Abstract
Background
Pulmonary arterial hypertension (PAH) is a progressive, cureless disease, characterized by increased pulmonary vascular resistance and remodeling, with subsequent ventricular dilatation and failure. New therapeutic targets are being investigated for their potential roles in improving PAH patients’ symptoms and reversing pulmonary vascular pathology.
Method
We aimed to address the available knowledge from the published randomized controlled trials (RCTs) regarding the role of Rho-kinase (ROCK) inhibitors, bone morphogenetic protein 2 (BMP2) inhibitors, estrogen inhibitors, and AMP-activated protein kinase (AMPK) activators on the PAH evaluation parameters. This systematic review (SR) was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CDR42022340658) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Overall, 5092 records were screened from different database and registries; 8 RCTs that met our inclusion criteria were included. The marked difference in the study designs and the variability of the selected outcome measurement tools among the studies made performing a meta-analysis impossible. However, the main findings of this SR relate to the powerful potential of the AMPK activator and the imminent antidiabetic drug metformin, and the BMP2 inhibitor sotatercept as promising PAH-modifying therapies. There is a need for long-term studies to evaluate the effect of the ROCK inhibitor fasudil and the estrogen aromatase inhibitor anastrozole in PAH patients. The role of tacrolimus in PAH is questionable. The discrepancy in the hemodynamic and clinical parameters necessitates defining cut values to predict improvement. The differences in the PAH etiologies render the judgment of the therapeutic potential of the tested drugs challenging.
Conclusion
Metformin and sotatercept appear as promising therapeutic drugs for PAH.
Clinical Trials Registration
This work was registered in PROSPERO (CDR42022340658).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Metformin and sotatercept are promising therapeutic drugs for pulmonary arterial hypertension (PAH). |
Additional long-term studies regarding fasudil and anastrozole in PAH are required. |
The possible implications of tacrolimus in the management of PAH are questionable. |
1 Introduction
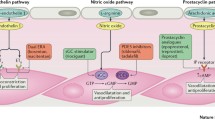
Pulmonary arterial hypertension (PAH) is a devastating disease with a 1-year mortality rate exceeding 20% in high-risk groups [1]. According to the underlying pathology, PAH is classified into idiopathic PAH (IPAH), heritable PAH (HPAH), drug- or toxin-induced PAH (D + T PAH), connective tissue disease-associated PAH (CTD-PAH), congenital heart disease-associated PAH (CHD-PAH), portal hypertension-associated PAH (PoPH), schistosomiasis-associated PAH, HIV-associated PAH (HIV-PAH), PAH with venous/capillary involvement, and persistent PH of the newborn. All of the aforementioned subtypes are characterized by pulmonary vascular obstructive disease and subsequent right ventricular failure. The currently approved therapeutic medications for PAH are endothelin receptor antagonists (ERAs), phosphodiesterase type 5 inhibitors (PDE5I), prostacyclin receptor agonists (PRAs), prostacyclin analogs (PAs), and soluble guanylate cyclase (sGC) [1, 2]. Being a disease of the pulmonary vasculature, these drugs target the vasoconstriction and vascular remodeling components of the disease. Although these drugs have relieved many of the PAH symptoms, and improved pulmonary vascular hemodynamics to some extent, the effects of the available therapeutic regimens as regards mortality are not satisfactory and cannot be considered a definitive cure [3]. This encouraged exceptional investigations to study this self-maintaining pathology and the possibility of reversing the already-existing pulmonary vascular disease.
Novel mechanisms, including the inhibition of Rho-kinase (ROCK) activity, estrogen synthesis and action, serotonin biosynthesis by the tryptophan hydroxylase inhibitor rodatristat (besides the bone morphogenetic protein 2 [BMP2] activators), the poly(ADP ribose) polymerase inhibitor olaparib, endothelin progenitor cells, metformin, and statins, emerged as new therapeutic approaches in PAH. Preliminary studies for statins did not show significant improvement in PAH patients [4], and rodatristat, olaparib, and endothelin progenitor cell investigations are still ongoing and their results are awaited. In contrast, ROCK, BMP2, estrogen signaling, and the cardioprotective potential of metformin all appeared as novel therapeutic targets in the management of PAH in many preclinical and clinical studies [5,6,7].
ROCK inhibitors are potent vasodilators that, by blocking ROCK activity, inhibit myosin light chain phosphorylation and relax contracted vascular smooth muscles [8]. Fasudil, a ROCK inhibitor, is approved in Japan and China to prevent cerebral vasospasm in aneurysmal subarachnoid hemorrhage [9]. According to many studies, ROCK activity has been demonstrated to be upregulated in PAH [10, 11]. Thus, ROCK inhibition by intravenous fasudil and oral fasudil hydrochloride (AT-877ER) have been investigated for their vasodilator effect that may limit PAH progression.
BMP2 belongs to the transforming growth factor-β (TGFβ) superfamily. BMP2 is known for its pleiotropic actions, including skeletal and extra-skeletal organogenesis, bone regeneration, and angiogenesis [12, 13]. Regarding PAH, the mutation in BMP2 has been observed mainly in HPAH. In contrast, reduced BMP2 expression in the absence of mutation has been reported in IPAH as well as other types of PAH. This BMP2 mutation/deficiency results in pulmonary endothelial cell dysfunction and apoptosis with cellular proliferation, vascular occlusive remodeling, and loss of the peripheral small pulmonary vessels, and hence a progressive increase in pulmonary arterial resistance, which initiates and maintains PAH [13,14,15]. Tacrolimus and sotatercept are potent BMP2 activators that have been investigated for their potential to improve PAH symptoms and reverse PAH pathology [13, 16, 17].
