Abstract
Background
Abrocitinib, an oral Janus kinase 1 inhibitor, provided significant itch relief by week 2 in patients with moderate-to-severe atopic dermatitis (AD) in the phase III JADE COMPARE trial.
Objectives
This post-hoc analysis of JADE COMPARE aimed to further characterize itch response and determined whether early itch relief could predict subsequent improvements in AD severity.
Methods
JADE COMPARE was a randomized, double-blind, double-dummy, placebo-controlled trial. Adult patients (aged ≥ 18 years) with moderate-to-severe AD were randomly assigned to receive oral abrocitinib 200 mg or 100 mg once daily, subcutaneous dupilumab 300 mg every other week (after a 600-mg loading dose), or placebo, plus medicated topical therapy for 16 weeks. Assessments were ≥ 4-point improvement in Peak Pruritus Numerical Rating Scale (PP-NRS4) from days 2 to 15, Eczema Area and Severity Index (EASI), Investigator’s Global Assessment (IGA) response, and Dermatology Life Quality Index (DLQI) scores at week 12. Association between week 2 PP-NRS4 and efficacy at week 12 was evaluated by chi-squared tests. The predictive value of early response for later efficacy was assessed by area under the receiver operating characteristic curve.
Results
As early as day 4 after treatment, a significantly greater proportion of patients achieved PP-NRS4 response with abrocitinib 200 mg (18.6%) versus dupilumab (5.6%; p < 0.001) and placebo (6.0%; p < 0.003). A similar trend was observed with abrocitinib at the 100-mg dose, with significantly greater PP-NRS4 response rates versus placebo as early as day 9. With both doses of abrocitinib, week 12 IGA 0/1, EASI-75, EASI-90, and DLQI 0/1 response rates were greater in week 2 PP-NRS4 responders than nonresponders; no differences were observed between week 2 PP-NRS4 responders and nonresponders in the dupilumab and placebo groups. Early improvement in PP-NRS at week 2 was associated with skin clearance at week 12 in abrocitinib-treated patients.
Conclusions
Abrocitinib resulted in rapid relief from itch in patients with moderate-to-severe AD, with significant improvement in itch as early as day 4 after treatment with abrocitinib 200 mg compared with dupilumab and placebo. Abrocitinib-induced itch relief by week 2 was associated with subsequent improvements at week 12. [Video abstract available.]
Trial registration
ClinicalTrials.gov identifier: NCT03720470.
Video abstract
Early itch response with abrocitinib is associated with later efficacy outcomes in patients withmoderate-to-severe atopic dermatitis: subgroup analysis of the randomized phase III JADE COMPARE trial (MP4 335,375kb)
Plain Language Summary: An investigation into whether quick relief from itch with abrocitinib, a new medicine for atopic dermatitis, leads to later improvements in skin rash
Atopic dermatitis (AD), also called atopic eczema, is a skin disease that affects people throughout their lives. About 10% of adults worldwide have AD. Itch is the most bothersome symptom reported by people with AD and scratching this itch can damage the skin, resulting in painful sores. It is unknown if relief from itch can influence other symptoms of AD. We analyzed data from the JADE COMPARE study, which included 837 people who received treatment with abrocitinib, dupilumab or placebo. We studied how fast itch relief occurred after people received these treatments. We also wanted to study if rapid itch relief was associated with improvement in other signs of AD later on with continued treatment. We found that as early as 4 days after treatment, abrocitinib 200 mg provided significant relief from itch compared with dupilumab or placebo. People who had rapid itch relief within 2 weeks of treatment with abrocitinib were more likely to have clear or almost clear skin and improved quality of life after 12 weeks of continued treatment with abrocitinib. Rapid itch relief did not appear to increase the likelihood of clear skin at week 12 in people who received dupilumab. Larger studies are needed to confirm this result. This study provides important evidence for physicians as they analyze itch relief and determine treatment options for people with AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this article can be found at https://doi.org/10.6084/m9.figshare.20506725 |
Atopic dermatitis can be associated with intense pruritus which negatively impacts patients’ quality of life; thus, rapid relief of itch is a key treatment goal. |
Abrocitinib, an oral Janus kinase 1 inhibitor, demonstrated significantly greater reduction in itch as early as day 4 versus placebo, and from day 4 to day 15 versus dupilumab. |
Early itch relief with abrocitinib was associated with skin clearance at week 12 and may inform the benefit:risk profile of continued treatment with abrocitinib in individual patients. |
1 Introduction
Atopic dermatitis (AD) is characterized by intense pruritus and eczematous lesions [1]. Itch is often the most burdensome symptom of AD [2, 3]. Intense pruritus associated with AD can worsen skin lesions by disrupting the skin barrier, causing significant discomfort for patients [4]. Associations between early itch relief and subsequent improvement in other symptoms of AD remain to be determined.
