Abstract
Background
We previously conducted a retrospective cohort study using chart review of oral anticoagulant-naïve Japanese patients with nonvalvular atrial fibrillation (NVAF) that assessed the risk of major bleeding and stroke/systemic embolism (SE) events of apixaban versus warfarin.
Methods
In this subgroup analysis, we compared the risk of major bleeding and stroke/SE events by stratifying patients into four subgroups matched 1:1 using propensity score matching (PSM) according to baseline creatinine clearance (CrCl; mL/min): ≥ 15 to < 30, ≥ 30 to < 50, ≥ 50 to < 80, and ≥ 80.
Results
Of the 7074 patients in the apixaban group and 4998 in the warfarin group eligible for inclusion in the analysis, 4385 were included in each group after PSM. Incidence rates of major bleeding and stroke/SE events were generally lower with apixaban versus warfarin across the CrCl subgroups. When all patients with a CrCl change of < 0 mL/min per year during the study period (apixaban, n = 3871; warfarin, n = 2635) were stratified into four subgroups based on the magnitude of CrCl decline (median CrCl change [mL/min] per year: − 1.09, − 3.48, − 7.54, and − 36.92 for apixaban, and − 1.10, − 3.65, − 7.85, and − 40.40 for warfarin), the incidence rates of major bleeding and stroke/SE events generally increased with an increasing CrCl decline per year in both groups.
Conclusions
In Japanese patients with NVAF, the safety and effectiveness of apixaban and warfarin were consistent across different renal subgroups, including those with severe renal impairment. Our results highlight the importance of monitoring renal function variations over time in patients with NVAF.
ClinicalTrials.gov identifier
NCT03765242.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This was a subgroup analysis of a retrospective cohort study that compared the risk of major bleeding and stroke/systemic embolism (SE) events between apixaban and warfarin in Japanese patients with nonvalvular atrial fibrillation (NVAF) who were naïve to oral anticoagulants based on their baseline creatinine clearance (CrCl) (≥ 15 to < 30, ≥ 30 to < 50, ≥ 50 to < 80, and ≥ 80 mL/min). In addition, the association between renal function decline during the study period and clinical outcomes of apixaban versus warfarin was investigated. |
After propensity score matching, patients treated with apixaban had generally lower incidence rates of major bleeding and stroke/SE events than those treated with warfarin across the CrCl subgroups. The incidence rates of major bleeding and stroke/SE events generally increased with an increasing annual decline in CrCl in both treatment groups. |
This real-world study revealed that the safety and effectiveness of apixaban and warfarin in Japanese patients with NVAF were consistent across different renal function subgroups, including those with severe renal impairment, and emphasized the importance of monitoring variations in renal function over time in patients with NVAF. |
1 Introduction
Atrial fibrillation (AF) has become a global public health challenge in aging societies and affects approximately 0.5% of the population worldwide [1]. Considering the aging population and the increase in common risk factors for AF and chronic kidney disease (CKD), such as hypertension and diabetes mellitus, the prevalence of CKD and AF is likely to increase in the future [2]. In the population-based REasons for Geographic and Racial Differences in Stroke (REGARDS) study, AF was associated with an increased risk of end-stage renal disease in the general population; however, this association was potentially explained by underlying CKD [3]. Moreover, AF and CKD have a bidirectional relationship, where the presence of CKD increases the risk of incident AF; the presence of AF is associated with the development and progression of CKD [4]. The risk of stroke, systemic embolism (SE), bleeding, and mortality is significantly increased in patients with co-existent AF and CKD compared with that in patients with either condition alone [5,6,7,8,9,10,11]. Therefore, managing patients with these co-existing conditions poses a challenging scenario for clinicians, especially in relation to the use of oral anticoagulants.
For the prevention of stroke/SE in patients with nonvalvular AF (NVAF), warfarin and direct oral anticoagulants (DOACs; apixaban, dabigatran, rivaroxaban, and edoxaban) are approved in Japan [12]. A systematic review and meta-analysis of studies involving patients with CKD treated with oral anticoagulants concluded that DOACs offer a more favorable safety and efficacy profile than warfarin for various cardiovascular outcomes such as intracerebral hemorrhage, combined ischemic and hemorrhagic stroke, stroke/SE, mortality, and major bleeding events [13].