Estrone (E1) and 17β-estradiol (E2) are formed by the action of the aromatase (cytochrome P450 [CYP] 19A1) enzyme on androstenedione and testosterone, respectively. E1 and E2 form the primary forms of estrogen in humans, besides estriol (E3), which is only found during pregnancy [18]. Interestingly, the marked increased female/male ratio among PAH patients pointed out the possible role of estrogen in the pathophysiology of PAH [19]. However, female PAH patients were observed to run a relatively more benign course than their matched male PAH patients. Moreover, animal studies have reported the protective effects of estrogen against PAH development. Therefore, the exact role of estrogen in PAH disease is not yet clear; however, current knowledge refers to estrogen paradox as a critical modifier of PAH pathology [20]. This was partially explained in the view of estrogen metabolites that showed contradictory actions on the pulmonary vasculature, where hydroxy- and methoxy estradiols possess an antiproliferative and proapoptotic action, and 16α-hydroxy-estrone, 16α-hydroxy-estradiol, and 4-hydroxy-estrogens and their metabolites possess proinflammatory, proliferative, antiapoptotic, and DNA-breaking properties. Certain diseases and pathological situations could favor proliferative metabolite accumulation, e.g., hypoxia and inflammation [21], predisposing to PAH. In the same context, E2 has been known to have both protective and detrimental properties for PAH. E2 can attenuate vascular proliferation and remodeling through its protective metabolites, acting on estrogen receptor β (ERβ). E2 can also modulate the transcription of the vascular endothelial growth factor (VEGF) gene and increase the expression and activity of nitric oxide (NO) synthase [22,23,24]. On the other hand, E2 can also induce endothelial cell proliferation and migration [25]. Anastrozole, an aromatase inhibitor, and fulvestrant, an estrogen antagonist, emerged as critical modifiers of PAH and have been tested in experimental and clinical PAH [5].
Metformin is a well-known antidiabetic drug that can improve insulin sensitivity, enhance fatty acid oxidation and reduce oxidative stress, making it a potentially effective metabolic form of therapy in PAH [26]. Previous studies suggested that metformin can reduce cardiovascular disease risks in PAH patients. These effects are not solely attributed to the antihyperglycemic properties of metformin and may involve its actions on improving lipid metabolism, inflammatory response, and endothelial and vascular smooth muscle cell functions. In addition, metformin-dependent, AMP-activated protein kinase (AMPK) activation can facilitate the vasodilator and antiproliferative actions of NO [27, 28].
This systematic review (SR) aims to focus on the impact of the novel therapeutic targets of ROCK inhibition using fasudil/AT-877ER, BMP2 inhibition using tacrolimus and sotatercept, estrogen inhibitors using anastrozole and fulvestrant, and AMPK activation using metformin, on clinical and laboratory parameters as well as on the pulmonary vascular hemodynamics of PAH patients.
2 Methods
The study protocol has been registered in the International Prospective Register of Systematic Reviews Database (PROSPERO; registration number CDR42022340658).
2.1 Search Strategy
We conducted a search of the PubMed, Scopus, Google Scholar, ClinicalTrials.gov, and Cochrane Library databases for all articles from inception until 1 August 2022, using the following search terms: [(pulmonary arterial hypertension) OR (PAH) OR (arterial hypertension AND pulmonary) OR (hypertension AND pulmonary arterial)] AND [(Estrogen Antagonists) OR (Estrogen Inhibitor) OR (Estrogen Antagonist) OR (Estrogen Receptor Modulator) OR (Estrogen Receptor Antagonist) OR (Aromatase Inhibitor) OR (Fulvestrant) OR (Tamoxifen) OR (Anastrazole) OR (DHEA) OR (1-5-isoquinolinesulfonyl homopiperazine) OR (fasudil) OR (HA-077) OR (HA-1077) OR (AT 877) OR (AT877) OR (AT-877) OR (Rho-kinase inhibitor) OR (ROCK) OR (Protein Kinases, ROCK) OR (ROCK Protein Kinases) OR (Morphogenetic Protein AND Bone) OR (BMP) OR (BMPs) OR (sotatercept) OR (ACE-011) OR (tacrolimus) OR (FK506) OR (Prograf) OR (FR-900506) OR (Anhydrous Tacrolimus) OR (Tacrolimus AND Anhydrous) OR (Tacrolimus Anhydrous) OR (Anhydrous AND Tacrolimus) OR (isorhamnetin) OR (isorhamnetine) OR (3,4′,5,7-tetrahydroxy-3′-methoxy-flavone) OR (iso-rhamnetin) OR (3-O-methylquercetin) OR (3,4′,5,7-tetrahydroxy-3'-methoxyflavone) OR (3-methyl-quercetin) OR (Metformin) OR (N,N-dimethylbiguanide)]. We also contacted the authors of relevant registered studies and published conference papers, and screened relevant references mentioned in the reference list one by one, with no language restrictions.
2.2 Selection Criteria
Included studies were randomized controlled trials (RCTs) with one of the novel therapies as a comparator versus a comparison group, e.g., placebo and/or conventional therapies or different drug doses. The studied population was adult patients ≥ 18 years of age, diagnosed with World Health Organization (WHO) Group I PAH, and with one of the following etiologies: IPAH, HPAH, D+T PAH, CTD-PAH, adult CHD-PAH, PoPH, schistosomiasis-associated PAH, HIV-PAH, PAH with venous/capillary involvement, and persistent PH of the newborn.
Exclusion criteria were studies that included PAH patients with other comorbidities of the cardiopulmonary systems or other pathologies that may show conflict with the other types of pulmonary hypertension (PH) other than Group 1, e.g., ischemic heart disease, chronic obstructive lung disease, asthma, lung fibrosis, thromboembolic disorders, and unclear/multifactorial PH.
2.3 Outcome Measures
In this SR, the primary outcome was assessment of the 6-min walk distance (6MWD), while the secondary outcome measures were brain natriuretic peptide (BNP)/N-terminal pro-BNP (NT-pro BNP), tricuspid annular plane systolic excursion (TAPSE), mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (PVR), and cardiac index (CI). Data were presented as frequency in numbers, or mean change ± standard deviation (SD). For data for which the mean and SD were not available, median (interquartile range) was reported. When data were available at different assessment points, they were mentioned accordingly, otherwise the change from baseline was mentioned.
