Abstract
Atopic dermatitis (AD) is a common, heterogeneous, inflammatory skin disorder characterized by chronic or relapsing eczematous lesions with intense pruritus and discomfort. Affected patients often experience significant impairment in their quality of life, and the treatment of moderate-to-severe AD forms remains challenging. In the past few decades, increasing knowledge on the AD pathogenesis has driven the development of novel targeted therapies. Interleukin (IL)-13 plays a central and pleiotropic role in AD pathogenesis, contributing directly or indirectly to epidermal barrier disfunction, type-2 inflammation, dysbiosis, fibrosis, and itch response. For this reason, agents selectively targeting IL-13, such as lebrikizumab, emerged as a potential therapy for AD. This article reviews the current evidence about lebrikizumab in the management of AD. The phase II and phase III trials seem to corroborate efficacy of lebrikizumab in the treatment of moderate-to-severe AD, as shown by significant improvement of Eczema Area and Severity Index, body surface area, and pruritus scores. Also, lebrikizumab demonstrated favorable safety and tolerability profiles, with the majority of patients experiencing no significant adverse events. Lebrikizumab seems to be a promising emerging targeted biological agent for patients with moderate-to-severe AD. More data on the long-term efficacy and safety, head-to-head comparisons with other agents, and real-world evidence will help to clarify its place in therapy in AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
IL-13 plays a central and pleiotropic role in the pathogenesis of AD. |
Lebrikizumab is a humanized monoclonal antibody that selectively binds to soluble IL-13, thus preventing IL‐4Rα/IL‐13Rα1 heterodimerization and downstream signaling. |
Phase II and phase III trials seem to corroborate the efficacy and safety of lebrikizumab in the treatment of moderate-to-severe AD. |
1 Introduction
Atopic dermatitis (AD) is the most common chronic inflammatory skin disorder, affecting up to 20% of children and 2–10% of adults worldwide, with an increasing global incidence and prevalence [1,2,3,4]. In most of cases, AD has an onset in early childhood and often precedes other atopic comorbidities such as asthma or allergic rhinitis [1, 4]. A family history of atopy is known to be the strongest risk factor for AD, estimated to increase a child’s risk of developing AD 1.5-fold, up to approximately 3- and 5-fold if one or both of the parents have AD, respectively [3].
AD has a broad clinical spectrum characterized by a persistent or relapsing course of intense itching and eczema lesions (erythema with exudation and crusting in early stages; desquamation, lichenification, and fissuring in later stages) that typically vary in distribution and morphology according to age [3, 4]. Natural history of AD can have heterogeneous trajectories, with active disease beyond childhood being common, including persistent and adult-onset forms [3, 4].
Itching and stigmatization due to visible skin lesions are the primary sources of morbidity in AD [4,5,6]. Both children and adults often suffer from significant impairment in daily activities, sleep, self-esteem, social functioning, and school/work productivity [4,5,6,7]. There is a well-established correlation between AD and an increased incidence of mental disorders, such as depression and anxiety, with a clear association with the severity of the disease [5, 6].
AD is a multifactorial disease resulting from complex gene–gene and gene–environment interactions. Known key factors underlying AD pathogenesis include genetic susceptibility, skin barrier disruption, T-helper 2 (Th2) cell-skewed immune dysregulation, immunoglobulin E (IgE)-mediated sensitization, dysbiosis, and an abnormal itching response [3, 4, 8,9,10]. Interleukin (IL-4) and IL-13 are the main drivers of the Th2 inflammatory axis, and they have a well-known central role in the pathogenesis of the disease [8,9,10,11,12].
As a lifelong disease, long-term therapy is often necessary. In mild-to-moderate AD, topical therapies, such as corticosteroids, calcineurin inhibitors, phosphodiesterase-4 inhibitors, and Janus kinase (JAK) inhibitors, in addition to skin emollients and avoidance of environmental triggers, remain as the mainstay of treatment [13, 14]. If the disease cannot be controlled with this approach or in the presence of moderate-to-severe forms, systemic therapy is required. Conventional immunosuppressant agents (cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil) are highly limited by their toxicities in chronic use [15, 16]. Moreover, none of these agents targets any specific element of the AD pathway.
In the past few decades, advances in the knowledge of the molecular basis behind AD physiopathology has driven the development of targeted biological therapies and small molecules, thus changing the treatment landscape of AD [3, 17]. Dupilumab, a human antibody that binds to the IL-4 alpha subunit receptor inhibiting the activity of both IL-4 and IL-13, was the first non-conventional immunomodulator agent approved for the treatment of moderate-to-severe AD in patients aged 6 months and older. More recently, JAK inhibitors (abrocitinib, baricitinib, and upadacitinib) and tralokinumab (a selective anti-IL‐13 human antibody) were also approved in Europe and the United States for moderate‐to‐severe AD.
Lebrikizumab is another investigational selective anti–IL‐13 monoclonal antibody which binds to a different epitope than tralokinumab [18, 19]. Here we review the role of IL-13 and the most recent data on lebrikizumab in the management of AD.
2 The Role of Interleukin-13 in the Pathogenesis of AD
A multiplicity of multidirectional and interconnected mechanisms are involved in the complex pathogenesis of AD [3, 4]. Epidermal barrier disruption, immune dysregulation (cutaneous and systemic), and disordered microbiome are thought to be the mechanistic drivers of the disease, interacting each other in a self-amplifying loop [3, 4].
