9 %; moderate–high risk: 10–14.9 and 15–19.9 %; high risk: ≥15 and ≥20 % in REGICOR and Framingham functions, respectively. CHD coronary heart disease
2.2 Cardio inCode® Effectiveness
For the purpose of the model, it was assumed that the patients classified as moderate risk using the standard method could be subjected to an evaluation of CHD risk with Cardio inCode®, being able to reclassify them correctly (in part) to a higher or lower risk, due to its higher sensitivity and specificity. For example, in the case of the REGICOR cohort, 23.14 % of patients with moderate coronary risk using the standard method are correctly reclassified [15]. In the model, only the patients reclassified to a higher risk (6.3 % of patients with moderate coronary risk using the standard method) are considered as that reclassification will have an economic impact since more demanding preventive and/or therapeutic objectives would be established for them. The reclassification values of the CHD risk (Table 2) were obtained from the validation study of Cardio inCode®, which found it to be linearly associated with CHD in the two population-based cohorts: the REGICOR Study (n = 2,351) and the Framingham Heart Study (n = 3,537) [meta-analyzed hazard ratio (95 % CI) ~1.13 (1.01–1.27), per unit]. Inclusion of the multilocus genetic risk score (Cardio inCode®) in the Framingham risk function improved its discriminative capacity in the Framingham sample (c-statistic 72.81 vs. 72.37, p = 0.042) but not in the REGICOR sample. According to both the net reclassification improvement (NRI) index and the integrated discrimination index (IDI), the genetic risk score improved re-classification among individuals with moderate CHD risk [meta-analysis NRI (95 % CI) 17.44 (8.04; 26.83)], but not overall [15]. The main characteristics of the patients included, corresponding to the REGICOR and Framingham populations, respectively, were as follows: (1) age 53.9 [standard deviation (SD) 11.2] and 56.0 (SD 9.3) years; (2) percentage males 47.8 and 43.5 %; (3) systolic blood pressure 132 (SD 20.8) and 127 (SD 18.3) mmHg; (4) diastolic blood pressure 79.5 (SD 10.4) and 75.0 (SD 9.8) mmHg; (5) percentage smokers 22 and 20.2 %; (6) total cholesterol 225 (SD 42.4) and 210 (SD 38.6) mg/dL; (7) high-density lipoprotein (HDL) cholesterol 51.7 (SD 13.3) and 51 (SD 15.2) mg/dL; and (8) percentage with diabetes mellitus 13.8 and 6.4 % [15].
2.3 Transition Probabilities
The transition probabilities between the health states for the REGICOR and Framingham cohorts were obtained from the results of the validation study [15] and other sources [2–4, 28–33] and are summarized in Table 3. The transition probabilities for Cardio inCode® effectiveness (defined as the correct reclassification to a higher risk) adopted in the model were calculated from patients reclassified to higher risk and who had coronary events. The transition probabilities between the health states were calculated using the following formula [34]:
where p is the transition probability from one determined state to another, and r is the percentage of patients who transit in the time t (annually in this model) between those states.
The annual probability of suffering a CHD event from each risk level was also obtained from the Cardio inCode® validation study [15]. According to the economic analysis by Wald et al. [2] and the two clinical trial meta-analyses by Law et al. [3, 4], the reduction of the risk of suffering from a coronary disease in patients with a plausible prevention strategy [standard treatment with statins (HMG-CoA reductase inhibitors) and antihypertensive drugs] was estimated to be 24.4 % in the first year of treatment, 53.3 % in the second year, 73.3 % in the third year, and 80 % annually in the following years. These risk reduction rates were applied to the patients reclassified to high CHD risk (Table 3).
The base-case analysis already includes adherence to drug treatment in the calculations, because (according to the meta-analysis by Law et al. [4]) over all of the trials, 25 % of participants stopped taking their allocated drugs. The probability of death unrelated to CHD from 56 years (mean age of Framingham cohort) or 54 years (mean age of REGICOR cohort) was estimated until 82 years (life expectancy in Spain) from data from the Spanish National Statistics Institute [28].