2.4 Data Extraction
Data extraction was conducted independently by two reviewers using an extraction form. To resolve discrepancies, group discussion was performed with resolution of disagreement by consensus.
2.5 Quality Assessment
The risk of bias in the included studies was evaluated according to the following domains: study design, randomization generation sequence, blinding for patients and researchers, allocation concealment, baseline comparability, post-randomization exclusion, duration of the study, and percentage of patients who completed the follow-up.
2.6 Synthesis
Although we intended to conduct a statistical synthesis/meta-analysis, we could not accomplish this due to the lack of uniformity between studies as regards the comparator, outcome measure, and reporting method. Therefore, we conducted a narrative synthesis where we analyzed the differences and similarity between the results of the individual studies as regards their therapeutic potential on PAH.
3 Results
Our search terms yielded 5092 records, of which 3138 were duplicates and were removed before screening. The remaining 1954 records were then screened by titles and 1850 records were subsequently excluded. Of 104 reports sought for retrieval by screening their abstracts, only 15 records were screened by full-text for eligibility and only 8 studies that fulfilled our inclusion and exclusion criteria were included (Fig. 1), with a total of 470 participants. The number of studies and number of participants in the eligible studies were as follows: ROCK inhibitors: fasudil/AT-877ER (3 studies with 133 participants); tacrolimus (1 study with 23 participants); sotatercept (2 studies with 203 participants); anastrozole (1 study with 18 participants); and metformin (1 study with 93 participants). Only one pilot study that analyzed the effect of fulvestrant on PAH was identified on the databases but was excluded for not meeting our inclusion criteria.
3.1 Baseline Patients and Study Characteristics
As shown in Table 1, participants in the different studies were predominantly females aged between 18 and 77 years. Most of the patients were diagnosed with CHD-PAH (n = 173) and IPAH (n = 165), however some patients had CTD-PAH (n = 72) and HPAH (n = 37), and only a few patients had either D+T PAH (n = 18), PoPH (n = 4) or HIV-PAH (n = 1). WHO functional class (FC) II and III were dominant among the participants, followed by WHO FC IV and WHO FC I.
Comparators varied greatly across studies. Regarding fasudil, Jiang et al. studied the effect of fasudil 30 mg against the inhalation of iloprost 5 μg jet [9], while Ruan et al. challenged fasudil 30 mg against fasudil 60 mg [30]. In these two studies, fasudil was administered by means of an intravenous infusion over 30 min. Fukumoto et al. studied the effect of oral fasudil hydrochloride (AT-877ER) [two capsules, increasing every 3 days to reach a total of six capsules/day, but the capsule dose was not mentioned] with standard of care (SOC) with either beraprost, bosentan, or sildenafil, in comparison with placebo in addition to the same SOC [29]. As regards bone morphogenic modulators, oral tacrolimus was investigated by Spiekerkoetter et al. in three different doses: < 2 ng/mL, 2–3 ng/mL, and 3–5 ng/mL in comparison with placebo in addition to SOC, i.e., ERAs, PDE5I, sGC, PAs, and PRAs [17]. Humbert et al. [13] studied the add-on effect of subcutaneous administration of sotatercept (0.3 or 0.7 mg/kg) every 3 weeks in addition to SOC, in comparison with placebo in addition to SOC. In the phase II PULSAR study, Humbert et al. [31] cross-randomized the placebo control group in their previous study to sotatercept (0.3 or 0.7 mg/kg), while the previous sotatercept-treated groups continued with their sotatercept doses. Only one trial, by Kawut et al. [32], investigated the effect on anastrozole 1 mg versus placebo in addition to SOC. One study addressed the effect of metformin 500 mg twice daily plus bosentan (initially at 62.5 mg twice daily for 4 weeks and then 125 mg twice daily) and compared it with bosentan alone in the same previously mentioned doses [33].
The duration of these RCTs varied widely. Jiang et al. [9] and Ruan et al. [30] focused on acute changes with intravenous fasudil infusion. The investigation by Jiang et al. lasted only 90 min (an additional 72 h were extended to observe any adverse drug reaction related to fasudil infusion), while the investigation by Ruan et al. lasted 30 min (with an additional 120 min for observation of any adverse drug reaction). In contrast, oral fasudil (AT877ER) was administered for 12 weeks [29]; tacrolimus was administered for 16 weeks [17]; sotatercept, according to Humbert et al. [13], was administered for 24 weeks (an additional 18 months were extended to exclude any chronic adverse drug reaction), and in the PULSAR study sotatercept was administered for 24 months [31]. Furthermore, anastrozole and metformin were studied for 3 months according to the protocols by Kawut et al. [32] and Liao et al. [33], respectively.
3.2 Primary Outcome, 6-Min Walk Distance
6MWD was assessed in six RCTs with a total of 370 patients. Humbert et al. reported significant improvement at 24 weeks with sotatercept 0.3 and 0.7 mg/kg doses compared with placebo [13]. This efficacy was again observed in the PULSAR trial in the placebo-crossed group, with no further improvement observed with the other sotatercept-treated group [31]. The aromatase inhibitor anastrozole also showed significant improvement after 3 months of therapy in comparison with the placebo group [32]. As regards metformin therapy, Liao et al. reported significant improvement with metformin in combination with bosentan for 3 months, compared with bosentan monotherapy [33]. Thus, three agents showed statistically significant improvement, namely sotatercept, anastrozole, and metformin. It is worth mentioning that the maximum improvement was observed with metformin/bosentan therapy, with around 31% improvement, followed by sotatercept and bosentan monotherapy, with improvement ranging between 12 and 16%, and finally anastrozole, with nearly 6.8% improvement. Others such as fasudil and tacrolimus showed no significant effect on 6MWD. The ROCK inhibitor AT-877ER showed a non-significant 4.8% improvement [29], but none of the three tested doses of tacrolimus showed improvement [17]. On the contrary, there was a marked deterioration, by 11.68%, with the low dose of the latter (Table 2).