AD is an immunologic disease characterized by an inappropriate activation of Th2 cell-mediated pathways, leading to overexpression of associated type 2 cytokines, such as IL‐13 and IL‐4 [4, 11]. These cytokines have been underlined as the central mediators of AD pathogenesis, exerting its effects through a shared heterodimeric receptor consisting of either the IL-4Rα subunit and the common γ-receptor chain (known as type 1 receptor) binding only IL-4 or the IL-4Rα subunit and IL-13Rα1 subunit (also known as the type 2 IL-4 receptor) binding both IL-4 and IL-13 [4, 11, 20, 21]. IL-13 also binds to IL-13Rα2, the function of which remains enigmatic. It has been thought to have an anti‐inflammatory decoy function via internalization of excessive IL‐13 circulant levels, lacking any known signal transduction function [21, 22].
Binding of IL-4 and IL-13 to their respective receptor subunits triggers its dimerization and subsequent downstream signaling via JAK-mediated phosphorylation of the signal transducer and activator of transcription 6 (STAT6). This transcription factor promotes Th2 cell differentiation, stimulates IgE class-switching, and suppresses the activity of regulatory T cells [4, 11, 20, 21]. Th2 cytokines also contributes to barrier disfunction by downregulating the expression of key structural proteins, such as fillagrin, involucrin, and loricrin [11, 21, 23]. Skin barrier damage and inflammation can be further enhanced by microbiotal abnormalities, in particular the overgrowth of Staphylococcus aureus (S. aureus) [4, 10]. IL-13 and IL-4 have been found to reduce antimicrobial peptide (AMP) expression by keratinocytes, increasing propensity to S. aureus colonization and infection [20, 23]. Damage to the skin barrier leads to increased transepidermal water loss and favors the penetration of potential irritants, allergens, and microorganisms into the skin, which can in turn enhance the inflammatory immune response [3, 24]. IL-13 and IL-4 are also pruritogens, contributing to itch response mainly through a histamine-independent sensory nerve stimulation, either directly or by amplifying the IL-31 pruritogenic effects [9, 23, 25]. Physical damage by scratching further increases skin disruption [9].
Recently, IL‐13 has been suggested as the primary cytokine involved in AD inflammation, rather than IL-4 [12]. Studies have shown that protein and mRNA levels of IL-13 are consistently dominant in lesional skin of AD patients, in contrast to the barely detectable levels of IL-4 [26,27,28,29]. Furthermore, levels of IL‐13 in lesional skin have been significantly correlated with disease severity, as measured by the Scoring Atopic Dermatitis (SCORAD) tool [28, 30]. IL-13-mediated inflammation also promotes recruitment of fibroblasts and collagen deposition, thus inducing skin fibrotic remodeling and lichenification [20, 31]. Because of its central role in pathogenesis, targeting IL-13 appears to be a valid therapeutic strategy for AD.
3 Mechanism of Action and Pharmacology of Lebrikizumab
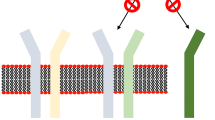
Lebrikizumab is a humanized IgG4κ monoclonal antibody which binds to soluble IL-13 with high affinity to an epitope that does not interfere with its binding to the receptor but instead prevents IL‐4Rα/IL‐13Rα1 heterodimerization, thus blocking downstream signaling [21, 22]. Unlike tralokinumab, lebrikizumab does not prevent IL-13 binding to the IL-13Rα2 decoy receptor, therefore allowing it to maintain this putative endogenous regulatory mechanism [21, 22]. Lebrikizumab’s mechanism of action is schematized in Fig. 1.
Lebrikizumab binds to soluble IL-13 with high affinity and prevents IL‐4Rα/IL‐13Rα1 heterodimerization, thus blocking downstream signaling. Lebrikizumab does not prevent IL-13 binding to the IL-13Rα2 decoy receptor, therefore allowing it to maintain this endogenous regulatory mechanism [12, 21, 22]. Adapted from Bieber [12]
Lebrikizumab was first investigated for the treatment of asthma. A meta-analysis of more than 2000 patients that received lebrikizumab for moderate-to-severe asthma aimed to characterize its pharmacokinetics [32]. Lebrikizumab is administered by subcutaneous injection and has consistently shown linear dose-proportional pharmacokinetics, high bioavailability (estimated as 85%), and a half-life of 19–26 days [18, 32].
4 Clinical Efficacy in AD
4.1 Phase II trials
TREBLE trial (NCT02340234) was a randomized, placebo-controlled, double-blind, phase IIa (proof of concept) multicenter study that evaluated efficacy and safety of lebrikizumab in different regimens as an add-on therapy to topical corticosteroids (TCS) in adults with moderate-to-severe forms of AD. Patients (n = 209) aged 18–75 years with the diagnosis of moderate-to-severe AD (required to have an Eczema Area and Severity Index (EASI) ≥ 14, an Investigator Global Assessment (IGA) ≥ 3, an affected body surface area (BSA) ≥ 10%, and a visual analogue scale for pruritus ≥ 3) inadequately controlled with TCS, and emollients were randomly distributed 1:1:1:1 into four arms to receive (1) lebrikizumab 125 mg single dose at baseline, (2) lebrikizumab 250 mg single dose at baseline, (3) lebrikizumab 125 mg every 4 weeks (Q4W) for 12 weeks, and (4) placebo Q4W for 12 weeks. All patients were concomitantly treated with medium-potency TCS twice daily to avoid study dropouts. The primary endpoint, defined as the achievement of a 50% reduction in EASI score (EASI 50) from baseline to week 12, was reached in a significantly greater proportion of patients treated with lebrikizumab 125 mg Q4W than those who received placebo (82.4% versus 62.3%; p = 0.026), with no statistically significant response in single-dose groups. Also, 125 mg Q4W group had significantly more patients achieving secondary endpoints, including an EASI 75 response (54.9% versus 34.0% placebo; p = 0.036), a 50% reduction in the SCORAD tool (SCORAD 50; 51.0% versus 26.4% placebo; p = 0.012), and an IGA score of 0 (clear) or 1 (almost clear; 33.3% versus 18.9% placebo; p = 0.098) at week 12. Besides, the response plateau might not have been reached by the end of the study, as suggested by upward sloping response curves. Again, no statistically significant response was seen in single-dose groups at week 12, but the 250 mg single-dose group have shown numerically higher and earlier responses in many outcomes evaluated, denoting a possible dose-response relationship [18].