Results of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial and its secondary analyses showed that the superiority of apixaban over warfarin in preventing stroke/SE is consistent across renal function subgroups [14, 15]. However, in the ARISTOTLE trial, the proportion of patients with moderate renal impairment (creatinine clearance [CrCl] > 30–50 mL/min) was approximately 15% in both the apixaban and warfarin groups, whereas that of patients with severe renal impairment (CrCl ≤ 30 mL/min) was 1.5% in both groups, suggesting limited efficacy and safety data for patients with moderate-to-severe renal impairment [14]. Several real-world studies have compared the safety and effectiveness of apixaban with warfarin in patients with AF and renal impairment and concluded that apixaban demonstrates similar effectiveness to warfarin in patients with CKD [16,17,18,19,20,21]. However, evidence in Japanese adults with AF is insufficient, especially in those with CrCl < 30 mL/min [22].
We previously conducted a retrospective cohort study using chart review of patients with NVAF that assessed the risk of bleeding and stroke/SE events of apixaban versus warfarin in oral anticoagulant-naïve Japanese patients with NVAF, of whom approximately 30% had CrCl < 50 mL/min (ClinicalTrials.gov identifier: NCT03765242) [22]. In patients with CrCl ≥ 50 mL/min, the risk of major bleeding and stroke/SE was not significantly different between groups. In patients with a baseline CrCl of < 50 mL/min, the risk of major bleeding was lower in the apixaban group versus the warfarin group, as was that of stroke/SE [22]. Therefore, in this subgroup analysis of the study, we compared the risk of major bleeding and stroke/SE events with apixaban versus warfarin by stratifying patients according to baseline renal function, including CrCl < 30 mL/min. Renal function often deteriorates over time in patients with AF [15, 23,24,25] and may increase the risk of stroke/SE and major bleeding [15]. We therefore aimed to investigate the association between the degree of decline in renal function during the study period and the clinical outcomes of apixaban versus warfarin.
2 Methods
2.1 Study Design and Eligibility Criteria
This study is the second analysis of a retrospective cohort study using a chart review [22]. Briefly, the study included Japanese patients with NVAF who were aged ≥ 20 years and were newly initiated on apixaban or warfarin (index date: 1 January 2010 to 31 December 2017). The incidence of major bleeding and stroke/SE events in the apixaban and warfarin groups was compared according to baseline CrCl (mL/min) ≥ 15 to < 30, ≥ 30 to < 50, ≥ 50 to < 80, and ≥ 80. Patients with CrCl decline (< 0 mL/min per year) during the study period were divided into quartiles, and the incidence rates of major bleeding and stroke/SE events within the apixaban and warfarin groups were compared.
2.2 Outcomes and Definitions
The primary outcome was major bleeding events defined based on the criteria established by the International Society on Thrombosis and Haemostasis [26]. In addition, stroke/SE events were evaluated as a composite secondary outcome, with stroke defined as ischemic, hemorrhagic, or unknown [22].
The key framework of this study was a comparison between the apixaban and warfarin groups, which were matched using propensity scores to balance the baseline characteristics. Patients were stratified into the following subgroups based on baseline CrCl (calculated using the Cockcroft–Gault formula: ≥ 15 to < 30, ≥ 30 to < 50, ≥ 50 to < 80, and ≥ 80 mL/min), and propensity score matching (PSM) was applied. The variables used for the calculation of propensity score and standardized mean difference (SMD) are shown in Online Resource 1. The incidence rates of major bleeding and stroke/SE events were compared between treatment groups for each CrCl subgroup. The change in CrCl per year was calculated for each patient in the full analysis set (FAS) for all eligible patients in the analysis. Patients who showed a CrCl decline (CrCl change < 0 mL/min per year) during the study period were stratified into four subgroups (quartiles) based on the magnitude of CrCl decline to assess the proportion of event-free patients (probability of having no event occurrences by month 36) and risks for major bleeding and stroke/SE events.