The mortality in the acute phase of a CHD event (throughout the 12 months following its occurrence) was obtained from two Spanish studies [29, 30]. The probability of a recurrent CHD was calculated from the studies by Ara et al. [31, 33] and according to available Spanish data [29, 32]. Finally, the probability of death by recurrent CHD was calculated from the study by Ahumada et al. [32] (Table 3).
2.4 Utilities
The QALYs were estimated from the utilities (measurement of the perceived quality of life by the patient), which were obtained by using the EQ-5D instrument in two previously published British studies [31, 35] (Table 4). Utilities obtained from Spanish patients were not used because they are not available.
2.5 Perspective and Costs
The analysis was made from the NHS perspective, and thus only direct health costs were included. The unit costs of the health states are from Spanish sources [36, 37] and are shown in Table 4.
The cost per determination of CHD risk with Cardio inCode® (€400) was provided by Ferrer inCode. To calculate the cost of one determination with the standard method, the following use of resources was assumed [37, 38]: (1) two consultations with a primary care physician (one to request the laboratory tests and another to inform the patient of the test results) [€34.24 each consultation]; (2) one determination in blood of total cholesterol (€11.23), HDL cholesterol (€14.88) and glucose (€10.02); and (3) one visit to the health center nurse to take blood pressure (€19.73). In the case of patients evaluated with Cardio inCode®, the costs also incurred as a result of the determination with the standard method were considered. The costs are expressed in euros (€) in year 2011 values. The actualization of the costs was made using the inflation rates in Spain.
2.6 Discount
In the long-term simulation performed in the model, an annual discount of 3.5 % was made in the costs as well as in the benefits (QALYs and LYs).
2.7 Cost-Effectiveness Analysis
Two types of analyses were performed: one deterministic, using the mean values of all the variables (base case), and a probabilistic analysis, by means of a Monte-Carlo simulation using the extreme values of all the variables at random [39, 40]. The following distributions were applied to the variables: (1) Log-normal to the costs; (2) Gamma to the utilities; and (3) Beta to the transition probabilities [30]. A hypothetical cohort of 10,000 patients was used in the Monte-Carlo simulation and 1,000 analyses were performed.
It was considered that Cardio inCode® would be cost effective compared with the standard method for an incremental cost-effectiveness ratio (ICER) (willingness of the NHS to pay) less than €30,000 (or €36,000 updating the 2002 value to the year 2011) per QALY gained or per LY gained without adjusting for its quality [41].
2.8 Sensitivity Analysis
Finally, deterministic sensitivity analyses were performed with extreme values of the following variables: (1) starting with patients with a moderate–low CHD risk (5–9.9 % in REGICOR; 10–15 % in Framingham) with a reclassification rate to a higher risk (moderate–high and high risk) and to a lower risk of 14.7 and 20.8 % for REGICOR and 9.2 and 10.7 % for Framingham [15], considering that the intensive prevention approach would be carried out not only on patients with a high CHD risk, but also in those classified as moderate–high risk; (2) starting with patients with a moderate–high CHD risk (10–14.9 % in REGICOR; 15–20 % in Framingham) with a reclassification rate to a higher risk and a lower risk (high and moderate–low) of 22.8 and 25.8 % for REGICOR and 12.0 and 15.6 % for Framingham [15]; (3) tornado analyses were performed, modifying separately the variables of each category (costs, utilities, and probabilities); and, finally (4) although the base-case analysis already includes adherence [42] to drug treatments in the calculations [3, 4], as in the study of Greving et al. [43], we assumed additional adherence rates of CHD prevention treatment derived from the literature (60 % adherence after year 1, 45 % after year 2, 40 % after year 3, and thereafter remaining stable) [44–46]. These rates were incorporated into the model by adjusting the reduction of the risk of suffering a CHD event in patients with a drug prevention strategy obtained in the meta-analyses of Law et al. [3, 4].
The Markov model was prepared using the program TreeAge Pro Healthcare Module 2009 [47].