A phase IIb randomized, placebo-controlled, double-blind, dose-ranging clinical trial (NCT03443024) was conducted to further elucidate efficacy, dose-response, and safety of lebrikizumab. Adult patients (n = 280) with chronic moderate-to-severe AD (defined as EASI ≥ 16, IGA ≥ 3, and an affected BSA ≥ 10%) that did not get disease controlled with standard topical treatment were randomized in a 3:3:3:2 ratio into four arms: (1) lebrikizumab loading dose (LD) of 250 mg, followed by 125 mg Q4W; (2) lebrikizumab LD of 500 mg, followed by 250 mg Q4W; (3) lebrikizumab LD of 500 mg at baseline and at week 2, followed by 250 mg Q2W; and (4) placebo Q2W. In that trial, lebrikizumab was studied as monotherapy, although rescue therapy with TCS was allowed if needed. In the primary endpoint, all lebrikizumab groups demonstrated a statistically significant dose-dependent least square mean percentage changes in EASI score from baseline to week 16 [−62.3% (p = 0.02) in 125 mg Q4W; −69.2% (p = 0.002) in 250 mg Q4W; −72.1% (p < 0.001) in 250 mg Q2W] when compared with placebo (−41.1%). Lebrikizumab 250 mg groups also showed significantly superior response rates in secondary endpoints, including IGA 0/1 response, EASI 50, EASI 75, and EASI 90. Dose-dependent differences between placebo and lebrikizumab groups were noted as early as week 4. All lebrikizumab groups experienced improvement in pruritus Numerical Rating Scale (NRS) score (percentage mean changes from baseline: −35.9% in 125 mg Q4W; −49.6% in 250 mg Q4W; and −60.6% in 250 mg Q2W) versus placebo (mean worsening of 4.3%), with a reduction of at least 4 points in NRS score seen as early as day 2 in the high-dose lebrikizumab-treated group (15.3% versus 4.3% in placebo group). Notably, in placebo-treated patients, rescue TCS use was approximately 3-fold higher, earlier, and for a longer period than lebrikizumab-treated patients. Study outcomes did not appear to have been confounded by TCS use [19]. The main results of phase II trials are summarized in Table 1.
4.2 Phase III trials
The lebrikizumab Phase III program consists of five key global studies, including two monotherapy pivotal duplicate studies (ADvocate 1 and 2 [33, 34]), a combination study (ADhere [35]), as well as long-term extension (ADjoin [36]) and pediatric studies [37]. Available results from phase III studies are included in Table 2.
The pivotal studies ADvocate 1 (NCT04146363) and ADvocate 2 (NCT04178967) are two randomized, double-blind, placebo-controlled, parallel-group, phase III trials designed to evaluate lebrikizumab as monotherapy in adult and adolescent patients (12 years or older and weighing at least 40 kg) with moderate-to-severe AD (required to have an EASI ≥ 16, IGA ≥ 3, and affected BSA ≥ 10%), consisting of a 16-week induction treatment period followed by a 36-week maintenance treatment period [33, 34].
During the induction phase, patients (n = 424 in ADvocate 1; n = 427 in ADvocate 2) were randomized 2:1 to receive either (1) 500 mg of lebrikizumab as a loading dose at baseline and week 2 followed by 250 mg Q2W from weeks 4 to 14, or (2) placebo Q2W. Topical treatment (such as TCS) was allowed as rescue therapy, but if systemic rescue therapy was deemed to be necessary, lebrikizumab was discontinued. In both trials, a significantly higher percentage of patients taking lebrikizumab achieved the primary outcome response, defined as an IGA score of 0 or 1, with a reduction of at least 2 points from baseline at week 16 [43% in ADvocate 1 (versus 13% of placebo) and 33% in ADvocate 2 (versus 11% of placebo)] [33].
Patients receiving lebrikizumab also experienced statistically significant improvements in skin clearance and pruritus, as well as improvements in interference of itch on sleep and quality of life, as measured by key secondary efficacy endpoints (EASI 75, EASI 90, pruritus NRS ≥ 4 points improvement, Sleep-Loss Scale Score ≥ 1 point improvement, and DLQI). A higher percentage of patients had an EASI 75 response at week 16 in the lebrikizumab group (59% in ADvocate 1; 52% in ADvocate 2) than in the placebo group (16% and 18%, respectively; p < 0.001). In both studies, statistical significance was achieved starting at week 4 for IGA 0/1, EASI 90, and pruritus NRS. In trial 1 (but not in trial 2), patients treated with lebrikizumab achieved clinically significant improvement in pruritus NRS versus placebo as early as week 2 [33].
Maintenance treatment period (weeks 16–52) is based on re-randomization 2:2:1 of responders in the induction period (patients who achieved an EASI 75 or IGA 0/1 with ≥ 2-point improvement, and no rescue medication use) to receive (1) lebrikizumab 250 mg Q2W, (2) lebrikizumab 250 mg Q4W, with one placebo injection 2 weeks after each lebrikizumab injection, or (3) placebo Q2W. Patients who needed rescue treatment for AD during the induction period or who did not meet protocol-defined response criteria at week 16 were eligible for treatment in an escape arm, where they received lebrikizumab 250 mg Q2W from week 16–52 [34].
