Abstract
Background
Most patients with chronic plaque psoriasis receive topical treatment; however, available options lack a balance of efficacy with long-term safety and tolerability. Roflumilast cream 0.3% is a highly potent phosphodiesterase 4 (PDE4) inhibitor approved by the US FDA for treatment of psoriasis.
Objective
The aim of this study was to define the pharmacokinetic (PK) profile of roflumilast delivered topically from a phase I maximal usage study and data from phase II and phase III studies.
Methods
PK data for roflumilast and the active metabolite, roflumilast N-oxide, were determined from a phase I PK and safety maximal usage study of roflumilast cream 0.3% applied once daily for 14 days in patients with plaque psoriasis affecting body surface area (BSA) ≥20% (N = 26). Serial plasma samples were obtained on Days 1 and 15 to determine maximum plasma concentration (Cmax) and area under the concentration-time curve (AUC). Plasma concentrations were also assessed at Weeks 3, 4, and 5 for terminal half-life (t½). Concentrations of roflumilast and roflumilast N-oxide in skin were assessed at Day 28 for 14 patients with psoriasis in a phase I/IIa study of once-daily roflumilast cream 0.5% and 0.15% for 28 days. Systemic exposure (Ctrough and AUC) of roflumilast and roflumilast N-oxide in two phase III trials (DERMIS-1, n = 245; DERMIS-2, n = 250) of roflumilast cream 0.3% for 8 weeks was assessed at Weeks 4 and 8.
Results
Bioavailability of roflumilast cream 0.3% after topical administration was 1.5%. Unlike after oral dosing, the plasma concentration-time curve was flat, with a peak-to-trough ratio of 1.2. Roflumilast N-oxide concentrations were eightfold higher than roflumilast concentrations. The t½ in adult patients was 4.0 days for roflumilast and 4.6 days for roflumilast N-oxide following the last dose administered. Steady state was reached by Day 15. Concentrations of roflumilast in skin were, on average, 126- and 61.8-fold higher than corresponding mean plasma Ctrough following administration of roflumilast cream 0.15% and 0.5% daily for 28 days. Roflumilast N-oxide was quantifiable in only one skin sample (N = 27). Following 8 weeks of treatment in DERMIS-1, mean plasma Ctrough of roflumilast was 1.78 ng/mL, and 9.86 ng/mL for roflumilast N-oxide. In DERMIS-2, mean plasma Ctrough was 1.72 ng/mL and 10.2 ng/mL, respectively. In the maximal usage study (mean BSA: 27.5%), eight patients (30.8%) experienced adverse events (AEs) and all were mild or moderate, with no reports of diarrhea, headache, insomnia, or application-site pain; no patients discontinued treatment due to an AE.
Conclusion
Topical administration of roflumilast cream 0.3% results in concentrations in skin 126- and 61.8-fold higher relative to plasma, which are much higher than expected to be achievable with oral dosing. PDE4 inhibition in the skin is likely due to roflumilast as compared with its active metabolite, as there is no significant conversion to roflumilast N-oxide in the skin. Consistent with reservoir formation and retention of drug in the stratum corneum, roflumilast is slowly released from the skin (t½ 4 days) and peak-to-trough ratio is 1.2.
ClinicalTrials.gov Identifiers
NCT04279119, NCT03392168, NCT04211363, NCT04211389.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Roflumilast cream 0.3% is a potent inhibitor of phosphodiesterase 4 (PDE4). |
Topical application results in higher concentrations in the skin than plasma, suggesting local PDE4 inhibition in the skin. |
1 Introduction
Psoriasis is a chronic, inflammatory, dermatologic condition that negatively impacts quality of life [1]. In patients with psoriasis receiving prescription medication for management of their disease, the vast majority receive topical treatment [2, 3]. However, there is a lack of topical medications that effectively provide a balance of high level of efficacy with long-term safety and tolerability. With the introduction of oral apremilast, phosphodiesterase 4 (PDE4) inhibitors have been shown to be an effective mechanism for treatment of psoriasis [4]; however, diarrhea and nausea, the most common adverse events (AEs), may require modification to initial dose titration [5]. Crisaborole is a topical PDE4 inhibitor approved for use in atopic dermatitis [6], but is associated with stinging and burning at the application site [7,8,9]. Oral roflumilast, a highly potent PDE4 inhibitor (approximately 25 to > 300 times more potent than apremilast or crisaborole) [10], is approved to reduce the risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD) at a daily dose of 500 µg [11]. However, the most common AEs with oral roflumilast are gastrointestinal, and diarrhea and nausea were the most common reasons for discontinuation from clinical trials [11].