3 Results
3.1 Deterministic Analysis
For each patient evaluated with Cardio inCode® instead of the standard method, 0.0256 and 0.0166 QALYs, or 0.0247 and 0.0160 LYs would be gained in the REGICOR and Framingham cohorts, respectively. For a Cardio inCode® price of €400, the additional cost per patient evaluated by this method would be €332 and €355, respectively. Thus, the cost of gaining a QALY compared with the standard method (ICER) would be €12,969 and €21,385 in the REGICOR and Framingham cohorts, respectively, and the cost of gaining a LY would be €13,441 and €22,187, respectively (Table 5). The threshold price of Cardio inCode® to achieve the generally accepted maximum ICER in Spain (€30,000/QALY) [41] would vary between €668 and €836. A large benefit would be achieved in the patient subgroup with a moderate–high risk, with a reclassification to high risk of 22.8 % of the patients, and an ICER of €1,652/QALY and €5,884/QALY (REGICOR and Framingham cohorts). The deterministic sensitivity analyses confirmed the stability of the study, obtaining ICER values in all cases lower than the acceptability threshold. The variables that most determined the result were the costs and utilities of an incident and a recurrent CHD event, the utility of the moderate CHD risk, and the probability of recurrent CHD, as well as the CHD preventive treatment adherence variable. When adherence rates of CHD prevention treatment derived from the literature were assumed, the cost per QALY gained compared with the standard method (ICER) would be €20,350 and €29,396 in the REGICOR and Framingham cohorts, respectively.
According to the tornado diagram (Fig. 2), the variables that most determine the outcome are the utilities of moderate, low, and high CHD risk health states (for REGICOR cohort) and the CHD event probability (moderate risk), CHD event cost, and CHD death probability (for Framingham cohort). However, the value of ‘net monetary benefit’ indicates that (for a willingness to pay €30,000) Cardio inCode® was a cost-effective option for all modified variables.
3.2 Probabilistic Analysis (Monte-Carlo Simulation)
Taking the QALY as the effectiveness variable, Cardio inCode® was the most cost-effective option compared with the standard method (for a willingness to pay less than €30,000 or €36,000 per QALY gained) in 82.0 and 89.5 %, respectively, of the simulations performed on the REGICOR cohort, and 65.7 and 73.7 %, respectively, of the simulations performed on the Framingham cohort (Fig. 3).
Results of the Monte-Carlo simulation: incremental cost-effectiveness expressed as cost per QALY gained (1,000 simulations in a hypothetical cohort of 10,000 patients). a REGICOR (Registre Gironí del Cor) cohort. Cardio inCode® was the most cost-effective option for a willingness to pay <€30,000 or <€36,000 (the latter are presented in this figure) in 82.0 and 89.5 % of the simulations. b Framingham cohort. Cardio inCode® was the most cost-effective option for a willingness to pay <€30,000 or <€36,000 (the latter are presented in this figure) in 65.7 and 73.7 % of the simulations. The results to the right of the diagonal dashed line indicate the cases in which Cardio inCode® was cost effective when compared with the standard option. ICER incremental cost-effectiveness ratio
4 Discussion
In accordance with the method used in this study, the Cardio inCode® clinical–genetic test is a cost-effective option in the evaluation of CHD risk, compared with the standard method, in patients with moderate CHD risk, with a higher benefit particularly in the moderate- to high-risk patients subgroup.
In assessing the results of the study we must take into account study limitations as well as strengths. The main limitation is that we have used a theoretical model, which is by definition a simplified simulation of reality. However, the cost-effectiveness analysis was made using a Markov model [26] with Monte-Carlo probabilistic analyses [40], thus providing a more realistic simulation of the long-term progression of the disease than deterministic models.
The utilities were obtained from two British studies using the EQ-5D instrument [31, 35] and these utilities may not be applicable to the Spanish population, which is another possible limitation. However, in a study based on 83,000 evaluations of 44 states of health with EQ-5D carried out in six European countries, including Spain, there was a higher variability between individuals than between countries [48].
Another possible limitation is that the minimum clinically significant difference in the utility of two health interventions is generally situated around 0.030 QALYs [49, 50] and the gains in QALYs observed in each patient evaluated with Cardio inCode®, in comparison with the standard method, were 0.0256 QALYs for REGICOR and 0.0166 QALYs for Framingham. Thus, it could be considered that the differences observed in utilities are very close to clinical significance in the Spanish cohort of the REGICOR study, although not in the Framingham cohort. However, these individual results, transferred to a hypothetical cohort of 1,000 patients initially classified as moderate CHD risk with the standard method would lead to a relevant gain of 25.6 and 16.6 QALYs in REGICOR and Framingham patients, respectively.