3.3 Secondary Outcome Parameters Brain Natriuretic Peptide/N-Terminal Pro-BNP (BNP/NT-proBNP)
As shown in Table 2, BNP/NT-proBNP was estimated in six RCTs, as Ruan et al. [30] and Jiang et al. [9] only reported the baseline BNP levels before initiating fasudil therapy. Sotatercept (0.3 and 0.7 mg/kg) significantly decreased NT-proBNP compared with placebo [13]. Similarly, in the placebo-crossed group of the PULSAR trial, sotatercept showed significant reduction similar to that of the continued treatment groups, with improvement ranging between 40 and 65% in both studies [31]. Both the metformin/bosentan combined group and the bosentan group showed a significant decrease in NT-proBNP values after 3 months of treatment [33], and followed sotatercept with a 23–30% improvement. Oral fasudil hydrochloride (AT-877ER) tended to improve BNP compared with baseline values, yet this improvement was not significant [29]. Tacrolimus [17] and anastrozole [32] failed to decrease NT-proBNP but showed marked deterioration, especially with low-dose tacrolimus followed by anastrozole.
3.3.1 Tricuspid Annular Plane Systolic Excursion (TAPSE)
TAPSE was not evaluated with either fasudil or sotatercept in the PULSAR trial, or with metformin. Moreover, no significant improvement was reported with the two sotatercept doses according to Humbert et al. [13]. In contrast, tacrolimus [17] and anastrozole [32] therapies showed worsening in this parameter.
3.3.2 Hemodynamics, Mean Pulmonary Artery Pressure, Pulmonary Vascular Resistance, and Cardiac Index
Regarding the study by Ruan et al. [30], the infusion of both fasudil 30 mg and 60 mg significantly decreased mPAP and PVR and increased the CI with no significant difference between the two groups. In the same context, Jiang et al. [9] reported that the infusion of fasudil 30 mg and the inhalation of iloprost succeeded in significantly decreasing mPAP and PVR and increasing the cardiac output. (CO). Moreover, Jiang et al. reported that the observed increase in CO was more related to fasudil than with iloprost, but the effect on the CI was not reported in their study. Oral fasudil hydrochloride (AT-877ER) did not show significant improvement in the mPAP or PVR, but showed significant improvement regarding the CI [29]. Sotatercept significantly reduced the mPAP from the baseline values, according to Humbert et al. [13], who also reported significant reduction in PVR compared with that observed in the placebo group. The PULSAR trial reported similar improvement with sotatercept in the placebo-crossed group with regard to the mPAP and PVR values, but not the CI. This effect was not observed in the group that continued sotatercept treatment [31]. Anastrozole did not show any significant improvement in these tested parameters [32]. Regarding metformin, Liao et al. reported significant improvement in the mPAP, PVR, and CI values in both the metformin/bosentan and bosentan groups, with no significant difference between both groups [33]. Interestingly, the maximum improvement in mPAP and PVR was observed with bosentan/metformin, bosentan monotherapy, sotatercept, and fasudil 60 mg followed by fasudil 30 mg. The CI was also maximally and significantly improved with bosentan/metformin, bosentan, and fasudil 60 mg (ranging between 13 and 16% improvement) followed by fasudil 30 mg, while sotatercept did not succeed in improving the CI.
3.4 Quality Assessment
Although all studies were reported as RCTs, the method of random sequence generation was not mentioned in four of the eight included studies. Most of the studies (six of eight) were double-blinded. Allocation concealment was reported in only two studies using sequential sealed envelopes [30] and pharmacy control [32]. Baseline comparability was not specified in two of eight studies. Regarding post randomization exclusion, 3/23 [29], 3/23 [17], 6/106 [13], and 7/97 [31] participants were excluded. The duration of follow-up exceeded 85% in all included studies and reached 100% in three studies, i.e. Jiang et al. [9], Kawut et al. [32], and Ruan et al. [30]. Detailed results are shown in Table 3.
4 Discussion
In this SR, eight RCTs that investigated the role of fasudil, tacrolimus, sotatercept, anastrozole, and metformin in adult PAH patients (≥ 18 years of age) were included. We relied on patients’ exercise tolerance (6MWD) and laboratory (BNP/NT-proBNP), echocardiographic (TAPSE) and hemodynamic (mPAP, PVR, CI) tools to assess the response to the studied drugs. Fasudil showed a positive impact on the pulmonary hemodynamics assessed by mPAP, PVR, and CI without improving 6MWD and BNP. Sotatercept improved 6MWD, NT-proBNP, mPAP, and PVR, but not CI. Anastrozole improved 6MWD significantly, however hemodynamic assessment was not performed. Metformin showed significant improvement in 6MWD, NT-proBNP, mPAP, PVR, and CI. Notably, TAPSE was only measured with tacrolimus, sotatercept and anastrozole but did not improve with any of these agents. Although it was difficult to compare among different drugs in the different studies statistically, it could be observed that metformin/bosentan therapy had the potential to improve all the examined parameters significantly and competitively with the other tested drugs, except for TAPSE, which was not evaluated in the study by Liao et al. [33]. Sotatercept also showed promising potential in PAH management by improving 6MWD, NT-proBNP, mPAP, and PVR. Although sotatercept did not show significant improvement in the CI, it protected against deterioration of the CI. Infused fasudil showed significant improvement in all the tested hemodynamic parameters, but these studies did not evaluate 6MWD or BNP. The small sample size and different PAH pathologies in the anastrozole study challenged these results. On the other hand, the worsening of 6MWD, NT-proBNP, and TAPSE observed with low-dose tacrolimus, as well as the deficient data regarding the effect of tacrolimus on the hemodynamic parameters, made it difficult to anticipate its impact on PAH management.