Topical cream and foam formulations of roflumilast are undergoing clinical development for the treatment of psoriasis as well as other inflammatory conditions of the skin. Roflumilast cream 0.3% was effective and well tolerated in vehicle-controlled, randomized, phase II studies [12] and two identical phase III trials (DERMIS-1 and DERMIS-2) in patients with chronic plaque psoriasis [13]. In both phase III trials, the primary endpoint (achievement of Clear or Almost Clear plus ≥ 2-grade improvement from baseline on the Investigator Global Assessment at Week 8, roflumilast cream 0.3% compared with vehicle) was met (DERMIS-1: 42.4% vs. 6.1%; DERMIS-2: 37.5% vs. 6.9%; p < 0.0001 for both). Statistically significant differences in favor of roflumilast cream versus vehicle were observed for multiple secondary endpoints. The incidence of treatment-emergent AEs (TEAEs), serious AEs, and TEAEs leading to discontinuation were low with similar rates between roflumilast and vehicle across studies. Topical application of roflumilast cream 0.3% was well tolerated [13].
The primary difference in the safety profiles with oral versus topical administration is occurrence of gastrointestinal AEs (oral roflumilast 500 µg: diarrhea 9.5%, weight decreased 7.5%, nausea 4.7% [11]; topical roflumilast cream 0.3%: diarrhea 3.5% in DERMIS-1 and 2.8% in DERMIS-2, weight decreased and nausea occurred in < 1.5% of patients in both DERMIS-1 and DERMIS-2) [13]. This could be explained by their distinct pharmacokinetic (PK) profiles. Following a 500 µg oral dose, roflumilast was rapidly absorbed (about 1.25 h) and oral bioavailability was approximately 79% [14]. With multiple dosing of oral roflumilast 500 µg in healthy volunteers, the peak plasma concentration (Cmax) was 6.01 ng/mL [14] (about 6% higher in COPD patients) with a peak-to-trough ratio greater than 10 [15]. Roflumilast undergoes gut and hepatic first-pass metabolism via the cytochrome P450 (CYP3A4 and CYP1A2) system to form an active, but approximately threefold less potent, N-oxide metabolite [16]. Following oral administration of roflumilast, systemic exposure of roflumilast N-oxide was 12.4 times greater than that of roflumilast; however, formation of N-oxide metabolite is less extensive (7.4 times greater than roflumilast) following intravenous administration [14, 16]. After roflumilast is administered orally, the elimination half-life of roflumilast is 17 h, and 30 h for roflumilast N-oxide [17].
The PK profile of roflumilast delivered topically in a 0.3% cream formulation was evaluated in a phase I maximal usage study. Additional data obtained during the clinical development program provide further information to define the PK of roflumilast cream in patients with psoriasis. In this study, we describe the PK profile of roflumilast cream in patients with psoriasis from these studies.
2 Methods
2.1 Maximal Usage Pharmacokinetic (PK) and Safety Study (Study 107)
The objective of this phase I study was to evaluate the systemic exposure and characterize the PK profile of roflumilast and its major N-oxide metabolite, when dosed under maximal usage conditions, e.g., in patients with psoriasis with extensive body surface area (BSA) involvement and at the upper range of disease severity. Safety and tolerability were also assessed in this open-label, single-arm, 2-week study of roflumilast cream 0.3% in adolescent and adult patients with chronic plaque psoriasis (ClinicalTrials.gov identifier: NCT04279119).
Enrolled patients included those diagnosed with psoriasis of at least 3 months’ duration, involving at least 10% of BSA in adolescents (12–17 years) and at least 20% of BSA (excluding the scalp) in adults (≥ 18 years of age), and Investigator Global Assessment score of at least moderate (3) at baseline. For this maximal usage study, a minimum of 20% BSA was chosen because it is considered the maximal feasible BSA for topical application. Additional inclusion and exclusion criteria are provided in Electronic Supplementary Table 1.