Combining the results of ADvocate 1 and 2, the proportion of patients who maintained an IGA score 0/1 at week 52 was greater in those treated with lebrikizumab Q2W (71%) and lebrikizumab Q4W (77%) than those in the withdrawal arm (48%). EASI 75 was maintained by 78% of patients treated with lebrikizumab Q2W, 82% of patients treated with lebrikizumab Q4W, and 66% of patients in the withdrawal arm at week 52. EASI 90 response rates were respectively 64%, 66%, and 42%. Regarding pruritus NRS, 85% of patients on lebrikizumab Q2W and Q4W maintained a ≥ 4-point improvement from baseline to week 52 versus 66% in the withdrawal arm. Most patients did not require topical rescue therapy, although use was permitted during the maintenance period [34].
ADhere (NCT04250337) is a 16-week randomized, double-blind, placebo-controlled, parallel-group, phase III study designed to evaluate the efficacy and safety of lebrikizumab when used in combination with TCS treatment, compared with placebo, in combination with TCS in adult and adolescent patients with moderate-to-severe AD. The study included 211 patients whose symptoms were inadequately controlled by TCS with or without topical calcineurin inhibitors, that were randomized (2:1, lebrikizumab: placebo) to treatment with either (1) lebrikizumab LD of 500 mg at baseline and week 2, followed by 250 mg Q2W, or (2) placebo Q2W. Patients were provided with mid-potency TCS (triamcinolone acetonide 0.1% cream) and low-potency TCS (hydrocortisone 1% cream, for sensitive skin areas) which could be tapered, stopped, or resumed as needed. The primary endpoints consisted of achieving an IGA score of 0 or 1 with a reduction of at least 2 points from baseline and EASI 75 score at 16 weeks. Key secondary endpoints were measured by EASI, the pruritus NRS score, sleep loss due to pruritus, and the DLQI. Among patients taking lebrikizumab plus TCS, 41% achieved an IGA 0/1 response at week 16 compared with 22% patients taking placebo plus TCS (p = 0.01). Also, 70% of patients taking lebrikizumab plus TCS achieved an EASI 75 response at week 16, compared with 42% taking placebo plus TCS (p < 0.001). Significant differences between patients receiving lebrikizumab with TCS and placebo with TCS were observed as early as 4 weeks for EASI 75. Patients in the lebrikizumab group also achieved statistically significant improvements across all key secondary endpoints when compared with placebo plus TCS, including percentage in EASI score from baseline to week 16 [−76.5% versus −53.1%; p < 0.001], ≥ 4-point improvement in pruritus NRS (51% versus 36%; p < 0.05), ≥ 2-point improvement in Sleep-Loss Scale Score (35% versus 18%; p < 0.05) and ≥ 4-point improvement in DLQI (79% versus 57%; p < 0.05), at week 16. Clinically meaningful differences were observed as early as 4 weeks [35].
ADjoin (NCT04392154) is a recruiting, phase III extension, 110-week clinical trial designed to assess the long-term safety and efficacy of lebrikizumab for moderate-to-severe AD. The study will include patients that have completed participation in a lebrikizumab parent study and will also be open to additional participants [36]. ADore (NCT04250350) is a 52-week, open-label, single arm study that will assess the safety and efficacy of lebrikizumab in adolescent participants (≥ 12–< 18 years and weighing at least 40 kg) with moderate-to-severe AD who are candidates for systemic therapy [37]. A study in pediatric population, 6 months to < 18 years of age, is already in progress. It consists of a 16-week randomized, double-blind study, which aims to measure the effect, safety, and pharmacokinetics of lebrikizumab in pediatric participants with moderate-to-severe AD [37]. The effect of lebrikizumab on vaccine immune responses in adult patients is being investigated in a phase III randomized, double-blind, placebo-controlled trial, ADopt (NCT04626297) [38]. Ongoing clinical trials investigating lebrikizumab for treatment of AD are available in Table 3.
5 Safety
Safety results from phase II and III clinical trials are summarized in Tables 1 and 2, respectively.
In the phase II TREBLE trial there were no imbalances in proportions of patients reporting adverse events (AE) between lebrikizumab-treated and placebo groups (67% in lebrikizumab groups versus 66% in placebo group), as well in serious AE, events leading to discontinuation, and overall infections. Reported AE were mild to moderate in intensity and lasted a median of 1–3 days. No dose-response relationship was found. Conjunctivitis was reported in 15 patients (9.6%) in the lebrikizumab group, but also in 4 patients (7.5%) in the placebo group. Herpetic infections and peripheral eosinophilia occurred only in lebrikizumab-treated patients but were infrequent (3.8% and 3.2%, respectively) and nonserious. All of these eosinophil-associated events were asymptomatic [18].
In the phase IIb trial, AE were reported by 55.7% of lebrikizumab-treated patients versus 46.2% of those who received placebo, most of which were also mild to moderate in intensity and did not lead to trial discontinuation. No serious AE were considered to be related to lebrikizumab. Most common AE in lebrikizumab groups (≥ 5% of patients) included upper respiratory tract infections, nasopharyngitis, headache, injection site pain, and fatigue. Low rates of AE of clinical interest were reported in the lebrikizumab-treated group, namely injection site reactions (5.7% versus 1.9% in placebo) and conjunctivitis (1.4%, 3.8%, and 2.7% for the lebrikizumab 125mg Q4W, 250mg Q4W, and 250mg Q2W groups, respectively, versus none in placebo). A similar rate of herpesvirus infection was seen in lebrikizumab-treated (3.5%) and placebo-treated (3.8%) patients. Transient small increases in eosinophil count were noted in lebrikizumab patients [18]. No anaphylactic reactions or deaths have been described in both trials [18, 19].
