Abstract
Background
Acne (syn: acne vulgaris) ranks as the most common inflammatory dermatosis treated worldwide. Acne typically affects adolescents at a time when they are undergoing maximum physical and social transitions, although prevalence studies suggest it is starting earlier and lasting longer, particularly in female patients. According to global burden of disease studies, acne causes significant psychosocial impact. Hence, identifying mechanisms to accurately measure the impact of the disease is important. Adopting an approach to harmonize and standardize measurements is now recognized as an essential part of any clinical evaluation and allows for better comparison across studies and meta-analyses.
Objective
The Acne Core Outcome Research Network (ACORN) has identified relevant domains as part of a core outcome set of measures for use in clinical studies. One of these is health-related quality of life (HRQoL). The aim of this systematic review was to provide information to inform the identification of the impacts most important to people with acne.
Methods
A synthesis of available evidence on acne impacts was constructed from a systematic review of the literature, with searches conducted in the MEDLINE, EMBASE and PsychInfo databases.
Results
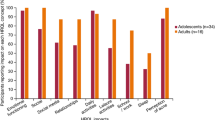
We identified 408 studies from 58 countries using 138 different instruments to detect the impacts of acne. Four of the five most commonly used instruments (Dermatology Life Quality Index [DLQI], Cardiff Acne Disability Index [CADI], Acne Quality of Life scale [Acne-QoL], Hospital Anxiety and Depression Scale [HADS] and Skindex-29) do not identify specific impacts but rather quantify to what extent acne affects HRQoL. Other studies identified one or more impacts using open-ended questions or tailor-made questionnaires.
Conclusion
This review serves as a rich data source for future efforts by groups such as ACORN (that include patients and health care providers) to develop a core set of outcome measurements for use in clinical trials.



Similar content being viewed by others
References
Layton AM, Eady EA, Thiboutot DM, Tan J, the Acne Core Outcomes Research Network (ACORN) Outcomes Identification Group. Identifying what to measure in acne clinical trials: first steps towards development of a core outcome set. J Investig Dermatol. 2017;137(8):1784–6.
Barnes LE, Levender MM, Fleischer AB, Feldman SR. Quality of life measures for acne patients. Dermatol Clin. 2012;30(2):293–300.
Alexis A, Daniels SR, Johnson N, Pompilus F, Burgess SM, Harper JC. Development of a new patient-reported outcome measure for facial acne: the acne symptom and impact scale (ASIS). J Drugs Dermatol. 2014;13(3):333–40.
Anderson R, Rajagopalan R. Responsiveness of the dermatology-specific quality of life (DSQL) instrument to treatment for acne vulgaris in a placebo-controlled clinical trial. Qual Life Res. 1998;7(8):723–34.
Girman CJ, Hartmaier S, Thiboutot D, et al. Evaluating health-related quality of life in patients with facial acne: development of a self-administered questionnaire for clinical trials. Qual Life Res. 1996;5(5):481–90.
Gupta MA, Johnson AM, Gupta AK. The development of an Acne Quality of Life scale: reliability, validity, and relation to subjective acne severity in mild to moderate acne vulgaris. Acta Derm Venereol. 1998;78(6):451–6.
Rapp SR, Feldman SR, Graham G, Fleischer AB, Brenes G, Dailey M. The Acne Quality of Life Index (Acne-QOLI): development and validation of a brief instrument. Am J Clin Dermatol. 2006;7(3):185–92.
US Department of Health and Human Services, US FDA. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009.
Chernyshov PV, Zouboulis CC, Tomas-Aragones L, Jemec GB, Manolache L, Tzellos T, et al. Quality of life measurement in acne. Position paper of the European Academy of Dermatology and Venereology Task Forces on quality of life and patient oriented outcomes and acne, rosacea and hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2018;32(2):194–208. https://doi.org/10.1111/jdv.14585.
Braun V, Clark V. Using thematic analysis in psychology. Qual Res Psychol. 2008;3:2:77–101.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–21.
Lewis-Jones M, Finlay A. The Children's Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942–9. https://doi.org/10.1111/j.1365-2133.1995.tb16953.x.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Rosenberg M. Conceiving the self. New York: Basic Books; 1979.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
Girman CJ, Hartmaier S, Thiboutot D, et al. Evaluating health-related quality of life in patients with facial acne: development of a self-administered questionnaire for clinical trials. Qual Life Res. 1996;5:481–90. https://doi.org/10.1007/BF00540020.
Chren MM, Lasek RJ, Sabek AP, Sands LP. Measurement properties of Skindex-16: a brief quality of life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105–10.
Nijsten TE, Sampogna F, Chren MM, Abeni DD. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Investig Dermatol. 2006;126:1244–50.
Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133:1433–40.
Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality of life measure for patients with skin disease: reliability, validity and responsiveness. J Investig Dermatol. 1996;107:707–13.
Smidt AC, Lai JS, Cella D, Patel S, Mancini AJ, Chamlin SL. Development and validation of Skindex-Teen, a quality-of-life instrument for adolescents with skin disease. Arch Dermatol. 2010;146:865–9.
Ware JE, Sherboume CD. The MOS36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Mad Care. 1992;30:473–83.
McLeod LD, Fehnel SE, Brandman J, Symonds T. Evaluating minimal clinically important differences for the acne-specific quality of life questionnaire. Pharmacoeconomics. 2003;21:1069–79.
Maloney JM, Arbit DI, Flack M, McLaughlin-Miley C, Sevilla C. Use of a low-dose oral contraceptive containing norethindrone acetate and ethinyl estradiol in the treatment of moderate acne vulgaris. Clin J Womens Health. 2001;1:123–31.
Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg. 2013;17:114–22.
Strauss JS, Leyden JJ, Lucky AS, et al. Safety of a new micronized formulation of isotretinoin in patients with severe recalcitrant nodular acne: a randomized trial comparing micronized isotretinoin with standard isotretinoin. J Am Acad Dermatol. 2001;45:196–207.
Webster GF, Leyden JJ, Gross JA. Results of a phase III double-blind, randomised, parallel group non-inferiority study evaluating the safety and efficacy of isotretinoin-lidose in patients with severe, recalcitrant nodular acne. J Drugs Dermatol. 2014;13:665–70.
Choi JM, Lew VK, Kimball AB. A single-blinded, randomized, controlled clinical trial evaluating the effect of face washing on acne vulgaris. Pediatr Dermatol. 2006;23:421–7.
Winkler UH, Ferguson H, Mulders JA. Cycle control, quality of life and acne with two low-dose oral contraceptives containing 20 microg ethinylestradiol. Contraception. 2004;69:469–76.
Tan J, Frey MP, Thiboutot D, Layton A, Eady EA. Identifying the impacts of acne: a Delphi survey of patients and clinicians. J Cutan Med Surg. 2020;24(3):259–66. https://doi.org/10.1177/1203475420907088.
Magin P, Adams J, Heading G, Pond D, Smith W. Psychological sequelae of acne vulgaris: results of a qualitative study. Can Fam Physician. 2006;52(8):978–9.
Kiassen A, Lipner S, O'Malley M, Longmire N, Cano S, Breitkopf T, et al. Development of a new patient-reported outcome measure to evaluate treatments for acne and acne scarring: the ACNE-Q. Br J Dermatol. 2019;181:1207–15. https://doi.org/10.1111/bjd.18005.
Schmitt J, Apfelbacker C, Spuls PI, Thomas KS, Simpson EL, Furue M, et al. The harmonizing outcome measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Investig Dermatol. 2015;135:24–30.
Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. San Jose: Consulting Psychologists Press, Inc.; 1983.
Derogitas RL, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605.
Skevington SM, Lotfy M, O'Connel KA, WHOQOL Group. The World Health Organization's WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13:299–310.
Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol. 1992;17:1–3.
Fenigstein A, Scheier MF, Buss AH. Public and private self-consciousness: assessment and theory. J Consult Clin Psychol. 1975;43:522–7.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5.
Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350–7.
Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, et al. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med. 1997;27:191–7.
Leibowitz MR. Social Phobia. Mod Probl Pharmacopsychiatry. 1987;22:141–73.
Martin AR, Lookinbill DP, Botek A, Light J, Thiboutot D, Gitman CJ. Health-related quality of life among patients with facial acne: assessment of a new acne-specific questionnaire. Clin Exp Dermatol. 2001;26:380–5.
Motley RJ, Finlay AY. How much disability is caused by acne? Clin Exp Dermatol. 1989;14:194–8.
Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401.
McNair DM, Heuchert JP, Shilony E. Profile of mood states: bibliography 1964–2002. Toronto: Multi-Health Systems; 2003.
Layton AM, Seukeran D, Cunliffe WJ. Scarred for life? Dermatology. 1997;195(Suppl 1):15–211.
Acknowledgements
The authors acknowledge Helen Weir, HDFT Librarian, and Esther Dell, Penn State Librarian.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alison Layton: Investigator and consultant for Galderma laboratories, Origimm, Proctor and Gamble, and La Roche Posay. Diane Thiboutot: Investigator and consultant for Galderma Laboratories, Biopharmix, and Cassiopea. Jerry Tan: Advisor, consultant, investigator and/or speaker for Allergan, Bausch, Botanix, Boots/Walgreens, Dermavant, Galderma, L’Oreal, and Novartis. Hayley Smith, Abbey Smith, Heather Whitehouse, Waseem Ghumra, Meenakshi Verma, Georgina Jones, Kathryn Gilliland, Megha Patel, Elaine Otchere, and Anne Eady have no conflicts of interest to declare.
Funding/Support
This work was funded by the US National Institutes of Health (NIH), award number 1U01AR065109-01.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Role of Funder/Sponsor
The NIH had no role in the design or conduct of this study, including data collection and analysis and preparation of this manuscript.
Availability of Data and Material
All data generated or analyzed during this study are included in this publication and the electronic supplementary files.
Code of Availability
Not applicable.
Author contributions
Study concept and design: Anne Eady, Alison Layton, Diane Thiboutot, Jerry Tan. Acquisition, analysis or interpretation of data: Kathryn Gilliland, Anne Eady, Megha Patel, Elaine Otchere, Waseem Ghumra, Hayley Smith, Abbey Smith, Heather Whitehouse, Meenakshi Verma, Georgina Jones, Alison Layton, and Diane Thiboutot. Drafting of the manuscript: Anne Eady, Hayley Smith, Alison Layton. Critical review of the manuscript for important intellectual content: All authors. Statistical analysis: Not applicable. Obtained funding: Diane Thiboutot. Administrative, technical or material support: Kathryn Gilliland. Study supervision: Diane Thiboutot and Alison Layton.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smith, H., Layton, A.M., Thiboutot, D. et al. Identifying the Impacts of Acne and the Use of Questionnaires to Detect These Impacts: A Systematic Literature Review. Am J Clin Dermatol 22, 159–171 (2021). https://doi.org/10.1007/s40257-020-00564-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-020-00564-6