Roflumilast cream 0.3% contains 50% water, 25% diethylene glycol monoethyl ether (Transcutol® P), 15% moisturizers (petrolatum and isopropyl palmitate), hexylene glycol, emulsifiers, and methylparaben and propylparaben as preservatives. It is formulated at physiological skin pH (5.5) without propylene glycol, polyethylene glycol, ethanol, or fragrances. Roflumilast cream was applied once daily (each evening) to areas with plaques, including body, face, and intertriginous/genital areas, but excluding the scalp. Patients were to apply roflumilast cream (2 mg/cm2) to all treated areas throughout the study, even if treatment areas cleared, and to any new lesions that appeared during the study.
Blood samples for PK analysis were obtained before application of roflumilast on Days 1 and 15; 1, 2, 4, 8, and 24 h post-dose on Days 1 and 15; and 7, 14, and 21 days after the last application (Weeks 3, 4, and 5; optional for adolescents). PK parameters included maximum concentration (Cmax), area under the curve until the last quantifiable timepoint (AUClast), time of maximum concentration (Tmax), and elimination half-life (t½) for roflumilast and its N-oxide metabolite. Cmax and AUClast values were normalized per dose to allow more accurate assessment of differences in adolescents and adults.
Local tolerability was assessed by the investigator at Baseline and before the last application at Day 15; patients assessed the level of stinging and burning at 10–15 min after the first and last application of roflumilast. Safety assessments included clinical laboratory, 12-lead electrocardiogram (ECG), vital signs, weight, Patient Health Questionnaire depression scale (PHQ-8 and modified PHQ-A), and Columbia-Suicide Severity Rating Scale (C-SSRS). BSA was assessed at Baseline and Day 15.
2.2 Assessment of Roflumilast and Roflumilast N-Oxide Levels in Skin from a Phase I/IIa Study (Study 101)
Levels of roflumilast and roflumilast N-oxide in the skin were assessed as part of a phase I/IIa study in two cohorts of patients with chronic plaque psoriasis (ClinicalTrials.gov identifier: NCT03392168). The safety and efficacy results of this study have been published [18]. Skin samples could be obtained in Cohort 2, which was a parallel-group, double-blind, vehicle-controlled study of once-daily roflumilast cream 0.5% and 0.15% for 28 days in patients with chronic plaque psoriasis for a duration > 6 months and ≤ 5% BSA involvement. Patients could opt to allow a punch biopsy (4 mm) for assessment of levels of roflumilast and roflumilast N-oxide in skin, taken from a target plaque on Day 28. The punch biopsy included the epidermis, dermis, and subcutaneous skin layers. Plasma concentrations of roflumilast and roflumilast N-oxide were assessed at Day 28 before application, and 1, 2, 4, 6 and 24 h after the last dose.
2.3 Phase III DERMIS-1 and DERMIS-2 PK Assessment (Studies 301 and 302)
DERMIS-1 (ClinicalTrials.gov identifier: NCT04211363) and DERMIS-2 (ClinicalTrials.gov identifier: NCT04211389) were identical, parallel-group, randomized (2:1), double-blind, vehicle-controlled trials of roflumilast cream 0.3% or vehicle applied once daily for 8 weeks to patients with chronic plaque psoriasis affecting 2–20% BSA. The safety and efficacy results of the DERMIS trials have been published [13]. Male or female children (2–11 years), adolescents (12–17 years), and adults (≥ 18 years) with chronic plaque psoriasis could be enrolled.
Roflumilast cream 0.3% or vehicle was applied once daily to all lesions (excluding the scalp) as identified by the investigator, even if lesions cleared during the study. New lesions were to be treated as well. Predose (Ctrough) plasma samples for roflumilast and roflumilast N-oxide were obtained at Baseline, Week 4, and Week 8. Ctrough concentrations were assessed from observed data, and given the flat PK profile of topical roflumilast, the AUC from time zero to 24 h (AUC24) was extrapolated by multiplying the predose concentration value by 24.