2.3 Statistical Analyses
Patients in each group were matched 1:1 based on propensity scores (caliper value = 0.15) calculated using a logistic regression model (PSM cohort). As reported previously, the covariate balance between the apixaban and warfarin groups in the PSM cohort was assessed using an SMD with a threshold of 0.1 [22]. For each CrCl subgroup in the PSM cohort, the incidence rates of major bleeding and stroke/SE events were estimated using the person-years method. Incidence rates were compared between the apixaban and warfarin groups using the Cox proportional hazards regression model by calculating the hazard ratio (HR) with 95% confidence interval (CI). The p value for the interaction between groups and by baseline CrCl (≥ 15 to < 30, ≥ 30 to < 50, ≥ 50 to < 80, or ≥ 80 mL/min) was calculated using the Cox proportional hazards model in the total PSM cohort.
The change in CrCl per year (mL/min) was calculated for each patient in the FAS by dividing the change in CrCl (mL/min) throughout the study period by the observation period (years), i.e., (last observation date − baseline date)/365.25. Patients with CrCl decline (CrCl change < 0 mL/min per year) during the study period were stratified into subgroups according to the magnitude of CrCl decline (CrCl-decline quartiles 1, 2, 3, and 4; quartiles 1 and 4 show the smallest and largest renal function decline, respectively), and the data were summarized for each quartile using descriptive statistics. The incidence rates of major bleeding and stroke/SE events were calculated for each quartile and compared between the apixaban and warfarin groups using the same Cox proportional hazards regression model (not compared directly). The incidence rates of the first major bleeding and stroke/SE events were estimated using the person-years method, and HRs with 95% CIs were calculated to compare the risk of the first event occurrence in quartiles 2, 3, and 4 versus quartile 1. Kaplan–Meier survival curves were generated for the time to the first event of major bleeding and stroke/SE in each CrCl-decline quartile; as these were exploratory analyses, the p values (log-rank test) were not reported. An additional Cox proportional hazards regression model was constructed to assess the impact of CrCl decline on the risks of major bleeding and stroke/SE events by including the CrCl quartile (1, 2, 3, and 4) as a continuous variable in the model.
Baseline variables were summarized for each CrCl subgroup and each CrCl-decline quartile. Continuous variables are expressed as mean ± standard deviation or median (interquartile range) and were compared between the groups in the FAS using Student’s t test or Wilcoxon rank-sum test (for CHADS2, CHA2DS2-VASc, and HAS-BLED scores). Categorical variables are expressed as the number and percentage of patients and were compared between the groups in the FAS using the Chi-square test.
As a post hoc analysis, the impact of the magnitude of renal function decline during the study period on the incidence rates of major bleeding and stroke/SE in the FAS was reported as HR and 95% CI.
All statistical tests were performed with a two-sided significance level of 0.05, and no imputation was performed for missing data. All analyses were performed using Statistical Analysis System (SAS) software version 9.2 (SAS Institute, Cary, NC, USA).
3 Results
3.1 Patient Disposition
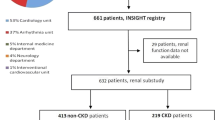
Of the 12,072 Japanese patients with NVAF who were naïve to oral anticoagulants at the index date (FAS), 7074 were prescribed apixaban and 4998 were prescribed warfarin. After PSM, 8770 patients (4385 each in the apixaban and warfarin groups) were included based on their baseline CrCl values (mL/min) (≥ 15 to < 30: 313 patients; ≥ 30 to < 50: 1049 patients; ≥ 50 to < 80: 1816 patients; and ≥ 80: 1207 patients). In the FAS, a total of 6506 patients (3871 in the apixaban group and 2635 in the warfarin group) experienced a decline in CrCl (CrCl change < 0 mL/min per year) during the study period. In the apixaban group, 968 patients were included in quartiles 1, 2, and 3 each, and 967 patients were included in quartile 4. In the warfarin group, 659 patients were included in quartiles 1, 2, and 3 each, and 658 patients were included in quartile 4 (Fig. 1).