Previous studies with novel therapies demonstrated rapid itch relief in patients with moderate-to-severe AD [5,6,7,8,9]. Abrocitinib is an oral, once-daily, selective Janus kinase 1 (JAK1) inhibitor approved for the treatment of adults [10,11,12,13] and adolescents [10, 12] with moderate-to-severe AD. Efficacy and short-term safety of oral abrocitinib as monotherapy was established in the phase III JADE MONO-1 (ClinicalTrials.com identifier: NCT03349060) and MONO-2 (NCT03575871) trials [14, 15]. The phase III JADE COMPARE trial (NCT03720470), designed to evaluate the efficacy and safety of abrocitinib versus placebo or dupilumab in adults with moderate-to-severe AD on background topical therapy, showed clinically meaningful and rapid itch relief with abrocitinib 200 mg and 100 mg compared with placebo; a significantly greater proportion of patients achieved an early itch response at week 2 with abrocitinib 200 mg compared with biweekly dupilumab 300 mg [16].
This analysis of JADE COMPARE further characterized early itch responses with abrocitinib in patients with moderate-to-severe AD. We assessed whether rapid reduction in itch was associated with subsequent treatment efficacy, including skin clearance and improved quality of life. The optimal threshold of itch relief needed to achieve meaningful improvements in these outcomes was also assessed.
2 Patients and Methods
2.1 Study Design and Patients
The JADE COMPARE study design was previously published [16]. Briefly, the study enrolled eligible patients ≥ 18 years of age who met the criteria for moderate-to-severe AD (affected body surface area ≥ 10%, Investigator Global Assessment [IGA] ≥ 3, Eczema Area and Severity Index [EASI] ≥ 16, Peak Pruritus Numerical Rating Scale [PP-NRS] score ≥ 4, representing moderate or worse pruritus on a scale of 0 [no itch] to 10 [worst itch imaginable] in the past 24 hours; PP-NRS ©Regeneron Pharmaceuticals, Inc. and Sanofi, 2017) and had a clinical diagnosis of chronic AD for ≥ 1 year. Patients were randomly assigned in a 2:2:2:1 ratio to receive once-daily oral abrocitinib (200 mg or 100 mg), subcutaneous dupilumab 300 mg once every 2 weeks (following a 600-mg loading dose), or placebo for 16 weeks. All patients used background medicated topical therapy once daily throughout the study.
2.2 Assessments
The proportion of patients who achieved an early itch response (defined as ≥ 4-point improvement from baseline in PP-NRS [PP-NRS4]) on day 2 through day 15 of treatment were assessed in a prespecified analysis. The impact of rapid itch relief on subsequent clinical efficacy was assessed post hoc in patients with and without PP-NRS4 response at week 2 by EASI-75 (≥ 75% improvement from baseline in EASI), EASI-90 (≥ 90% improvement from baseline in EASI), IGA response of 0 (clear) or 1 (almost clear) and ≥ 2-grade improvement from baseline, and Dermatology Life Quality Index score of 0 or 1 (DLQI 0/1; representing no effect on patients’ quality of life) on a scale of 0–30, where higher scores indicate greater impairment in patients’ quality of life [17]. Association between PP-NRS4 response at week 2 and IGA score and EASI percentage change from baseline category (< 50%, ≥ 50% to <75%, ≥ 75% to < 90%, ≥ 90% improvement from baseline) at week 12 was assessed post hoc. Predictability of week 12 efficacy response (IGA 0/1 and EASI-75) by improvement in PP-NRS score at week 2 was also assessed post hoc.