2.4 PK Analyses
In Study 107, the PK analysis was performed for roflumilast and the roflumilast N-oxide metabolite using noncompartmental analysis and following the Linear Trapezoidal Linear Interpolation calculation method. A minimum of three consecutive quantifiable concentrations were required to include a patient in the analysis. Cmax and the corresponding Tmax values were determined by direct assessment of the concentration versus time data. Nominal dose values and sampling times were used for PK parameter estimations. The terminal elimination rate constant (lambda z, λz) was calculated following the last dose administered using the actual analysis day values. The Week 2, 24-h plasma concentration value, along with any quantifiable concentration at 1, 2, and 3 weeks post the last dose administered, were used to derive a λz value by determining the slope of the regression line of the natural log-transformed concentrations versus time. Data from patients with at least two values were used. The terminal half-life (t½) was calculated as ln (2)/λz. Analysis of the PK data collected was performed with a CFR 21 Part 11 compliant software package (Phoenix WinNonlin version 8.3; Certara, Princeton, NJ, USA), which is in full compliance with International Council for Harmonization Good Clinical Practice.
2.5 Drug Analysis
Roflumilast and roflumilast N-oxide plasma concentrations were measured using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay. The lower limit of quantification (LLOQ) was 0.100 ng/mL for roflumilast and roflumilast N-oxide. Skin samples from punch biopsies were immediately frozen until samples were assayed at a central location. Roflumilast and roflumilast N-oxide concentrations were quantified in skin homogenates using an LC-MS/MS assay for assessment of tissue concentrations of roflumilast and roflumilast N-oxide.
2.6 Study Ethics
These studies were conducted in accordance with the ethical principles set forth in the Declaration of Helsinki and the International Council for Harmonization harmonized tripartite guideline regarding Good Clinical Practice (2016 guidance). Approval by the investigational review board was obtained from each investigator’s institution and written informed consent or assent was obtained before enrollment. In the skin-level assessment from the phase I/IIa study (Study 101), the protocol was approved by Research Review Board, Inc., Richmond Hill, ON, Canada, for all sites [18].
3 Results
3.1 Maximal Usage PK and Safety Study (Study 107)
A total of 26 patients were enrolled (6 adolescents, 20 adults); 2 withdrew from the study (both adults, 1 withdrew consent after completing the 2-week treatment, 1 was lost to follow-up), and 24 completed the study. The PK and safety populations consisted of all 26 patients. Baseline disease and demographic characteristics are shown in Table 1.
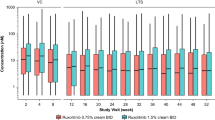
Mean values of the PK parameters of roflumilast and roflumilast N-oxide for adolescents and adults at Day 15 are shown in Table 2. Following a single roflumilast cream 0.3% topical dose, the bioavailability of roflumilast was 1.5% (based on a mean BSA of 27.5%, with a mean dose applied of 28.5 mg). The AUC normalized by dose suggests that systemic exposure to roflumilast and roflumilast N-oxide was similar between adolescents and adults, with a trend for higher levels in adults. The mean plasma concentration versus time plot of roflumilast and roflumilast N-oxide in adolescents and adults at Days 1 and 15 is shown in Fig. 1A, and B, respectively. These graphs illustrate that plasma concentrations of roflumilast and roflumilast N-oxide increased after the first application of roflumilast cream 0.3% and steady state was achieved by Day 15. In addition, at steady state (Day 15), the concentration versus time plots were relatively flat, with a peak-to-trough ratio of 1.2. The roflumilast N-oxide concentrations were generally eightfold over roflumilast concentrations, which was more consistent with the ratio observed following intravenous administration than oral administration. The mean concentration versus time plot after the last application of roflumilast cream 0.3% illustrates elimination of roflumilast and N-oxide in adolescents and adults (Fig. 2). Mean elimination half-life for roflumilast and roflumilast N-oxide in adolescents was 2.9 (n = 1) and 2.8 (n = 2) days, respectively, and 4.0 (n = 16) and 4.6 (n = 18) days in adults, respectively. The longer half-life following topical administration relative to oral administration suggests significant retention in the skin.