In this SR, ROCK inhibition using infused, but not oral, fasudil improved mPAP and PVR, while both infused and oral fasudil significantly improved the CI; however, oral fasudil did not improve the 6MWD or BNP. However, the authors stated that their calculations predicted a significant improvement in these parameters if the sample size included ≥ 100 participants [29]. ROCK inhibition has gained much attention recently due to its established role in various cardiovascular diseases such as hypertension, ischemic heart disease, and PAH [34]. The therapeutic potential of fasudil in PAH has also been extensively investigated in many preclinical and clinical studies that showed the potential of fasudil to relax the abnormally contracted pulmonary vascular smooth muscle [11, 35, 36]. Intravenous [9, 30], inhaled [11], and oral fasudil forms [29] were all evaluated as therapeutic dosage forms that have shown promising results. Fasudil also showed better response than that of calcium channel blockers due to the selective action of the latter on the hyper-contracting muscular cells. ROCK inhibition can also enhance the expression of endothelial NO synthase (eNOS) and inhibit chemotaxis and angiotensin II-mediated activation of plasminogen activator inhibitor-1, and hence ameliorate PAH [35].
Regarding drugs that affect BMP2, Spiekerkoetter et al. reported that tacrolimus failed to significantly improve 6MWD, NT-proBNP, and TAPSE [17]. This came about in spite of the fact that Spiekerkoetter et al. reported significant improvement using tacrolimus in BMP2 knockout mice and monocrotaline-induced PAH rats [16]. They also observed that patients with higher BMP2 levels showed better 6MWD results, which emphasizes the direct association between BMP2 levels and PAH. This encourages additional future studies targeting the role of tacrolimus in PAH, but with a larger sample size and possibly a uniform PAH pathology.
With respect to sotatercept, in their two RCTs, Humbert et al. reported significant improvement in 6MWD, NT-proBNP, and PVR using sotatercept doses of 0.3 and 0.7 mg/kg. This improvement was also achieved in the placebo-crossed group, which also showed improvement in the mPAP values. As previously mentioned, sotatercept can restore the TGFβ/BMPII balance, which is speculated to reverse pulmonary vascular pathology and guard against right ventricular failure [13, 31]. Following this finding, Joshi et al. studied the effect of sotatercept on isolated human pulmonary artery smooth muscle cells (PASMc) and human pulmonary artery endothelial cells in preclinical models of PAH. They stated that sotatercept attenuating the TGFβ/BMPII-dependent Smad2/3 signaling pathway reversed pulmonary vascular remodeling and restored the right ventricular proper geometry and function [37]. It should be mentioned that in the RCTs conducted by Humbert et al., the achievement of sotatercept referred to, at least in part, the relatively short disease period (average of 8 years from the time of the diagnosis) and the characteristics of the population who had received intensive SOC before and during the trial period [13, 31]. However, this points to the promising therapeutic potential of sotatercept in the management of PAH.
Anastrozole showed significant improvement in the 6MWD as a percentage and an absolute change from baseline [32]. It has previously been reported that high E2 levels correlated to an increased risk for PAH in men [38]. The deleterious effect of the proliferative estrogen metabolites on hematopoietic progenitor cells that are encountered in the pathophysiology of PAH, and the attenuation of this pathway with anastrozole, can explain this positive finding [21]. In concordance with this, Kuwait et al. reported a positive impact of the estrogen receptor α (ERα) antagonist fulvestrant on the 6MWD in a pilot study that enrolled five patients with PAH, which confirms the potential of estrogen inhibitors to attenuate PAH disease. Additionally, the add-on metabolic actions of anastrozole may have improved muscle perfusion, which was reflected in the observed changes in the 6MWD [32, 39].
Liao et al. reported significant improvement in the 6MWD, NT-proBNP, mPAP, PVR, and CI when using metformin [33]. Besides its imminent role in lowering blood glucose and improving insulin resistance, metformin-dependent activation of AMPK seems beneficial in PAH therapy. Previous studies have shown that metformin can activate AMPK actions and suppress endothelin gene (EDN1) expression, where the latter is a potent proliferative cytokine with strong vasoconstriction properties. This action can attenuate PASMc proliferation and reverse PAH [40, 41]. Furthermore, AMPK activation by metformin was shown to increase eNOS activity and potentiate the pulmonary vascular reactivity and hemodynamics through the vasodilator and antiproliferative actions of NO [27]. Notably, metformin can inhibit the action of the aromatase enzyme, decreasing estrogen signaling with the potential to reverse the development of PH [28]. Metformin has been used for decades in the management of type 2 diabetes mellitus. Due to the approved safety and euglycemic potential of metformin, the latter has been approved for the management of other non-diabetic disorders, e.g., polycystic ovary, weight reduction, and prevention of weight gain, with some antipsychotic drugs. It has also been widely investigated for lowering the risk of cancer, dementia and stroke in vulnerable patients [42]. However, a significant concern when contemplating the use of metformin in the management of PAH is the increased risk of developing lactic acidosis in patients with acute heart failure or end-organ tissue hypoperfusion, as these conditions can be frequently observed in some PAH patients [43].
Many parameters are currently used to evaluate the therapeutic potential of PAH-targeted therapies, which can also help to predict the prognosis and monitor improvement during the treatment journey. The 6MWD has been used for decades as a primary endpoint in the evaluation process of new PAH therapies. Being safe, inexpensive, and generally acceptable for patients, aided in its wide-use scale as one of the independent predictors of mortality among severely compromised PAH patients [44, 45], the 6MWD was extensively used to evaluate the currently approved PAH therapies, namely endothelin antagonists, phosphodiesterase inhibitors, and prostacyclins [46]. According to the European Society of Cardiology (ESC) and European Respiratory Society (ERS) 2022 PH guidelines, the 6MWD is included as a prognostic tool in the simplified four-strata risk assessment of PAH patients, together with BNP/NT-proBNP and WHO FC, as well as in the three-strata model of risk assessment [47]. Hence, in this SR, 6MWD was chosen as a primary endpoint to assess the impact of the tested drugs on PAH patients and to investigate how the speculated improvement would improve patients’ exercise capacity.
The Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) enrolled 1798 patients for whom the 6MWD was consistently evaluated over a period of up to 1 year. Researchers concluded that worsening of 6MWD can predict lower 1-year survival, but stationary or no improvement in the 6MWD had a similar outcome [48]. In the same context, a meta-analysis conducted on 22 RCTs enrolling 3112 PAH patients concluded that one could not rely on the 6MWD as a predictor of major clinical events in short-term therapies up to 4 months [49], which is the treatment period range of all the included RCTs in this SR that failed to show significant changes in the 6MWD values. In parallel, the ESC/ERC 2022 PH guidelines suggested a timing schedule for 6MWD, i.e. to be performed at baseline and after 3–6 months after changing therapies as well as in stable patients. This confirms the shortage of data derived from short-term clinical trials. Moreover, to accurately describe the available data regarding the 6MWD, we should exclude any musculoskeletal or systemic comorbidities that could have affected physical performance during the 6MWD evaluation [50]. However, the positive results gained with sotatercept, anastrozole, and metformin deserve additional clinical studies to confirm their positive impact on exercise capacity.
In this SR, the CI was used to evaluate cardiac performance against progressive pulmonary artery resistance. In this SR, BNP/NT-proBNP and TAPSE have been used as indicators of right ventricular function, which strongly predicts patients’ survival. In contrast, mPAP and PVR were used to estimate the response of the pulmonary vascular component of PAH pathology.
In addition to being one of the risk assessment tools for PAH patients, according to the ESC/ERC 2022 PH guidelines, BNP/NT-proBNP confers advantages through being non-subjective, cost effective, and reducing the time to diagnosis [47]. Moreover, it can reduce hospital admission rates, with BNP better correlated with PAH hemodynamics and NT-proBNP correlated with the prognosis [51]. Thus, by showing significant improvement in the NT-proBNP values, sotatercept and metformin tend to be novel therapeutic targets of PAH in the future.
By analyzing data from 517 PAH patients from seven observational studies from Europe and the United States, Ghio et al. [52], found that TAPSE is one of the practical tools in the stratification of all-cause mortality. Similarly, improvement in TAPSE ≥ 15 mm has been associated with lower mortality risk even when this change in TAPSE values was not closely related to a similar improvement in vascular resistance of hemodynamics [53]. This may explain the negative results with tacrolimus, sotatercept, anastrozole, and metformin studies in the current SR that included TAPSE as an outcome measure and showed relative improvement in some hemodynamic parameters, but not with TAPSE. However, it should be mentioned that in the ESC/ERC 2022 PH guidelines, TAPSE/systolic artery pressure (sPAP), but not TAPSE, is currently used in the risk stratification of PAH [47]. Therefore, future studies should use TAPSE/sPAP instead of the classic TAPSE.
Some studies investigated the impact of their tested drug using, preferably, non-invasive parameters, i.e., 6MWD, BNP/NT-proBNP, and TAPSE. In contrast, others used hemodynamic changes as their primary outcome measure, with only four of the eight included studies relying on both non-invasive and invasive parameters. In these four studies, the discrepancy in the correlation of the outcome measures raised the question of the sensitivity of these parameters. In concordance with these findings, in the REVEAL trial, 6MWD was correlated with CO, but no significant correlation was found with mPAP or PVR [48]. This may encourage the need for determining cut-points for mPAP and PVR similar to those of 6MWD, BNP/NT-proBNP, TAPSE, and CI mentioned in the ESC/ERC 2022 PH guidelines when being accepted to be used as predictors of outcome or for risk stratification. It should be noted that although right atrial pressure (RAP), mixed venous oxygen saturation (Sv02), and stroke volume index (SVI) are good prognostic tools that are well correlated to the risk stratification of PAH patients, these parameters did not receive much attention by most of the investigators, with only some parameters evaluated in only three of the eight included RCTs. Ruan et al. [30] reported a non-significant improvement with fasudil intravenous infusion on RAP, while Jiang et al. [9] also mentioned that fasudil intravenous infusion did not improve RAP but significantly improved Sv02. On the other hand, Liao et al. [33] reported significant improvement on RAP using metformin/bosentan, but this improvement was not associated with a similar improvement in SvO2 and was also achieved in the bosentan monotherapy group, which makes the add-on value of metformin on these parameters doubtful and needing further evaluation.
The limitations of the included studies include the lack of uniformity in the underlying pathology of PAH in the studied groups, except for Ruan et al. [30] and Liao et al. [33], who chose CHD-PAH as their targeted studied population, which may render the analysis of the acquired data not fully accurate. Even if targeting the same pathology, i.e., pulmonary vascular disease, the pathophysiology with each PAH subtype differs markedly, and so may the outcome parameters that evaluated the therapeutic potential of the tested drugs collectively. Moreover, the participant’s age/sex, the WHO FC, and the duration of the disease/therapy should all be taken into consideration, together with any other related comorbidities, while judging whether or not the tested therapy is of significant impact on PAH [47,48,49,50,51,52,53,54]. Thus, the absolute numbers of the tested parameters, or even the change from baseline, are not always the best tool to estimate patients’ response to tested drugs. It should also be taken into consideration that TAPSE is no longer used in the risk stratification of PAH patients and TAPSE/sPAP should instead be used [47]. In addition, RAP, SvO2, and SVI should be included as part of the hemodynamic assessment during right heart catheterization (RHC). Nevertheless, during RHC, performing hemodynamic assessments at the end of expiration is preferred as the end expiratory intrathoracic pressure is closely correlated with the atmospheric pressure [55]. Therefore, whether the recording timing during RHC influenced the hemodynamic results or not, is questionable.