This data seems to be consistent with the phase III results of ADvocate 1 and ADvocate 2 [33, 34]. During the induction period, patients taking lebrikizumab reported an overall lower frequency of AE in both ADvocate 1 (lebrikizumab: 45%, placebo: 52%) and ADvocate 2 (lebrikizumab: 53%, placebo: 66%), and no notable differences in discontinuation rates due to AE were observed between lebrikizumab group (1.4%) and placebo (1.7%). The most common adverse event consistently reported with higher frequency in patients who received lebrikizumab (versus placebo) was conjunctivitis (7.4% versus 2.8% in trial 1; 7.5% versus 2.1% in trial 2). On the other hand, the incidence of skin infections was lower among patients receiving lebrikizumab (2.8% versus 5.7% in trial 1 and 1.4% versus 6.2% in trial 2) [33].
Considering all lebrikizumab treated patients through the 52 weeks, 63% reported any treatment emergent AE. The most common AE in ADvocate 1 and 2 for those on lebrikizumab were conjunctivitis (8% in both studies), nasopharyngitis (7% and 10%, respectively), headache (3% and 6%, respectively), herpes infection (5%), injection site reactions (1.8% and 2.9%, respectively), and eosinophilia (1.3% and 1.7%, respectively) [33, 34].
Most AE across the two studies were mild or moderate in intensity and nonserious and did not lead to treatment discontinuation. Serious AE were reported by 3% of patients, which includes 1 death that was assessed as being unrelated to the study drug. Across both trials, 3.1% of patients reported an AE leading to treatment discontinuation [33, 34].
In ADhere, patients taking lebrikizumab plus TCS reported a higher frequency of AE (43%) when compared with placebo plus TCS (35%). Also, most AE were mild or moderate in intensity and nonserious and did not lead to treatment discontinuation. The most common AE in patients taking lebrikizumab were headache (5%), conjunctivitis (5%), herpes infection (3%), injection site reactions (3%), and hypertension (3%) [35].
ADjoin is a 110-week ongoing clinical trial that will assess the long-term safety of lebrikizumab in AD as the primary endpoint [36] (Table 2). Data on the drug–drug interactions of lebrikizumab are currently unavailable.
6 Discussion
AD is a heterogeneous skin inflammatory disease with a complex pathogenesis and therapeutic challenges. Although multiple molecules are implicated in the pathogenesis of AD, the central and pleiotropic role of IL‐13 has been elucidated. IL-13 directly contributes to epidermal barrier disruption, induces Th2-mediated inflammation, reduces synthesis of AMP favoring skin dysbiosis, promotes fibrosis, and mediates itch response either directly or through IL-31 [3, 4, 8,9,10,11,12, 31]. Both IL-13 and IL-4 share overlapping biological functions, with recent data suggesting a greater relative importance of IL-13 in the pathogenesis of AD [12, 20, 29]. Therefore, agents specifically targeting IL-13, namely tralokinumab and lebrikizumab, emerged as potential therapeutic alternatives to dupilumab, which antagonizes both IL-4 and IL-13 signaling. In fact, inhibition of IL-13 alone seems to be sufficient to reach adequate therapeutic responses in AD, as demonstrated by the clinical efficacy of tralokinumab (already approved by Food and Drug Administration and European Medicines Agency) and lebrikizumab.
In the phase II study TREBLE, lebrikizumab 125 mg Q4W led to significant improvement of all disease severity scores assessed at week 12, with no significant response seen in single-dose groups. However, the lebrikizumab 250 mg single-dose group has shown numerically higher and earlier responses for several outcomes, thus suggesting a dose-response relationship or even a potential benefit of a loading dose [18]. In the phase IIb study, lebrikizumab showed rapid onset of action and dose-dependent efficacy, with statistically significant improvements in skin lesions, pruritus, and quality of life scores at week 16. Notably, a reduction in pruritus severity was observed as early as day 2 in patients receiving a high dose of lebrikizumab (500 mg) [19].
The results of available phase III trials seem to corroborate effectiveness of lebrikizumab in the treatment of moderate-to-severe AD. Data from ADvocate and ADhere trials demonstrate the potential for lebrikizumab to reduce disease burden for patients with uncontrolled AD, when used either alone or combined with TCS, respectively. Lebrikizumab demonstrated rapid onset of action. Clinically meaningful differences were observed as early as week 4 for skin clearance, pruritus, and quality of life measures. At week 16, more than 50% of patients receiving lebrikizumab achieved an EASI 75 response, increasing to 70% when combined with TCS. At this time, in all trials, more than one-third of the patients had clear or almost clear skin [33,34,35].
Results from 52-week ADvocate analyses demonstrated that around 80% of lebrikizumab responders maintained improvements in skin clearance, disease severity, and pruritus. Notably, during the maintenance period, dosing with lebrikizumab Q4W provided similar clinical responses to dosing lebrikizumab Q2W. These results may support less frequent dosing regimens, which translates into greater comfort for patients. It was also observed that loss of clinical response following withdrawal of lebrikizumab was slow, with about half of patients re-randomized to placebo still showing clear or almost clear skin at week 52. More than 60% maintained improvement on disease severity and pruritus scores [34].
The use of rescue medication (mostly TCS) was protocoled and allowed to avoid drop-outs, limiting the evaluation of the efficacy of lebrikizumab as a monotherapy. On the other hand, add-on TCS therapy reflects real-life clinical practice in the management of AD. Across all studies, lebrikizumab-treated patients showed less requirement of rescue medication [18, 19, 33,34,35]. Also, data reported in ADhere suggest an added benefit with TCS combination therapy [35].