Patient disposition. aA total of 2689 patients were excluded after PSM and analyzed for the incidence of major bleeding and stroke/SE events. bA total of 613 patients were excluded after PSM and analyzed for the incidence of major bleeding and stroke/SE events. The PSM cohort was used for comparing the incidence rates of major bleeding and stroke/SE events between the apixaban and warfarin groups. The quartiles were used for comparing the incidence rates of major bleeding and stroke/SE events within the apixaban and warfarin groups among patients with CrCl decline (<0 mL/min per year) during the study period. CrCl creatinine clearance, FAS full analysis set, PSM propensity score matching, SE systemic embolism
3.2 Demographics and Baseline Clinical Characteristics
The demographics and baseline clinical characteristics of the overall cohort have been previously published [22]. Overall, there were 4113 males and 2961 females in the apixaban group, and 3062 males and 1936 females in the warfarin group. For most baseline variables, including the proportion of women or elderly patients, body mass index (BMI), CrCl, and risk scores (CHADS2, CHA2DS2-VASc, and HAS-BLED), the SMDs in patient characteristics between the apixaban and warfarin groups were within the threshold of 0.1 (Table 1). Most patients with CrCl ≥ 15 to < 30 mL/min (89.4%) or ≥ 30 to < 50 mL/min (77.6%) were prescribed apixaban at a reduced dose (2.5 mg twice daily), whereas 83.2% of patients with CrCl ≥ 80 mL/min were prescribed apixaban at a regular dose (5 mg twice daily) (Online Resource 2).
In the PSM cohort, patient age and risk scores tended to be higher, whereas BMI tended to be lower in patients with a lower baseline CrCl value. The proportions of women, patients with comorbidities, and patients using concomitant medications were generally higher in patients with a lower baseline CrCl value. The proportion of patients with a history of stroke/transient ischemic attack (TIA) was higher in those with a lower baseline CrCl value (≥ 15 to < 30 mL/min: 20.4% for apixaban and 18.5% for warfarin; ≥ 80 mL/min: 6.3% for apixaban and 6.6% for warfarin), whereas that of patients with a history of bleeding was similar across the four CrCl subgroups (Table 1).
3.3 Clinical Outcomes of Apixaban Versus Warfarin Stratified by Baseline CrCl
The incidence rate of major bleeding was higher with a lower baseline CrCl in both treatment groups. The incidence rate of major bleeding (per 100 person-years) in the CrCl ≥ 15 to < 30 mL/min subgroup was lower with apixaban than with warfarin (3.75 vs. 7.34; HR 0.48, 95% CI 0.26–0.87; p = 0.02) (Table 2).
The incidence rate of stroke/SE was generally higher with a lower baseline CrCl in both treatment groups, and was the highest in patients with CrCl ≥ 30 to < 50 mL/min in the warfarin group. The incidence rate of stroke/SE (per 100 person-years) in the CrCl ≥ 30 to < 50 mL/min subgroup was lower with apixaban than with warfarin (1.54 vs. 2.69; HR 0.58, 95% CI 0.36–0.92; p = 0.02) (Table 2).
The p value for interaction was not statistically significant between the CrCl subgroups (p = 0.55 for major bleeding and p = 0.69 for stroke/SE), suggesting a consistent effect of apixaban in preventing major bleeding and stroke/SE over warfarin across CrCl subgroups (Fig. 2).
3.4 Clinical Outcomes of Apixaban Versus Warfarin in Patients with CrCl Decline During the Study Period
The median CrCl change per year for patients assigned to quartiles 1, 2, 3, and 4 was − 1.09, − 3.48, − 7.54, and − 36.92 mL/min, respectively, in the apixaban group, and − 1.10, − 3.65, − 7.85, and − 40.40 mL/min, respectively, in the warfarin group (Table 3). Most of the patient characteristics were comparable among patients in different quartiles. The mean systolic and diastolic blood pressures were higher in the apixaban group than in the warfarin group in quartile 4, and the proportion of patients with paroxysmal AF was higher in the apixaban group than in the warfarin group in quartiles 1–3. Almost half of the patients in each quartile were prescribed apixaban 2.5 mg twice daily (Online Resource 3).