2.3 Statistical Analysis
Analysis of the proportion of patients achieving PP-NRS4 from day 2 to day 15 was performed with the Cochran-Mantel-Haenszel method, and nonresponder imputation as described previously [16]. Associations between PP-NRS4 response at week 2 and week 12 IGA score and EASI percentage change from baseline category were evaluated by chi-squared tests. Area under the receiver operating characteristic (ROC) curve from logistic regression was used to assess the predictability of week 12 IGA 0/1 and EASI-75 by week 2 change from baseline in PP-NRS score.
The Youden Index was used to determine the optimum PP-NRS improvement needed at week 2 to predict week 12 efficacy responses for sensitivity and specificity. The Youden Index ranges from 0 to 1, with values approaching 1 indicating higher predictiveness (i.e., no false positives or false negatives).
Statistical testing sequences for PP-NRS4 response at week 2 and at time points on or earlier than day 15 are detailed in the Supplementary Appendix S1 (see electronic supplementary material [ESM]). For all other analyses, a p value less than the nominal significance level of 5% was considered statistically significant, and no adjustments were made for multiplicity.
For PP-NRS4 response on day 2 to day 15, and week 12 binary outcomes (IGA 0/1, EASI-75, EASI-90, and DLQI 0/1), patients who withdrew from the study were considered nonresponders after that time point. Any observations that were missing intermittently were considered missing completely at random and remained as missing in the analysis. Categorical values including week 12 IGA score and EASI percentage change from baseline categories were based on observed values. For assessments of associations and predictiveness, week 2 PP-NRS–related endpoints were based on observed values.
3 Results
3.1 Baseline Demographics and Disease Characteristics
A total of 837 patients were included in this analysis. Baseline demographics and disease characteristics were balanced across treatment groups (Table S1, see ESM). At study baseline, 64.6% of patients had a moderate IGA score (3), mean EASI score was 30.9 (SD 12.8), mean PP-NRS score was 7.3 (SD 1.7), and mean DLQI score was 15.7 (SD 6.6).
3.2 Early PP-NRS4 Response
As early as day 4 after treatment, a significantly greater proportion of patients in the abrocitinib 200-mg group (18.6%) achieved PP-NRS4 compared with the placebo (6.0%; p < 0.003) or dupilumab (5.6%; p < 0.001) groups (Fig. 1). This response was sustained through day 15, with a significantly greater proportion of patients achieving PP-NRS response with abrocitinib 200 mg (49.0%) compared with placebo (11.1%) or dupilumab (26.5%) (p < 0.001 for both; Fig. 1). A similar trend was observed in the abrocitinib 100-mg group, with 11.6% of patients achieving a PP-NRS4 response on day 4 compared with 6.0% with placebo and 5.6% with dupilumab. PP-NRS4 responses with abrocitinib 100 mg were significantly greater than placebo from day 9 (24.0% vs 13.0%; p < 0.03) through day 15 (30.4% vs 11.1%; p < 0.001).
Proportion of patients achieving PP-NRS4 response from day 1 to day 15. CI confidence interval, PP-NRS4 ≥ 4-point improvement from baseline in Peak Pruritus Numerical Rating Scale score, QD once daily, Q2W once every 2 weeks. *p < 0.05; †p ≤ 0.01; ‡p ≤ 0.001 vs placebo. §p ≤ 0.01; ¶p ≤ 0.001 vs dupilumab
3.3 Associations Between PP-NRS4 Response at Week 2 and Efficacy Outcomes at Week 12
Significant associations (p ≤ 0.05) were identified between PP-NRS4 response at week 2 and week 12 IGA score and EASI percentage change from baseline category with abrocitinib 200 mg and 100 mg (Table S2, see ESM). No significant associations were identified with either dupilumab or placebo.