Rapid and consistent efficacy of lebrikizumab in the relief of pruritus is worthy of note, since it is considered the most burdensome symptom among patients with AD [19, 33]. To better clarify the mechanisms underlying this clinical finding, a recent preclinical study [25] was performed to test the direct effects of IL-13 and lebrikizumab in neuronal itch pathways, using a human dorsal root ganglion model. The data obtained provides additional support to the hypothesis that IL-13 is a potent enhancer of neuronal responses to several pruritogenic stimuli, which are effectively blocked by lebrikizumab. Lebrikizumab shown to successfully reverse IL-13-elicited neuronal itch sensitization and excitability in a dose-dependent fashion, and also shown to downregulate some gene transcriptional changes driven by IL-13, exerting significant effects in the first 24 h after administration. Thus, lebrikizumab seems to directly address the IL-13-enhanced neuronal itch, which can explain its antipruritogenic efficacy in AD patients [25].
Regarding the safety profile, lebrikizumab seems to be well tolerated in AD patients. The majority of patients experienced none or only mild side effects, which is consistent with what was previously observed in various phase II and phase III clinical trials for asthma [39,40,41]. The most commonly reported AE were conjunctivitis (2–10%), nasopharyngitis (5–10%), headache (3–6%), and injection site pain [18, 19, 33,34,35]. Transient and mild eosinophilia was also noted, but with no clinical implications. These changes are probably related to the decrease of eosinophil trafficking from the blood to the tissues as a result of reduced chemotaxis by inhibiting IL-13 activity [18, 40, 42]. Of note, the incidence of skin infections in ADvocate studies were lower in the lebrikizumab groups than in the placebo groups, thus suggesting a possible improvement of the skin barrier function and immune-response in lebrikizumab-treated patients [33].
Dupilumab-induced conjunctivitis has emerged as a considerable issue with regard to the tolerability profile in AD patients, affecting approximately 1 in 4 dupilumab-treated patients based in clinical trials and in real-world experience [43]. In patients receiving lebrikizumab, the occurrence of conjunctivitis was lower than 10% in both phase II and III trials, with no clear dose-response pattern. These rates were only slightly higher than those of placebo groups, remembering that the prevalence of conjunctivitis is increased in AD by itself [18, 19, 33,34,35]. In ADvocate trials, one-third of patients who reported conjunctivitis also reported a prior history of conjunctivitis [34].
Similar results have been reported in tralokinumab clinical trials, thus suggesting that selective IL-13 inhibitors may be associated with a minor increased risk of conjunctivitis in AD patients, but lower than dupilumab [19, 44]. The pathogenesis behind these findings is not fully understood. It has been suggested that inhibition of IL-4 signaling may induce a Th1 response, which leads to interferon γ-mediated goblet cell apoptosis and reduction in mucin production, thus causing eye dryness and conjunctivitis [19, 43]. The lower incidence of conjunctivitis with selective IL-13 inhibitors can reveal a potential advantage over dupilumab, although head-to-head studies are missing.
The vast majority of AE were nonserious, mild to moderate in intensity, resolved with simple general measures, and did not lead to treatment discontinuation [18, 19, 33,34,35]. With the 110-week trial ADjoin, it is expected that further data on long-term efficacy, posology, and safety of lebrikizumab in AD will become available [36]. Clinical trials in the pediatric population are also ongoing [37].
JAK inhibitors also emerged as an effective and fast acting therapeutic option, but their safety profile may raise some concern, as JAK/STAT signaling pathway plays a role in several crucial immunological and cellular processes. These agents were associated with an increased risk of infections (including infection by opportunistic agents), hematopoietic dysfunction resulting in cytopenias, thromboembolic events, malignancies, and lipid abnormalities [45].
Lebrikizumab and tralokinumab are both selective IL-13 inhibitors that differ in their binding epitopes [44]. Cendakimab [46] is another investigational IL-13 antibody currently in phase II clinical trials, with a mechanism of action similar to tralokinumab. While tralokinumab and cendakimab prevent IL-13 from binding to both IL-13Rα1 and IL-13Rα2, lebrikizumab blocks downstream signaling through the IL‐4Rα/IL‐13Rα1 heterodimeric receptor and does not prevent IL-13 binding to the IL-13Rα2 decoy receptor, thus allowing putative neutralization of excessive IL-13 through the decoy receptor IL-13Rα2 [47,48,49]. In vitro, lebrikizumab has shown a stronger binding affinity and a higher inhibitory potency of IL-13 than tralokinumab or cendakimab [47,48,49]. However, it is not clear that these differences have clinical implications.
Direct comparison between lebrikizumab and other targeted therapies, such as dupilumab, tralokinumab, and JAK inhibitors, is difficult because no head-to-head studies have been carried out, and individual studies have different designs, durations, population sizes, selection criteria of the participants, and concomitant TCS use. Head-to-head studies and network metanalyses comparing the efficacy and safety of lebrikizumab with these agents is warranted in the future.
Current data suggests that targeting IL-13 alone may be enough to achieve adequate therapeutic responses in patients with AD, maybe with less side effects, and supports the hypothesis that IL-13 is the pivotal cytokine in the pathogenesis of AD. Lebrikizumab seems to be a promising targeted biological agent for patients with moderate-to-severe AD.
7 Conclusion
IL-13 is a key cytokine involved in the pathogenesis of AD. The results of phase II and phase III trials seem to corroborate the efficacy of the selective IL-13 inhibitor lebrikizumab in the treatment of moderate-to-severe AD, with a favorable safety profile. More data on the long-term efficacy and safety, head-to-head comparisons with other agents, and real-world evidence will help to clarify its place in the therapeutic ladder of AD.
References
Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66:8–16. https://doi.org/10.1159/000370220.
Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–93. https://doi.org/10.1111/all.13401.
Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018. https://doi.org/10.1038/s41572-018-0001-zReviews.
Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–60. https://doi.org/10.1016/S0140-6736(20)31286-1.