The last possible limitation is that the efficiency of treating all patients with moderate CHD risk has not been compared with the option of treating only those patients who were reclassified to high CHD risk after applying Cardio inCode® in this model. This would be an interesting future analysis.
One of the strengths of this study is that the reclassification rates with Cardio inCode® were obtained in a validation study [15] that included 2,351 Spanish patients from the cohort of the REGICOR study and 3,537 US patients from the cohort of the Framingham study. Another strength is that the transition probabilities between different health states were calculated from that validation study, clinical trials meta-analyses [3, 4], and Spanish studies [29, 30, 32].
Another strength is that all of the costs used in the study were taken from Spanish sources [36–38]. In particular, the costs associated with the CHD risk states were obtained from a Spanish cross-sectional study using a retrospective review of the medical records of 12,828 patients followed up as outpatients in seven primary care centers [36].
A final strength is that the results of the model were very stable, with probabilities of obtaining ICERs lower than €30,000 or €36,000 per QALY gained of 82.0 and 89.5 %, respectively, in the REGICOR cohort and 65.7 and 73.7 %, respectively, in the Framingham cohort. The ICERs were even lower in the scenario of adherence rates of CHD prevention treatment derived from the literature without considering the expected positive effect of genetic information on the motivation to adhere to risk-reducing behaviors and treatment based on published results for other conditions [51, 52].
To our knowledge, this is the first economic analysis on the efficiency of a genetic-based CHD risk score. This study indicates that the clinical–genetic evaluation of CHD risk with Cardio inCode® is cost effective compared with the standard evaluation method in patients with moderate risk.
According to the European Guidelines on Cardiovascular Prevention and its Spanish adaptation by the Spanish Interdisciplinary Committee for Cardiovascular Prevention (CEIPC) of 2008 (http://www.CEIPC.org), pharmacological treatment for the prevention of CHD and therapeutic objectives in relation to the CHD risk in high-risk patients are one of the priorities in cardiovascular prevention. For this reason, the patients who would most benefit from Cardio inCode® would be those with a moderate–high CHD risk, as it will identify a high number of these patients who have, in reality, a higher risk and, therefore, the most adequate preventive strategies and therapeutic objectives will be established for the most effective prevention of CHD.
5 Conclusion
Cardio inCode® improves risk reclassification for moderate CHD risk and particularly in the population at moderate–high CHD risk. This potential value, if confirmed in clinical practice [53], would be cost effective.
References
Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, for the Cuarto Grupo de Trabajo Conjunto de la Sociedad Europea de Cardiología y otras Sociedades sobre Prevención de la Enfermedad Cardiovascular en la Práctica Clínica, et al. Guías de práctica clínica sobre prevención de la enfermedad cardiovascular: versión resumida. Rev Esp Cardiol. 2008;61:e1–49.
Wald NJ, Simmonds M, Morris JK. Screening for future cardiovascular disease using age alone compared with multiple risk factors and age. PLoS ONE. 2011;6(5):e18742. doi:10.1371/journal.pone.0018742.
Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423–6.
Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665.
De la Peña A. Epidemiología de la enfermedad cardiovascular y de sus factores de riesgo en España. In: Suárez C (Coord.) Protocolos de riesgo cardiovascular. Madrid: Sociedad Española de Medicina Interna; 2004. p. 15–24.
Instituto Nacional de Estadística. Defunciones según la causa de muerte. Año 2009. Madrid: INE; 2012.
Pyörälä K, de Backer G, Graham I, Pooloe-Wilson P, Word D. Prevention of coronary heart disease in clinical practice: recommendation of the task force of the European Society of Cardiology, European Atherosclerosis and European Society of Hypertension. Atherosclerosis. 1994;2:121–61.
Riesgo cardiovascular: Guía de actuación en Atención Primaria. Palma de Mallorca: Gerencia de Atención Primaria de Mallorca; 2006.
Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framminghan study. Ann Intern Med. 1971;74:1–12.
Marrugat M, Vila J, Baena-Díez JM, Grau JM, Ramos R, Subirana I, et al. Relative validity of the 10-year cardiovascular risk estimate in a population cohort of the REGICOR study. Rev Esp Cardiol. 2011;64:385–94.
Marrugat J, Solanas P, D’Agostino R, Sullivan L, Ordovas J, Cordón F, et al. Estimación del riesgo coronario en España mediante la ecuación de Framingham calibrada. Rev Esp Cardiol. 2003;56:253–61.
Sans S, Fitzgerald AP, Royo D, Conroy R, Graham I. Calibración de la tabla SCORE de riesgo cardiovascular para España. Rev Esp Cardiol. 2007;60:476–85.
Lluís-Ganella C, Lucas G, Subirana I, Sentí M, Jiménez-Conde J, Marrugat J, et al. Efecto aditivo de diferentes variantes genéticas en el riesgo de cardiopatía isquémica. Rev Esp Cardiol. 2010;63:925–33.
Elosua R, Lluís C, Lucas G. Estudio del componente genético de la cardiopatía isquémica: de los estudios de ligamiento al genotipado integral del genoma. Rev Esp Cardiol. 2009;9(Suppl B):B24–38.
Lluis-Ganella C, Subirana I, Lucas G, Tomás M, Muñoz D, Sentí M, et al. Predictive capacity of the Framingham coronary risk function improved by including a genetic score. Atherosclerosis. 2012;222:456–63.
Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;2:210–5.
Erdmann J, Grosshennig A, Braund PS, Konig IR, Hengstenberg C, Hall AS, et al. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet. 2009;41:280–2.
Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–3.
Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–41.
McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–91.
Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, et al. Genome wide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–53.
Tregouet DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41:283–5.
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–78.
Gerszten RE, Wang TJ. The search for new cardiovascular biomarkers. Nature. 2008;451:949–52.
Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16.
Rubio-Terrés C, Echevarría A. La herramienta clave: modelos de Markov. Pharmacoecon Spa Res Art. 2006;3(Suppl. 2):71–8.
Esperanza de vida al nacimiento. Ambos sexos. Enero/Diciembre de 2010. Instituto Nacional de Estadística. http://www.ine.es. Accessed Dec 2011.
Defunciones según la causa de muerte. Año 2009. Instituto Nacional de Estadística. http://www.ine.es. Accessed Dec 2011.
García-García C, Sanz G, Valle V, Molina L, Sala J, Subirana I, et al. Evolución de la mortalidad intrahospitalaria y el pronóstico a seis meses de los pacientes con un primer infarto agudo de miocardio. Cambios en la última década. Rev Esp Cardiol. 2010;63:1136–44.
Hurtado-Martínez J, Pinar-Bermúdez E, Teruel-Carrillo F, Gimeno-Blanes JR, Lacunza-Ruiz J, Valdesuso R, et al. Mortalidad a corto y largo plazo en mujeres con infarto de miocardio tratado con angioplastia primaria. Rev Esp Cardiol. 2006;59:1113–22.
Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al. Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation. Health Technol Assess. 2008;12(21):iii, xi–xiii, 1–212.
Ahumada M, Cabadés A, Valencia J, Cebrián J, Payá E, Morillas P, Investigadores del registro PRIMVAC, et al. El reinfarto como complicación del infarto agudo de miocardio. Datos del registro PRIMVAC. Rev Esp Cardiol. 2005;58:13–9.
Ara R, Pandor A, Stevens J, Rees A, Rafia R. Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation. Health Technol Assess. 2009;13(34):1–74, 75–118
Petitti DB. Meta-analysis, decision analysis and cost-effectiveness analysis. Methods for quantitative synthesis in medicine. New York: Oxford University Press; 1994.
Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13:509–18.
Sicras A, Velasco S. González n, Rodríguez JL. Evaluación clínica y económica según el grado de riesgo cardiovascular en sujetos pertenecientes a un ámbito poblacional español. Med Clin (Barc). 2008;131:158–9.