5 Conclusion
In this SR, we compared the effect of fasudil, tacrolimus, sotatercept, anastrozole, and metformin as therapeutic options for PAH. Metformin appears to be a critical modifier of PAH pathology, with promising results that need to be validated with different PAH pathologies and for longer durations. Similarly, sotatercept emerges as a powerful novel therapy, yet the least effective dose should be defined to avoid its unpleasant adverse drug reactions. The long-term therapeutic potential of fasudil and anastrozole is of great interest and needs further long-term investigations that should include different assessment parameters. Reports about the role of tacrolimus in PAH are conflicting and therefore robust studies are needed. The least effective dose of these novel drugs and the combination regimen with the available approved PAH therapies remain an attractive area of investigation. It is also highly recommended to account for any confounding variables, such as different PAH pathology, age, sex, duration since diagnosis, and previous/concomitant interventions, while designing a clinical trial and interpretating its results.
References
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RM, Brida M, Carlsen J, Coats AJ, Escribano-Subias P, Ferrari P, Ferreira DS. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022;43(38):3618–731.
Vazquez ZG, Klinger JR. Guidelines for the treatment of pulmonary arterial hypertension. Lung. 2020;198(4):581–96.
Fu W, He W, Li Y, Chen Y, Liang J, Lei H, Fu L, Chen Y, Ren N, Jiang Q, Shen Y. Efficacy and safety of novel-targeted drugs in the treatment of pulmonary arterial hypertension: a Bayesian network meta-analysis. Drug Deliv. 2021;28(1):1007–19.
Rysz-Górzynska M, Gluba-Brzózka A, Sahebkar A, Serban MC, Mikhailidis DP, Ursoniu S, Toth PP, Bittner V, Watts GF, Lip GY, Rysz J. Efficacy of statin therapy in pulmonary arterial hypertension: a systematic review and meta-analysis. Sci Rep. 2016;6(1):1.
Condon DF, Agarwal S, Chakraborty A, Auer N, Vazquez R, Patel H, Zamanian RT, de Jesus Perez VA. Novel mechanisms targeted by drug trials in pulmonary arterial hypertension. Chest. 2022;161(4):100–1072.
Dunmore BJ, Jones RJ, Toshner MR, Upton PD, Morrell NW. Approaches to treat pulmonary arterial hypertension by targeting BMPR2: from cell membrane to nucleus. Cardiovasc Res. 2021;117(11):2309–25.
Lazarus HM, Denning J, Wring S, Palacios M, Hoffman S, Crizer K, Kamau-Kelley W, Symonds W, Feldman J. A trial design to maximize knowledge of the effects of rodatristat ethyl in the treatment of pulmonary arterial hypertension (ELEVATE 2). Pulm Circ. 2022;12(2): e12088.
Odagiri K, Watanabe H. Effects of the Rho-kinase inhibitor, fasudil, on pulmonary hypertension. Circ J. 2015;79(6):1213–4.
Jiang X, Wang YF, Zhao QH, Jiang R, Wu Y, Peng FH, Xu XQ, Wang L, He J, Jing ZC. Acute hemodynamic response of infused fasudil in patients with pulmonary arterial hypertension: a randomized, controlled, crossover study. Int J Cardiol. 2014;177(1):61–5.
Fukumoto Y, Takaki A, Tawara S, Ohashi J, Nakano M, Tada T, Saji K, Sugimura K, Fujita H, Hoshikawa Y, Nawata J. Evidence for Rho-kinase activation in patients with pulmonary arterial hypertension. Circ J. 2009;73(9):1731–9.
Fujita H, Fukumoto Y, Saji K, Sugimura K, Demachi J, Nawata J, Shimokawa H. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels. 2010;25(2):144–9.
Riley EH, Lane JM, Urist MR, Lyons KM, Lieberman JR. Bone morphogenetic protein-2: biology and applications. Clin Orthop Relat Res. 1996;324:39–46.
Humbert M, McLaughlin V, Gibbs JS, Gomberg-Maitland M, Hoeper MM, Preston IR, et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021;384(13):1204–15.
de Jesus Perez VA, Alastalo TP, Wu JC, Axelrod JD, Cooke JP, Amieva M, Rabinovitch M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt–β-catenin and Wnt–RhoA–Rac1 pathways. J Cell Biol. 2009;184(1):83–99.
Guignabert C, Humbert M. Targeting transforming growth factor-β receptors in pulmonary hypertension. Eur Respir J. 2021;57(2).
Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Investig. 2013;123(8):3600–13.
Spiekerkoetter E, Sung YK, Sudheendra D, Scott V, Del Rosario P, Bill M, et al. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J. 2017;50(3):1602449.
Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res. 1999;84(11):1285–91.
Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–87.
Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol-Lung Cell Mol Physiol. 2014;307(1):L7-26.
Mueck AO, Seeger H. 2-methoxyestradiol—biology and mechanism of action. Steroids. 2010;75(10):625–31.
Austin ED, Lahm T, West J, Tofovic SP, Johansen AK, MacLean MR, Alzoubi A, Oka M. Gender, sex hormones and pulmonary hypertension. Pulm Circ. 2013;3(2):294–314.
Tofovic SP. Estrogens and development of pulmonary hypertension-interaction of estradiol metabolism and pulmonary vascular disease. J Cardiovasc Pharmacol. 2010;56(6):696.
Tofovic SP, Jackson EK. Estradiol metabolism: crossroads in pulmonary arterial hypertension. Int J Mol Sci. 2019;21(1):116.
White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin L, McClure JD, Thomas M. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126(9):1087–98.
Hemnes A, Niswender K, Burke K, Fan R, Mallugari R, Newman JH, et al. Clinical trial of metformin in pulmonary arterial hypertension. American Thoracic Society International Conference Abstracts. C97. Don’t stop believing: clinical trials in pulmonary vascular medicine. Am J Respir Crit Care Med. 2019;199:A5588.
Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55(2):496–505.
Dean A, Nilsen M, Loughlin L, Salt IP, MacLean MR. Metformin reverses development of pulmonary hypertension via aromatase inhibition. Hypertension. 2016;68(2):446–54.