Rønnstad ATM, Halling-Overgaard AS, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79(3):448-456.e30. https://doi.org/10.1016/j.jaad.2018.03.017.
Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131(2):428–33. https://doi.org/10.1016/j.jaci.2012.10.041.
Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The Burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. https://doi.org/10.1016/j.jid.2016.07.012.
Fujii M. Current understanding of pathophysiological mechanisms of atopic dermatitis: interactions among skin barrier dysfunction, immune abnormalities and pruritus. Biol Pharm Bull. 2020;43(1):12–9. https://doi.org/10.1248/bpb.b19-00088.
Furue M, Ulzii D, Vu YH, Tsuji G, Kido-Nakahara M, Nakahara T. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16(2):97–107. https://doi.org/10.22034/iji.2019.80253.
David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21–37. https://doi.org/10.1007/978-3-319-64804-0_3.
Silverberg JI, Kantor R. The role of interleukins 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol Clin. 2017;35(3):327–34. https://doi.org/10.1016/j.det.2017.02.005.
Bieber T. Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy Eur J Allergy Clin Immunol. 2020;75(1):54–62. https://doi.org/10.1111/all.13954.
Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: sectin 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32. https://doi.org/10.1016/j.jaad.2014.03.023.
Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–82. https://doi.org/10.1111/jdv.14891.
Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: sectin 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–49. https://doi.org/10.1016/j.jaad.2014.03.030.
Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–78. https://doi.org/10.1111/jdv.14888.
Renert-Yuval Y, Guttman-Yassky E. What’s new in atopic dermatitis. Dermatol Clin. 2019;37(2):205–13. https://doi.org/10.1016/j.det.2018.12.007.
Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(5):863-871.e11. https://doi.org/10.1016/j.jaad.2018.01.017.
Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156(4):411–20. https://doi.org/10.1001/jamadermatol.2020.0079.
Tubau C, Puig L. Therapeutic targeting of the IL-13 pathway in skin inflammation. Expert Rev Clin Immunol. 2021;17(1):15–25. https://doi.org/10.1080/1744666X.2020.1858802.
Moyle M, Cevikbas F, Harden JL, Guttman-Yassky E. Understanding the immune landscape in atopic dermatitis: the era of biologics and emerging therapeutic approaches. Exp Dermatol. 2019;28(7):756–68. https://doi.org/10.1111/exd.13911.
Ultsch M, Bevers J, Nakamura G, et al. Structural basis of signaling blockade by anti-IL-13 antibody lebrikizumab. J Mol Biol. 2013;425(8):1330–9. https://doi.org/10.1016/j.jmb.2013.01.024.
Dubin C, del Duca E, Guttman-Yassky E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev Clin Immunol. 2021;17(8):835–52. https://doi.org/10.1080/1744666X.2021.1940962.
Torres T, Ferreira EO, Gonçalo M, Mendes-Bastos P, Selores M, Filipe P. Update on atopic dermatitis. Acta Med Port. 2019;32(9):606–13. https://doi.org/10.20344/amp.11963.
Miron Y, Miller PE, Hughes C, Indersmitten T, Lerner EA, Cevikbas F. Mechanistic insights into the antipruritic effects of lebrikizumab, an anti–IL-13 mAb. J Allergy Clin Immunol. 2022;150(3):690–700. https://doi.org/10.1016/j.jaci.2022.01.028.
Tsoi LC, Rodriguez E, Degenhardt F, et al. Atopic dermatitis Is an IL-13–dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 2019;139(7):1480–9. https://doi.org/10.1016/j.jid.2018.12.018.
Koppes SA, Brans R, Ljubojevic Hadzavdic S, Frings-Dresen MHW, Rustemeyer T, Kezic S. Stratum corneum tape stripping: monitoring of inflammatory mediators in atopic dermatitis patients using topical therapy. Int Arch Allergy Immunol. 2016;170(3):187–93. https://doi.org/10.1159/000448400.
Szegedi K, Lutter R, Res PC, et al. Cytokine profiles in interstitial fluid from chronic atopic dermatitis skin. J Eur Acad Dermatol Venereol. 2015;29(11):2136–44. https://doi.org/10.1111/jdv.13160.
Tazawa T, Sugiura H, Sugiura Y, Uehara M. Relative importance of IL-4 and IL-13 in lesional skin of atopic dermatitis. Arch Dermatol Res. 2004;295(11):459–64. https://doi.org/10.1007/s00403-004-0455-6.
Ungar B, Garcet S, Gonzalez J, et al. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol. 2017;137(3):603–13. https://doi.org/10.1016/j.jid.2016.09.037.
Oh MH, Oh SY, Yu J, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011;186(12):7232–42. https://doi.org/10.4049/jimmunol.1100504.
Zhu R, Zheng Y, Dirks NL, et al. Model-based clinical pharmacology profiling and exposure-response relationships of the efficacy and biomarker of lebrikizumab in patients with moderate-to-severe asthma. Pulm Pharmacol Ther. 2017;46:88–98. https://doi.org/10.1016/j.pupt.2017.08.010.
Silverberg JI, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. 2023;388:1080–91. https://doi.org/10.1056/NEJMoa2206714.
Blauvelt A, et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br J Dermatol. 2023. https://doi.org/10.1093/bjd/ljad022.
Simpson EL, et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: a randomized clinical trial (ADhere). JAMA Dermatol. 2023;159(2):182–91. https://doi.org/10.1001/jamadermatol.2022.5534.
ClinicalTrials.gov. Long-term safety and efficacy study of lebrikizumab (ly3650150) in participants with moderate-to-severe atopic dermatitis (ADjoin). https://clinicaltrials.gov/ct2/show/NCT04392154. Accessed 4 Apr 2023.