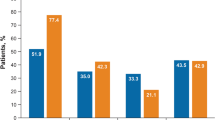
In the abrocitinib 200-mg group, the proportion of PP-NRS responders was greater than PP-NRS nonresponders for achieving week 12 IGA 0/1 (63.1% vs 38.3%), EASI-75 (81.6% vs 65.4%), EASI-90 (59.2% vs 37.4%), and DLQI 0/1 (43.8% vs 18.5%; Fig. 2). Similar trends were observed in the abrocitinib 100-mg group for week 12 IGA 0/1 (57.4% vs 30.9%), EASI-75 (73.5% vs 57.9%), EASI-90 (54.4% vs 32.2%), and DLQI 0/1 response rates (38.2% vs 16.7%). In the dupilumab group, the proportions of PP-NRS responders were similar to PP-NRS nonresponders for achieving week 12 IGA 0/1 (35.0% vs 39.2%), EASI-75 (66.7% vs 59.0%), EASI-90 (36.7% vs 36.7%), and DLQI (26.7% vs 23.5%). A similar trend was observed in the placebo group for IGA 0/1 (20.0% vs 14.6%), EASI-75 (33.3% vs 29.1%), and EASI-90 (13.3% vs 10.7%) response, although a greater proportion of PP-NRS responders than PP-NRS nonresponders achieved DLQI 0/1 (26.7% vs 7.7%).
The proportion of patients with a IGA 0/1 and ≥ 2-grade improvement from baseline in IGA score, b EASI-75, c EASI-90, and d DLQI 0/1 responses at week 12 by PP-NRS4 response status at week 2. CI confidence interval, DLQI 0/1 Dermatology Life Quality Index score of 0 or 1, EASI-75 ≥ 75% improvement from baseline in Eczema Area and Severity Index, EASI-90 ≥ 90% improvement from baseline in Eczema Area and Severity Index, IGA 0/1 Investigator’s Global Assessment of clear (0) or almost clear (1) and ≥ 2-grade improvement from baseline, PP-NRS4 ≥ 4-point improvement from baseline in Peak Pruritus Numerical Rating Scale score, QD once daily, Q2W once every 2 weeks
3.4 Predictive Value of Week 2 Change from Baseline in PP-NRS for Week 12 Outcomes
By measuring area under the ROC curve, the predictive value of early itch relief (as measured by change from baseline in PP-NRS at week 2) to achieve additional improvements at week 12 was found to be 0.668 for IGA 0/1, 0.657 for EASI-75, 0.663 for EASI-90, and 0.694 for DLQI 0/1 with abrocitinib 200 mg (Fig. 3). Similarly, the predictive value of early itch relief with abrocitinib 100 mg was 0.688 for IGA 0/1, 0.602 for EASI-75, 0.660 for EASI-90, and 0.663 for DLQI 0/1. The predictability of week 12 outcomes was generally lower with dupilumab (IGA 0/1: 0.521; EASI-75: 0.600; EASI-90: 0.539; and DLQI 0/1: 0.580) and placebo (IGA 0/1: 0.520; EASI-75: 0.516; EASI-90: 0.539; and DLQI: 0.774) compared with abrocitinib at either dose (Table S3, see ESM).
Predictability of week 12 a IGA 0/1, b EASI-75, c EASI-90, and d DLQI 0/1 responses by PP-NRS change from baseline at week 2 with abrocitinib. DLQI 0/1 Dermatology Life Quality Index score of 0 or 1, EASI-75 ≥ 75% improvement from baseline in Eczema Area and Severity Index, EASI-90 ≥ 90% improvement from baseline in Eczema Area and Severity Index, IGA 0/1 Investigator’s Global Assessment of clear (0) or almost clear (1) and ≥ 2-grade improvement from baseline, PP-NRS Peak Pruritus Numerical Rating Scale, ROC receiver operating characteristic curve
3.5 Optimal PP-NRS Cutoff for Predicting Week 12 IGA 0/1, EASI-75, EASI-90, and DLQI 0/1 Responses
ROC curves were further summarized using the Youden Index scale of 0 to 1 (with values approaching 1 indicating higher predictiveness) to determine the optimal PP-NRS improvement needed at week 2 to predict week 12 efficacy responses for sensitivity and specificity (Table 1). The optimal threshold for PP-NRS improvement at week 2 to predict IGA 0/1 at week 12 was estimated to be 4 points with abrocitinib 200 mg, with a sensitivity/specificity of 61.3%/63.4%, and 3 points with abrocitinib 100 mg, with a sensitivity/specificity of 64.0%/66.4%. The lack of differences in week 12 efficacy assessments between week 2 PP-NRS4 responders and nonresponders with either dupilumab or placebo precluded further analysis in these treatment groups.