The incidence rate of major bleeding (per 100 person-years) was generally higher with a greater decline in CrCl per year in both the apixaban (1.97 for quartile 1 vs. 7.06 for quartile 4; HR 4.46, 95% CI 2.40–8.29) and warfarin (3.57 for quartile 1 vs. 9.66 for quartile 4; HR 3.42, 95% CI 1.88–6.21) groups (Table 4). Similarly, the incidence rate of stroke/SE (per 100 person-years) was generally higher with a greater decline in CrCl per year in both the apixaban (2.23 for quartile 1 vs. 3.20 for quartile 4; HR 2.31, 95% CI 1.19–4.46) and warfarin (2.44 for quartile 1 vs. 7.51 for quartile 4; HR 4.05, 95% CI 2.09–7.83) groups (Table 4).
The probability of having no occurrences of major bleeding by 36 months was substantially lower in quartile 4 than in quartiles 1–3 in both the apixaban (Fig. 3a) and warfarin (Fig. 3b) groups. The probability of having no occurrences of stroke/SE by month 36 was lower in quartiles 3 and 4 than in quartile 1 in the apixaban group (Fig. 3c) and lower in quartile 4 than in quartiles 1–3 in the warfarin group (Fig. 3d). The risks of major bleeding and stroke/SE events increased with deterioration of renal function in the treatment groups (Online Resource 4).
Event-free rate (probability of having no event occurrences by month 36) in patients with renal function decline (CrCl change < 0 mL/min per year) during the study period for major bleeding in the a apixaban and b warfarin groups, and for stroke/SE in the c apixaban and d warfarin groups (FAS). CrCl creatinine clearance, FAS full analysis set, SE systemic embolism
4 Discussion
The current study is a subgroup analysis of the data derived from a retrospective cohort study involving Japanese patients with NVAF who were naïve to oral anticoagulants (apixaban and warfarin) [22]. By collecting clinical data essential for oral anticoagulant therapy (e.g., body weight and renal function) using a medical cohort approach, the current analysis enabled patient stratification by CrCl at baseline or its decline over time. The findings of this study revealed lower incidence rates of major bleeding and stroke/SE in the apixaban group than in the warfarin group, regardless of baseline kidney function, including in patients with advanced CKD (baseline CrCl ≥ 15 to < 30 mL/min). A majority of patients in both treatment groups experienced some degree of decline in CrCl per year. Stratification of patients into quartiles based on the magnitude of CrCl decline revealed that the incidence rates of major bleeding and stroke/SE events generally increased with an increasing decline in CrCl per year in both treatment groups. The findings of this real-world study highlight the effectiveness of apixaban in reducing the risk of stroke/SE and major bleeding in patients with CrCl < 30 mL/min, a population for whom limited data are available [16,17,18,19,20,21]. The decrease in CrCl observed in this study, along with the increased risk of stroke/SE and major bleeding with reduced CrCl, suggests the need to routinely monitor kidney function in patients with NVAF treated with apixaban or warfarin.
When oral anticoagulation is to be started in a patient with AF who is eligible for DOACs, the 2020 Japanese Circulation Society/Japanese Heart Rhythm Society guidelines recommend a DOAC in preference to warfarin (class I recommendation: evidence and/or general agreement that a given procedure or treatment is useful and effective) [12]. In a cohort study that used a national database representing the Japanese population, the annual number of new warfarin users declined 74% from fiscal year (FY) 2011 to FY 2015, whereas users of any DOAC increased by 6.8-fold (FY 2011: 26.7%; and FY 2015: 83.4%) [27]. The 2020 European Society of Cardiology guidelines recommend DOACs as the first choice for patients with CrCl ≥ 30 mL/min [28]. In Japanese patients with NVAF, DOACs are as effective and safe as warfarin [7, 8, 29, 30]. For patients with CrCl < 15 mL/min, the only anticoagulant of choice is warfarin; however, thorough consideration of the necessity of anticoagulant use is required.