The overall percentage of patients with at least one TEAE was 30.8%; all the TEAEs were reported by eight adults (no AEs were reported by adolescents). TEAEs included nausea (three patients), ear discomfort, application-site paresthesia, upper respiratory tract infection, back pain, and hematuria (all one patient each). Most TEAEs (five) were considered mild, while three were moderate in severity; none were rated as serious or Grade 5. No patients interrupted or discontinued treatment due to a TEAE. All three TEAEs of nausea were reported as mild (Grade 1), occurred early during treatment, were considered possibly related to roflumilast cream, and resolved during the course of the study without any action being taken. There were no reports of diarrhea, headache, insomnia, or application-site pain. Treatment with roflumilast cream 0.3% did not appear to have any influence on vital signs, physical examination findings, clinical laboratory assessments, ECG parameters, or weight/body mass index. All patients had a score of 0 on the C-SSRS throughout the study. Shifts in PHQ-8 and PHQ-A did not indicate any safety concerns.
At Week 2, there was no significant change from Baseline in investigator-assessed local tolerability, with ‘no evidence of irritation’ reported for all patients. For patient assessment of local tolerability, all adolescents reported ‘no sensation’ (0 = none) after application at both Baseline and Week 2. For adult subjects, most patients reported ‘no sensation’ (0 = none), including 17/20 at Baseline and 17/19 at Week 2. Two adults reported at Baseline a slight warm tingling sensation and one had a definite warm, tingling sensation after roflumilast application. Similarly, at Week 2, one patient had a slight warm tingling sensation and one had a definite warm tingling sensation after application of roflumilast cream 0.3%.
3.2 Assessment of Roflumilast and Roflumilast N-Oxide Concentrations in Skin from a Phase I/IIa Study (Study 101)
Skin punch biopsy samples for assessment of roflumilast and roflumilast N-oxide concentrations were obtained from 14 patients receiving roflumilast cream 0.15% and 13 patients receiving roflumilast cream 0.5%. All but one sample (patient receiving roflumilast cream 0.5%) had measurable levels of roflumilast, whereas only one sample (patient receiving roflumilast cream 0.15%) had a measurable level for roflumilast N-oxide. In patients receiving roflumilast cream 0.15%, the mean (standard deviation [SD]) concentration of roflumilast in the skin was 120 (185) ng/g and the concentration of measurable roflumilast N-oxide was 0.216 ng/g (one patient), near the LLOQ. In patients receiving roflumilast cream 0.5%, the mean (SD) concentration of roflumilast in the skin was 56.1 (43.1) ng/g. No patients receiving the 0.5% dose had measurable concentrations of roflumilast N-oxide.
The mean (SD) roflumilast plasma concentrations with roflumilast cream 0.15% (n = 21) and roflumilast cream 0.5% (n = 20) were 1.03 (0.903) ng/mL and 1.15 (0.736) ng/mL, respectively, while the skin concentrations of roflumilast N-oxide were at least 30-fold lower (skin LLOQ of 0.2 ng/g) than the circulating plasma concentrations (6.52 [4.04] ng/mL and 5.44 [4.95] ng/mL following 0.5% and 0.15%, respectively). The data suggest that there is no significant conversion of roflumilast to the N-oxide metabolite in the skin, and that efficacy in the skin is due to the higher skin roflumilast concentrations following topical administration.
There were 11 patients per treatment group with matched skin biopsy and plasma concentration at 24 h after the last dose. In the 0.15% and 0.5% roflumilast cream subgroups, the mean (SD) plasma concentration was 1.15 (1.14) ng/mL and 0.805 (0.307) ng/mL, and the mean concentration in skin was 84.0 (171) ng/mL and 51.0 (44.4), respectively. The ratio of mean (SD) concentration of roflumilast in the skin to the mean (SD) plasma concentration was 126 (263) and 61.8 (47.1) in the roflumilast cream 0.15% and 0.5% treatment groups, respectively.
3.3 Phase III DERMIS-1 and DERMIS-2 PK Assessment (Studies 301 and 302)
The PK population for DERMIS-1 was comprised of 241 adults, 3 adolescents, and 1 child, while the PK population for DERMIS-2 comprised 244 adults, 5 adolescents, and 1 child. Systemic exposure of roflumilast and roflumilast N-oxide based on Ctrough and AUC24 are shown in Table 3 for the three age categories from DERMIS-1 and DERMIS-2. Concentrations were consistent between the two phase III trials.