4 Discussion
In the JADE COMPARE study, a significantly higher proportion of patients receiving abrocitinib 200 mg achieved a clinically meaningful itch response at week 2 compared with patients receiving dupilumab or placebo [16]. Further characterization of itch responses in this analysis showed that onset of itch relief was more rapid in patients treated with abrocitinib than those with dupilumab or placebo, with improvement occurring as early as 4 days after treatment with abrocitinib 200 mg and 9 days after treatment with abrocitinib 100 mg. Importantly, these results were achieved in patients with AD with a mean baseline PP-NRS score of > 7.0 and DLQI score of > 15.0, which corresponded to severe itch and a substantial quality-of-life impairment. Furthermore, early improvements in itch predicted later clinical efficacy, with significant associations identified between PP-NRS4 at week 2 and IGA score and EASI percentage change from baseline category at week 12 with both abrocitinib doses but not with dupilumab. In this analysis, a 3- to 4-point improvement from baseline in PP-NRS score was estimated to be the optimal threshold for achieving skin clearance at week 12 in patients treated with either dose of abrocitinib, albeit with modest sensitivity and specificity.
Itch is one of the most burdensome symptoms reported by patients with moderate-to-severe AD, and rapid improvement in itch intensity is a key treatment goal [2, 3, 18]. Treatments that rapidly reduce itch and provide clinically meaningful improvements in disease severity and patients’ quality of life are important additions to the therapeutic armamentarium. Results from this post-hoc analysis suggest that patients who experience rapid itch relief with abrocitinib are also more likely to experience an improvement in skin clearance after 12 weeks of treatment than those who do not achieve a rapid response in itch. Notably, this trend did not hold for patients treated with dupilumab or placebo. Given the chronic nature of AD, early indicators of treatment outcomes may help inform the benefit:risk profile of continued treatment in individual patients. However, it should be highlighted that itch relief alone may not be sufficient to achieve improvement in lesional severity and skin clearance, and patients who do not experience early itch relief can still experience later improvements in skin clearance. This analysis does not fully address the relationship between itch relief and skin clearance, which is likely to be multifactorial in nature. Therefore, further study is warranted to determine the clinical utility of rapid itch response to help guide treatment decisions, including whether or not to continue treatment to achieve optimal outcomes for patients with AD.
Although the pathophysiology of itch in AD has not been fully elucidated, the differences in onset of itch relief between abrocitinib and dupilumab observed in this study may be attributable to their respective mechanisms of action. Through selective inhibition of JAK1, abrocitinib directly inhibits the signaling of multiple inflammatory cytokines that are associated with inducing itch, including interleukin (IL)-31, IL-4, IL-13, and thymic stromal lymphopoietin (TSLP) [19,20,21]. Dupilumab binds to the shared alpha chain subunit of the IL-4 and IL-13 receptors, thereby inhibiting the signaling of IL-4 and IL-13 [22]. Previous studies with dupilumab showed a delayed itch response, with most patients experiencing improvement after 4 weeks of treatment [22], supporting the findings in this analysis.
The main limitation of this study is the post-hoc nature of the efficacy assessments by PP-NRS response in the relatively small sample sizes of the subgroups. Results from patients in clinical trials may not be applicable to those in real-world clinical practice. This analysis was not designed as a head-to-head comparison of abrocitinib and dupilumab (with the exception of PP-NRS4 itch response from day 2 to day 15). Nevertheless, the week 12 response rates observed with abrocitinib 200 mg were numerically greater than dupilumab in the subset who achieved an early itch response. The differences in efficacy and quality-of-life outcomes between PP-NRS responders and nonresponders were assessed ad hoc and not powered for statistical significance when designing the study; hence the results should be interpreted accordingly. Finally, the high response rates observed with placebo should be contextualized with the use of emollients and topical medicated therapy for active lesions [23].