ClinicalTrials.gov. Study to assess the safety and efficacy of lebrikizumab (ly3650150) in adolescent participants with moderate-to-severe atopic dermatitis (ADore). https://clinicaltrials.gov/ct2/show/NCT04250350. Accessed 4 Apr 2023.
ClinicalTrials.gov. A study of lebrikizumab (LY3650150) on vaccine response in adults with atopic dermatitis (ADopt-VA). https://clinicaltrials.gov/ct2/show/NCT04626297. Accessed 4 Apr 2023.
Hanania NA, Noonan M, Corren J, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70(8):748–56. https://doi.org/10.1136/thoraxjnl-2014-206719.
Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4(10):781–96. https://doi.org/10.1016/S2213-2600(16)30265-X.
Korenblat P, Kerwin E, Leshchenko I, et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir Med. 2018;134:143–9. https://doi.org/10.1016/j.rmed.2017.12.006.
Wechsler ME, et al. Effect of dupilumab on blood eosinophil counts in patients with asthma, chronic rhinosinusitis with nasal polyps, atopic dermatitis, or eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2022;10(10):2695–709. https://doi.org/10.1016/j.jaip.2022.05.019.
Ferreira S, Torres T. Conjunctivitis in patients with atopic dermatitis treated with dupilumab. Drugs Context. 2020. https://doi.org/10.7573/DIC.2020-2-3.
Freitas E, Guttman-Yassky E, Torres T. Tralokinumab for the treatment of atopic dermatitis. Am J Clin Dermatol. 2021;22(5):625–38. https://doi.org/10.1007/s40257-021-00613-8.
Nogueira M, Torres T. Janus kinase inhibitors for the treatment of atopic dermatitis: focus on abrocitinib, baricitinib, and upadacitinib. Dermatol Pract Concept. 2021. https://doi.org/10.5826/dpc.1104a145.
ClinicalTrials.gov. A study to evaluate safety and effectiveness of cendakimab (CC-93538) in participants with moderate to severe atopic dermatitis. https://clinicaltrials.gov/ct2/show/NCT04800315. Accessed 4 Apr 2023.
Okragly A, Wulur I, Daniels M et al. Characterization of binding, neutralization, and internalization of the IL-13 binding antibody lebrikizumab. Presented at 7th SPIN Congress; 2022; P054. Paris, France.
Okragly A, Ryuzoji A, Daniels M, Patel C, Benschop R. Comparison of the affinity and in vitro activity of lebrikizumab, tralokinumab, and cendakimab primary human cell assays-IL-13 induced periostin. Presented at Inflammatory Skin Disease Summit; Nov 2021; P#89. Virtual/New York, USA.
Wulur I, Van Horn RD, Ryuzoji A et al. Lebrikizumab allows Interleukin (IL)-13 membrane binding and subsequent internalization through IL-13 receptor alpha 2 (Rα2) (IL-13 Decoy Receptor). Presented at Inflammatory Skin Disease Summit; Nov 2021; P#96. Virtual/New York, USA.
ClinicalTrials.gov. A study of lebrikizumab in adult and adolescent participants with moderate-to-severe atopic dermatitis previously treated with dupilumab. https://clinicaltrials.gov/ct2/show/NCT05369403. Accessed 4 Apr 2023.
ClinicalTrials.gov. A study of lebrikizumab to assess the safety and efficacy of adult and adolescent participants with moderate-to-severe atopic dermatitis and skin of color. https://clinicaltrials.gov/ct2/show/NCT05372419. Accessed 4 Apr 2023.
ClinicalTrials.gov. A study of lebrikizumab in combination with topical corticosteroids in participants having atopic dermatitis (ad) that are not adequately controlled or non-eligible for cyclosporine. https://clinicaltrials.gov/ct2/show/NCT05149313. Accessed 4 Apr 2023.
ClinicalTrials.gov. A study of lebrikizumab (LY3650150) in combination with topical corticosteroids in Japanese participants with moderate-to-severe atopic dermatitis (ADhere-J). https://clinicaltrials.gov/ct2/show/NCT04760314. Accessed 4 Apr 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
DB, TB, and TT had the idea for the article, performed the literature search and data analysis, and drafted and critically revised the work.
Funding
Open access funding provided by FCT|FCCN (b-on).
Ethics approval
Not applicable.
Patient consent to participate/publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Conflicts of interest
Diana Bernado has no conflicts of interest. Thomas Bieber was speaker and/or consultant and/or Investigator for AbbVie, Affibody, Almirall, AnaptysBio, Arena, Asana Biosciences, ASLAN pharma, Bayer Health, BioVerSys, Boehringer Ingelheim, Bristol-Myers Squibb, Connect Pharma, Dermavant, DIECE Therapeutics, Domain Therapeutics, EQRx, Galderma, Glenmark, GSK, Incyte, Innovaderm, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, Lilly, L’Oréal, MSD, Novartis, Numab, OM-Pharma, Pfizer, Pierre Fabre, Q32bio, RAPT, Sanofi/Regeneron, and UCB. He is founder and chairman of the board of the non-profit biotech “Davos Biosciences.” Tiago Torres has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials sponsored by AbbVie, Amgen, Almirall, Arena Pharmaceuticals, Biocad, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius-Kabi, Janssen, LEO Pharma, Eli Lilly, MSD, Mylan, Novartis, Pfizer, Samsung-Bioepis, Sanofi-Genzyme, Sandoz, and UCB.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bernardo, D., Bieber, T. & Torres, T. Lebrikizumab for the Treatment of Moderate-to-Severe Atopic Dermatitis. Am J Clin Dermatol 24, 753–764 (2023). https://doi.org/10.1007/s40257-023-00793-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-023-00793-5