4 Discussion
The maximal usage PK and safety study, required by the US FDA, evaluated the safety and exposure of roflumilast cream 0.3% under maximal use conditions; therefore, the mean BSA affected (27.5%) was approximately four times greater in this study than in the DERMIS trials (6.0% and 6.9%), which represents the target population for this topical therapy. It was anticipated that a greater BSA affected would correspond to higher Cmax and AUC; however, the relationship with BSA was not directly proportional. Relative to the DERMIS trials, AUC in the maximal usage study was approximately 1.6-fold higher, not the four times that would be expected if there was a direct relationship. Safety and tolerability under maximal usage conditions were similar to the AE profiles in the phase IIb [12] and two phase III studies [13]. Despite a greater BSA, Cmax, and AUC than in DERMIS-1 and DERMIS-2, there were no reported cases of diarrhea, headache, insomnia, or application site pain in the maximal usage study.
The PK profiles of roflumilast and roflumilast N-oxide with topical administration have distinct differences in comparison with oral dosing, which may explain the excellent safety and tolerability profile of roflumilast cream relative to the oral formulation. Roflumilast was more bioavailable with oral dosing (79%) and absorbed rapidly, with peak levels occurring in 1 h. After single topical administration, roflumilast had bioavailability of approximately 1.5% and was slowly absorbed. Under steady-state conditions in the maximal usage study (Study 107, 27.5% BSA treatment), despite the 55-fold higher dose (27.5 mg vs. 0.5 mg oral) with the topical formulation, peak roflumilast and roflumilast N-oxide concentrations were 3.72 and 30.6 ng/mL, respectively, following topical administration. These values were within twofold of the peak Cmax values following repeat oral roflumilast 500 µg dose administration in healthy volunteers, in which peak roflumilast and roflumilast N-oxide concentrations were 6.01 ng/mL and 21.7 ng/mL, respectively [14]. Following 8 weeks of treatment with roflumilast cream 0.3% in the DERMIS trials, the Ctrough concentrations were 1.78 ng/mL and 9.9 ng/mL for roflumilast and roflumilast N-oxide, respectively, in DERMIS-1. Values in DERMIS-2 were similar to those in DERMIS-1. With oral administration, roflumilast peak-to-trough ratios were > 10 [15], while there was minimal fluctuation following topical administration—the ratio was 1.2-fold at steady state. The relatively flat concentration-time curve with topical roflumilast, lower Cmax, or action of bypassing the gastrointestinal tract with topical administration likely contribute to the low rate of gastrointestinal AEs observed during clinical trials with roflumilast cream in psoriasis [18, 19].
Roflumilast is lipophilic (roflumilast logP = 3.53), water-insoluble (roflumilast water solubility = 0.52–0.56 mg/L at 22 °C) and has an affinity for protein. These three properties of topically applied actives correlate well with reservoir formation and retention of drug in the stratum corneum [20]. Quantitation of human skin reservoir function in vivo has been determined for a lipophilic, water-insoluble chemical ultraviolet (UV) filter delivered from oil-in-water emulsions [21] and has often been observed using in vitro techniques for topically applied actives dissolved in diethylene glycol monoethyl ether [22]. The longer half-life of roflumilast after topical administration (4 days) relative to oral (17–30 h) and flat concentration versus time curve is reflective of the prolonged release out of the stratum corneum and into the epidermis, dermis, and systemic circulation. This supports the efficacy profile observed with once-daily application of roflumilast cream 0.3% in psoriasis and could mitigate any impact of occasional missed doses.
With repeat daily dosing of roflumilast cream, roflumilast N-oxide plasma concentrations were approximately eightfold higher than roflumilast at steady state, which was evident by Day 15. This is consistent with the ratio of 7.4-fold observed following a single intravenous administration. With oral administration, the ratio is 12.4-fold; the greater proportion of roflumilast N-oxide is due to increased contribution from first-pass metabolism [14]. Accordingly, the ratio of roflumilast N-oxide to roflumilast plasma concentrations is distinct and lower with topical versus oral dosing, and results in relatively lower overall systemic PDE4 inhibition at a given roflumilast concentration.