5 Conclusions
Abrocitinib provides rapid improvement in itch, with significant relief as early as day 4 after treatment, in patients with moderate-to-severe AD. Early itch relief was associated with additional treatment benefits of skin clearance and improvement in quality-of-life outcomes by week 12.
References
Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10-22.e2.
Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–7.
Vilsbøll AW, Anderson P, Piercy J, Milligan G, Kragh N. Extent and impact of inadequate disease control in us adults with a history of moderate to severe atopic dermatitis following introduction of new treatments. Dermatol Ther (Heidelb). 2021;11(2):475–86.
Rerknimitr P, Otsuka A, Nakashima C, Kabashima K. The etiopathogenesis of atopic dermatitis: barrier disruption, immunological derangement, and pruritus. Inflamm Regen. 2017;37:14.
Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–55.
Buhl T, Rosmarin D, Serra-Baldrich E, Fernandez-Peñas P, Igarashi A, Konstantinou MP, et al. Itch and sleep improvements with baricitinib in patients with atopic dermatitis: a post hoc analysis of 3 phase 3 studies. Dermatol Ther (Heidelb). 2021;11(3):971–82.
Blauvelt A, Ardern-Jones MR, Bieber T, Hong CH, Chu CY, Liu M, et al. 28032 Rapid itch improvement with upadacitinib with or without concomitant topical corticosteroids (TCS) in moderate-to-severe atopic dermatitis (AD): results from 3 phase 3 studies (Measure Up 1, Measure Up 2, and AD Up). J Am Acad Dermatol. 2021;85(3, Supplement):AB171.
Silverberg JI, Yosipovitch G, Simpson EL, Kim BS, Wu JJ, Eckert L, et al. Dupilumab treatment results in early and sustained improvements in itch in adolescents and adults with moderate to severe atopic dermatitis: analysis of the randomized phase 3 studies SOLO 1 and SOLO 2, AD ADOL, and CHRONOS. J Am Acad Dermatol. 2020;82(6):1328–36.
Simpson E, Wollenberg A, Soong W, Mark T, Kuznetsova A, Abildgaard Steffensen L, et al. Rapid and sustained improvements in itch and sleep with tralokinumab treatment in patients with moderate-to-severe Atopic Dermatitis, a post hoc analysis of pooled data from ECZTRA 1 and 2. SKIN J Cutan Med. 2021;5(6):S61.
CIBINQO 100 mg film-coated tablets. Summary of product characteristics. Pfizer, Limited: Kent; 2021. Updated September 10, 2021.
European Medicines Agency. CIBINQO® (abrocitinib). Summary of product characteristics. Beligium: Pfizer Europe MA EEIG; 2021.
JAPAN’S MHLW approves Pfizer's CIBINQO® (abrocitinib) for adults and adolescents with moderate to severe atopic dermatitis. News release. Pfizer Inc.; 2021.
CIBINQO (abrocitinib) tablets, for oral use. Prescribing information. New York: Pfizer Inc.; 2022.
Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–66.
Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–73.
Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–12.
Barrett A, Hanh-Pedersen J, Kragh N, Evans E, Gnanasakthy A. Patient-reported outcome measures in atopic dermatitis and chronic hand eczema in adults. Patient. 2019;12(5):445–59.
Boeri M, Sutphin J, Hauber B, Cappelleri JC, Romero W, Di Bonaventura M. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatol Treat. 2020;2:1–10.
Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342.
Cartron AM, Nguyen TH, Roh YS, Kwatra MM, Kwatra SG. JAK inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46(5):820–4.
Sutaria N, Adawi W, Goldberg R, Roh YS, Choi J, Kwatra SG. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86(1):17–34.
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–48.
Leshem YA, Bissonnette R, Paul C, Silverberg JI, Irvine AD, Paller AS, et al. Optimization of placebo use in clinical trials with systemic treatments for atopic dermatitis: an International Eczema Council survey-based position statement. J Eur Acad Dermatol Venereol. 2019;33(5):807–15.
Acknowledgements
Editorial/medical writing support under the guidance of the authors was provided by Megan K. Elder, PhD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Pfizer Inc.