Base de datos de costes sanitarios españoles. Versión 3.1. Madrid: Health Value; 2011.
Ruiz I, Ramos JR, Hernández I. Variaciones en la prevención del riesgo cardiovascular: estudio poblacional. Gac Sanit. 2003;17:20–6.
Rubio-Terrés C, Cobo E, Sacristán JA, Prieto L, del Llano J, Badia X, Grupo ECOMED. Análisis de la incertidumbre en las evaluaciones económicas de intervenciones sanitarias. Med Clin (Barc). 2004;122:668–74.
Brigss A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. ¿Qué es una tecnología eficiente en España? Gac Sanit. 2002;16:334–43.
Kadambi A, Leipold RJ, Kansal AR, Sorensen S, Getsios D. Inclusion of compliance and persistence in economic models: past, present and future. Appl Health Econ Health Policy. 2012;10:365–79.
Greving JP, Visseren FLJ, de Wit GA, Algra A. Statin treatment for primary prevention of vascular disease: whom to treat? Cost-effectiveness analysis. BMJ. 2011;342:d1672. doi:10.1136/bmj.d1672.
Helin-Salmivaara A, Lavikainen P, Korhonen MJ, Halava H, Junnila SY, Kettunen R, et al. Long-term persistence with statin therapy: a nationwide register study in Finland. Clin Ther. 2008;30:2228–40.
Avorn J, Monette J, Lacour A, Bohn RL, Monane M, Mogun H, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279:1458–62.
Hiatt JG, Shamsie SG, Schectman G. Discontinuation rates of cholesterol-lowering medications: implications for primary care. Am J Manage Care. 1999;5:437–44.
TreeAge Pro Healthcare Module 2009. Williamstown: TreeAge Software Inc.; 2009.
Greiner W, Weiinen T, Nieuwenhuizen M, Oppe S, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ. 2003;4:222–31.
Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI®): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54.
Wee H-L, Machin D, Loke W-C, Li S-C, Cheung Y-B, Luo N, et al. Assessing differences in utility scores: a comparison of four widely used preference-based instruments. Value Health. 2007;10:256–65.
Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ, Kastelein JJ. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet. 2001;357:165–8.
Arkadianos I, Valdes AM, Marinos E, Florou A, Gill RD, Grimaldi KA. Improved weight management using genetic information to personalize a calorie controlled diet. Nutr J. 2007;18(6):29.
Ashley EA, Hershberger RE, Caleshu C, Ellinor PT, Garcia JG, Herrington DM, American Heart Association Advocacy Coordinating Committee, et al. Genetics and cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2012;126:142–57.
Acknowledgments
We acknowledge Jaume Marrugat and Roberto Elosua from IMIM-Hospital del Mar, Barcelona, Spain, for the fruitful discussion of the results.
Conflict of interest
A. Ramírez de Arellano, A. Gracia, and A. Boldeanu were employees of the company Ferrer Incode at the time of preparation of this manuscript. J. Puig-Gilberte and E. Salas were employees of the company Gendiag at the time of preparation of this manuscript. A. Coca and M. de la Figuera received a research grant from Ferrer for studies in connection with the development of this manuscript. C. Rubio-Terrés and D. Rubio-Rodríguez received an honorarium from Ferrer Internacional in connection with the development of this manuscript.
Contributions of the authors
C. Rubio-Terrés and D. Rubio-Rodríguez made the economic model. A. Ramírez de Arellano and J. Puig-Gilberte reviewed in depth the economic model. C. Rubio-Terrés, D. Rubio-Rodríguez, A. Ramírez de Arellano and E. Salas wrote the first and subsequent versions of the manuscript. All authors contributed to the fruitful discussion of the results and to the review of the different versions of the manuscript. C. Rubio-Terrés is the guarantor for the overall content of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Ramírez de Arellano, A., Coca, A., de la Figuera, M. et al. Economic Evaluation of Cardio inCode®, a Clinical-Genetic Function for Coronary Heart Disease Risk Assessment. Appl Health Econ Health Policy 11, 531–542 (2013). https://doi.org/10.1007/s40258-013-0053-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-013-0053-x