The 61.8- to 126-fold higher roflumilast concentrations in the skin relative to plasma coupled with the lack of roflumilast N-oxide in the skin suggest that dermal conversion of roflumilast to roflumilast N-oxide does not occur and the activity of roflumilast cream 0.3% in psoriasis is due to local PDE4 inhibition by the parent compound. This is in line with data from Snape et al. [23], who demonstrated that the efficacy of a precursor formulation of roflumilast cream for psoriasis was due to local effects. Using a modified psoriasis plaque test, roflumilast cream (using the precursor formulation) reduced skin infiltrate thickness, which correlated with a reduction in psoriasis severity. Moreover, both the roflumilast levels in the skin and plasma PK profile seen with roflumilast cream are not expected to be achievable with oral dosing of roflumilast.
Systemic exposure of roflumilast and roflumilast N-oxide with topical application was observed in adolescents and adults, with a trend for greater exposure in adults. Plasma concentrations of roflumilast and roflumilast N-oxide were similar when normalized per dose in adolescents and adults, consistent with no dosage adjustment being needed in adolescents.
Limitations of these studies include the small number of adolescents in the maximal usage study and number of skin samples (N = 27), contributing to the high level of variability in concentrations. Additionally, concentrations in skin were not determined separately for the epidermis, dermis, and subcutaneous tissue.
5 Conclusion
The PK profile of roflumilast cream provides support to the favorable safety and tolerability observed when administered topically to patients with psoriasis and is distinct from that of the oral formulation used in COPD. Diarrhea and nausea are the most common AEs leading to discontinuation with oral treatment in patients with COPD; although the rates of these events were less commonly associated with the topical application of roflumilast in the treatment of psoriasis. However, the risks of weight loss, depression, and suicidal ideation and behavior have not been associated with the topical application of roflumilast in the treatment of psoriasis. The lack of variation in peak-to-trough levels, approximately threefold lower peak concentrations with typical topical dosing in psoriasis relative to oral dosing in COPD, and lack of potential for local irritation in the gastrointestinal tract are likely contributing factors to the excellent tolerability with topical administration.
References
Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–9.
Elmets CA, Korman NJ, Prater EF, Wong EB, Rupani RN, Kivelevitch D, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432–70.
Feldman SR, Goffe B, Rice G, Mitchell M, Kaur M, Robertson D, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benef. 2016;9:504–13.
Amgen Inc. OTEZLA (apremilast) prescribing information. Thousand Oaks: Amgen Inc.; 2020.
Narla S, Silverberg JI, Simpson EL. Management of inadequate response and adverse effects to dupilumab in atopic dermatitis. J Am Acad Dermatol. 2022;86(3):628–36.
Pfizer Labs. EUCRISA (crisaborole) prescribing information. New York: Pfizer Labs; 2020.
Pao-Ling Lin C, Gordon S, Her MJ, Rosmarin D. A retrospective study: application site pain with the use of crisaborole, a topical phosphodiesterase 4 inhibitor. J Am Acad Dermatol. 2019;80(5):1451–3.
Zane LT, Kircik L, Call R, Tschen E, Draelos ZD, Chanda S, et al. Crisaborole topical ointment, 2% in patients ages 2 to 17 years with atopic dermatitis: a phase 1b, open-label, maximal-use systemic exposure study. Pediatr Dermatol. 2016;33(4):380–7.
Schlessinger J, Shepard JS, Gower R, Su JC, Lynde C, Cha A, et al. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild-to-moderate atopic dermatitis: a phase IV open-label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21(2):275–84.
Dong C, Virtucio C, Zemska O, Baltazar G, Zhou Y, Baia D, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413–22.
AstraZeneca Pharmaceuticals LP. Daliresp (roflumilast) prescribing information. Wilmington: AstraZeneca Pharmaceuticals LP; 2020.
Lebwohl MG, Papp KA, Stein Gold L, Gooderham MJ, Kircik LH, Draelos ZD, et al. Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020;383:229–39.