Conflict of interest
SS is an investigator for Dermasence, Galderma, Kiniksa, Menlo Therapeutics, Novartis, Sanofi Genzyme, Trevi Therapeutics, and Vanda; a member of scientific advisory boards, consultant, and/or speaker for Pfizer Inc., AbbVie, Almirall, Beiersdorf, Bellus Health, Benevolent, Bionorica, Cara, Clexio, Eli Lilly, Escient, Galderma, Grünenthal, Kiniksa, LEO Pharma, Menlo Therapeutics, P.G. Unna Academy, Sanofi Genzyme, Trevi Therapeutics, and Vifor. SGK is an advisory board member/consultant for Pfizer Inc., AbbVie, Celldex Therapeutics, Galderma, Incyte Corporation, Kiniksa Pharmaceuticals, Novartis Pharmaceuticals Corporation, Regeneron Pharmaceuticals, and Sanofi Genzyme; and has served as an investigator for Pfizer Inc., Galderma, Kiniksa Pharmaceuticals, and Sanofi Genzyme. JIS has served as an investigator for Celgene, Eli Lilly, F. Hoffmann-La Roche, Menlo Therapeutics, Realm Therapeutics, Regeneron, and Sanofi Genzyme; as a consultant for Pfizer Inc., AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Glenmark, Incyte, Kiniksa Pharmaceuticals, LEO Pharma, Menlo Therapeutics, Novartis, Realm Therapeutics, Regeneron Pharmaceuticals and Sanofi Genzyme; and as a speaker for Regeneron Pharmaceuticals and Sanofi Genzyme. ELS has received grants from Pfizer Inc., Eli Lilly, Kyowa Kirin, LEO Pharma, Merck, and Regeneron Pharmaceuticals and personal fees from Pfizer Inc., Bausch Health (Valeant), Dermira, Eli Lilly, Galderma, LEO Pharma, Menlo Therapeutics, Novartis, Regeneron, and Sanofi Genzyme. JPT is an advisor for Pfizer Inc., AbbVie, Almirall, Arena Pharmaceuticals, Aslan Pharmaceuticals, Eli Lilly, LEO Pharma, OM Pharma, Regeneron Pharmaceuticals, Sanofi Genzyme, and Union Therapeutics; a speaker for Pfizer Inc., AbbVie, Almirall, Eli Lilly, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme; and received research grants from Pfizer Inc., Regeneron Pharmaceuticals, and Sanofi Genzyme. GY has been a consultant and an advisor for Pfizer Inc., Bellus, Eli Lilly, Galderma, Kiniksa, LEO Pharma, Novartis, Sanofi Regeneron, and Trevi Therapeutics and a principal investigator for Pfizer Inc., Galderma, Kiniksa, LEO Pharma, and Sanofi Regeneron. HV, MDB, and CF are employees and shareholders of Pfizer Inc. FZ and RRC are former employees and shareholders of Pfizer Inc. MC is a former employee and shareholder of Pfizer Inc. and has been a speaker, member of scientific advisory boards, and/or a consultant for AbbVie, Eli Lilly, and Incyte.
Ethics approval
The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice guidelines. All local regulatory requirements were followed. This research was approved by the institutional review board or ethics committee at each trial site. This article does not contain any studies with animals performed by any of the authors.
Consent to participate
All patients provided written informed consent to participate in the study.
Consent to publish
Not applicable.
Availability of data
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Author contributions
SS, JIS, ELS, JPT, GY, MCC, RRC, HV, and CF contributed to the study conception and design. FZ performed the data acquisition and analysis. All authors contributed to the data interpretation, had full editorial control of the manuscript, and provided input and approval of all drafts, including the final submitted version.
Additional information
Fan Zhang, Michael C. Cameron and Ricardo Rojo Cella: Affiliation at the time of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ständer, S., Kwatra, S.G., Silverberg, J.I. et al. Early Itch Response with Abrocitinib Is Associated with Later Efficacy Outcomes in Patients with Moderate-to-Severe Atopic Dermatitis: Subgroup Analysis of the Randomized Phase III JADE COMPARE Trial. Am J Clin Dermatol 24, 97–107 (2023). https://doi.org/10.1007/s40257-022-00738-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00738-4