Fukumoto Y, Yamada N, Matsubara H, Mizoguchi M, Uchino K, Yao A, et al. Double-blind, placebo-controlled clinical trial with a Rho-kinase inhibitor in pulmonary arterial hypertension—a pilot efficacy trial. Circ J. 2013;77(10):2619–25.
Ruan H, Zhang Y, Liu R, Yang X. The acute effects of 30 mg vs 60 mg of intravenous Fasudil on patients with congenital heart defects and severe pulmonary arterial hypertension. Congenit Heart Dis. 2019;14(4):645–50.
Humbert M, McLaughlin V, Gibbs JS, Gomberg-Maitland M, Hoeper MM, Preston IR, et al. Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension. Eur Respir J. 2023;61(1):2201347.
Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, Palevsky HI, Palmisciano AJ, Patel M, Pinder D, Propert KJ. Anastrozole in pulmonary arterial hypertension. A randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2017;195(3):360–8.
Liao S, Li D, Hui Z, McLachlan CS, Zhang Y. Metformin added to bosentan therapy in patients with pulmonary arterial hypertension associated with congenital heart defects: a pilot study. Erj Open Research. 2018;4(3).
Surma M, Wei L, Shi J. Rho kinase as a therapeutic target in cardiovascular disease. Future Cardiol. 2011;7(5):657–71.
Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2002;39(3):319–27.
Qi L, Lv T, Cheng Y, Yu M, Han H, Kong H, et al. Fasudil dichloroacetate (FDCA), an orally available agent with potent therapeutic efficiency on monocrotaline-induced pulmonary arterial hypertension rats. Bioorg Med Chem Lett. 2019;29(14):1812–8.
Joshi SR, Liu J, Pearsall RS, Li G, Kumar R. ACTRIIA-Fc (Sotatercept) reverses pulmonary vascular remodeling to attenuate pulmonary arterial hypertension (PAH) by rebalancing TGF-b/BMP signaling in a preclinical model. American Thoracic Society International Conference Abstracts. C26. Let it bleed: endothelial injury and angiogenesis in pulmonary hypertension [poster]. Am J Respir Crit Care Med. 2019;199:A4395.
Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, Palmisciano AJ. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med. 2016;193(10):1168–75.
Kawut SM, Pinder D, Al-Naamani N, McCormick A, Palevsky HI, Fritz J, Smith KA, Mazurek JA, Doyle MF, MacLean MR, DeMichele A. Fulvestrant for the treatment of pulmonary arterial hypertension. Ann Am Thorac Soc. 2019;16(11):1456–9.
Liu L, Pan Y, Song Y, Su X, Ke R, Yang L, Gao L, Li M. Activation of AMPK α2 inhibits airway smooth muscle cells proliferation. Eur J Pharmacol. 2016;791:235–43.
Deng M, Su D, Xu S, Little PJ, Feng X, Tang L, Shen A. Metformin and vascular diseases: a focused review on smooth muscle cell function. Front Pharmacol. 2020;11:635.
Scherger JE. Is Metformin a Wonder Drug?. Int Med Alert. 2021;43(8).
Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321(19):1926–7.
Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, Rainisio M, Simonneau GÉ. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40(4):780–8.
Vachiery JL, Yerly P, Huez S. How to detect disease progression in pulmonary arterial hypertension. Eur Respir Rev. 2012;21(123):40–7.
Demir R, Küçükoğlu MS. Six-minute walk test in pulmonary arterial hypertension. Anatol J Cardiol. 2015;15(3):249.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RM, Brida M, Carlsen J, Coats AJ, Escribano-Subias P, Ferrari P, Ferreira DS. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61(1):2200879.
Farber HW, Miller DP, McGoon MD, Frost AE, Benton WW, Benza RL. Predicting outcomes in pulmonary arterial hypertension based on the 6-minute walk distance. J Heart Lung Transplant. 2015;34(3):362–8.
Savarese G, Paolillo S, Costanzo P, D’Amore C, Cecere M, Losco T, et al. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension? A meta-analysis of 22 randomized trials. J Am Coll Cardiol. 2012;60(13):1192–201.
Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, Meyer FJ, Karger G, Buss J, Juenger J, Holzapfel N. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114(14):1482–9.
Lewis RA, Durrington C, Condliffe R, Kiely DG. BNP/NT-proBNP in pulmonary arterial hypertension: time for point-of-care testing?. Eur Resp Rev. 2020;29(156).
Ghio S, Mercurio V, Fortuni F, Forfia PR, Gall H, Ghofrani A, Mathai SC, Mazurek JA, Mukherjee M, Richter M, Scelsi L. A comprehensive echocardiographic method for risk stratification in pulmonary arterial hypertension. Eur Resp J. 2020;56(3).
Ghio S, Pica S, Klersy C, Guzzafame E, Scelsi L, Raineri C, Turco A, Schirinzi S, Visconti LO. Prognostic value of TAPSE after therapy optimisation in patients with pulmonary arterial hypertension is independent of the haemodynamic effects of therapy. Open heart. 2016;3(1): e000408.
Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J. 2009;39(8):495–501.
Rosenkranz S, Preston IR. Right heart catheterisation: best practice and pitfalls in pulmonary hypertension. Eur Respir Rev. 2015;24(138):642–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Omnia Azmy Nabeh, Alaa I. Saud, Basma Amin, Amira Samy Khedr, Alaa Amr, Aml Medhat Faoosa, Eshraka Esmat, Yasmeen Magdy Mahmoud, Aya Hatem, Mariam Mohamed, Alaa Osama, Youssef Mohamed Amin Soliman, Reem Ibrahim Elkorashy, and Soha Aly Elmorsy declare they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author contributions
All authors contributed to the study conception, design, data collection, analysis and writing, and read and approved the final manuscript.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Consent to participate
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nabeh, O.A., Saud, A.I., Amin, B. et al. A Systematic Review of Novel Therapies of Pulmonary Arterial Hypertension. Am J Cardiovasc Drugs 24, 39–54 (2024). https://doi.org/10.1007/s40256-023-00613-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00613-5