Lebwohl M, Kircik L, Moore A, Stein Gold L, Draelos Z, Godderham M, et al. Once-daily roflumilast cream in patients with chronic plaque psoriasis (DERMIS-1 and DERMIS-2): two randomized clinical trials. J Am Med Assoc. 2022;328(11):1073–84. https://doi.org/10.1001/jama.2022.15632.
Bethke TD, Böhmer GM, Hermann R, Hauns B, Fux R, Mörike K, et al. Dose-proportional intraindividual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol. 2007;47(1):26–36.
Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review, Application Number: 022522Orig1s000. 2010. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022522Orig1s000ClinPharmR.pdf. Accessed 25 Jun 2022.
Bethke TD, Lahu G. High absolute bioavailability of the new oral phosphodiesterase-4 inhibitor roflumilast. Int J Clin Pharmacol Ther. 2011;49(1):51–7.
Baye J. Roflumilast (daliresp): a novel phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease. Pharm Ther. 2012;37(3):149–61.
Papp KA, Gooderham M, Droege M, Merritt C, Osborne DW, Berk DR, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19(8):734–40.
Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048.
Miselnicky SR, Lichtin JL, Sakr A, Bronaugh RL. The influence of solubility, protein binding, and percutaneous absorption on reservoir formation in skin. J Soc Cosmet Chem. 1988;39:169–77.
Teichmann A, Jacobi U, Weigmann H-J, Sterry W, Lademann J. Reservoir function of the stratum corneum: development of an in vivo method to quantitatively determine the stratum corneum reservoir for topically applied substances. Skin Pharmacol Physiol. 2005;18:75–80.
Osborne DW, Musakhanian J. Skin penetration and permeation properties of Transcutol®-neat or diluted mixtures. AAPS PharmSciTech. 2018;19(8):3512–33.
Snape SD, Wigger-Alberti W, Goehring UM. A phase I randomized trial to assess the effect on skin infiltrate thickness and tolerability of topical phosphodiesterase inhibitors in the treatment of psoriasis vulgaris using a modified psoriasis plaque test. Br J Dermatol. 2016;175(3):479–86.
Acknowledgements
This research was supported by Arcutis Biotherapeutics. Medical writing support was provided by Susan Sutch, PharmD, for Alligent Biopharm Consulting LLC, funded by Arcutis Biotherapeutics.
Author information
Authors and Affiliations
Contributions
AWT Jr, DWO, SS, RCH, PB, and DRB developed the protocol, including formulation of the search strategy; contributed to data interpretation; were involved in drafting the manuscript; approved the final version for submission; and agree to be accountable for all aspects of this work.
Corresponding author
Ethics declarations
Funding
The study was funded by Arcutis Biotherapeutics Inc.
Conflicts of interest/competing interests
Archie W. Thurston Jr is an employee of Toxicology Solutions and a consultant with Arcutis Biotherapeutics, Inc. David R. Berk, David W. Osborne, Scott Snyder, Robert C. Higham, and Patrick Burnett are employees of Arcutis Biotherapeutics, Inc.
Ethics approval
These studies were conducted in accordance with the ethical principles set forth in the Declaration of Helsinki and the International Council for Harmonization harmonized tripartite guideline regarding Good Clinical Practice (2016 guidance). Approval by the Investigational Review Board was obtained from each investigator’s institution, and written informed consent or assent was obtained before enrollment. In the skin-level assessment from the phase I/IIa study (Study 101), the protocol was approved by Research Review Board, Inc, Richmond Hill, ON, Canada, for all sites.
Consent to participate
Written informed consent or assent was obtained before enrollment in each of the studies included in this manuscript.
Consent for publication
Not applicable.
Availability of data and material
Data collected for these studies will be made available to others. Proposals for data requests will be reviewed and considered for sharing following approval of the indication. Information about when data availability will begin and end will be provided following approval of the indication.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Thurston, A.W., Osborne, D.W., Snyder, S. et al. Pharmacokinetics of Roflumilast Cream in Chronic Plaque Psoriasis: Data from Phase I to Phase III Studies. Am J Clin Dermatol 24, 315–324 (2023). https://doi.org/10.1007/s40257-022-00741-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00741-9