In the current subgroup analysis, the incidence rates of major bleeding and stroke/SE were lower with apixaban than with warfarin, after matching patients’ baseline characteristics between the groups, and the results were consistent across CrCl subgroups, including patients with CrCl ≥ 15 to < 30 mL/min, which is consistent with previous findings derived from real-world settings [16,17,18,19,20,21]. Our data support that the safety and effectiveness of apixaban and warfarin in Japanese patients with NVAF were consistent across different renal function subgroups, including those with CrCl ≥ 15 to < 30 mL/min. Patients with AF often experience a decline in renal function over time [15, 23, 25], which may increase the risk of stroke/SE and major bleeding events. Depending on the equation used to estimate kidney function, there may be significant discordance in eligibility for and the dose adjustment of DOACs [31]. In this study, the Cockcroft–Gault equation was employed to calculate the CrCl for DOAC dosing, which was also employed in the ARISTOTLE trial [14]. A total of 13.6% of patients enrolled in the ARISTOTLE trial experienced renal function decline (> 20% annual decrease in estimated glomerular filtration rate [eGFR]) during a median follow-up of 1.8 years and had a higher risk of cardiovascular and bleeding events than those without renal function decline [15]. Patients registered in a substudy of the Japanese SAKURA AF Registry also showed a mean annual eGFR decrease of 1.07 mL/min/1.73 m2 during a median follow-up of 39.3 months [32]. The change in CrCl per year observed in this study was higher in the warfarin group than in the apixaban group across most quartiles, which may be attributed to the lower risk of CKD progression in patients treated with DOACs [33]. Furthermore, major bleeding and stroke/SE events occurred most frequently in quartile 4 in both groups, which was the patient population showing the highest decline in renal function (median CrCl change of approximately − 37 mL/min per year with apixaban and − 40 mL/min per year with warfarin). Our findings underscore the need for careful monitoring of renal function decline in patients with AF and renal impairment treated with anticoagulants in clinical practice. The median CrCl change in quartile 4 observed in the current analysis may be a useful indicator of an increased risk of major bleeding or stroke/SE, to consider additional preventive interventions.
All DOACs undergo some degree of renal elimination (ranging from 25% for apixaban to 80% for dabigatran), which can have significant implications for their overall effectiveness and safety [34]. In an administrative claims study conducted in the United States, compared with warfarin, apixaban and rivaroxaban were associated with a significantly lower risk of stroke, major bleeding, and mortality, whereas dabigatran was associated with a similar risk of stroke but a lower risk of major bleeding and mortality [35]. Real-world studies on Japanese patients with NVAF and CKD comparing the effects of different DOACs on major bleeding and stroke/SE events are warranted.
One of the strengths of the current study is its retrospective cohort design, which enabled the collection of renal function data from a substantial number of patients with NVAF. Second, the large sample sizes allowed for the correction of variability in values and provided reliable mean values. Moreover, the current study included patients treated at general practitioner clinics, which reflects Japanese real-world practice regarding the administration of anticoagulant therapy. This study verified the effectiveness and safety of apixaban using real-world data from patients with poor renal function, and the results were highly reliable because the data were collected from physicians. The current data also confirmed the result of previous randomized controlled trials. However, this study has some limitations. As described previously, some variables (e.g., left atrial dimension, blood pressure, smoking history, and alcohol abuse) were not considered for PSM, and patients’ loss to follow-up due to clinic/hospital transfer may have resulted in an underestimation of the incidence of major bleeding or stroke/SE events [22]. There is also the possibility of drug selection bias because not all DOACs are used in patients with renal impairment. Moreover, CrCl was measured at each site and not centrally, which may have resulted in some errors. However, this study has a large sample size and therefore the mean value is reliable. Thus, the generalizability of our results to the entire population of Japanese patients with severe renal impairment may be limited.
5 Conclusion
The current subgroup analysis of a retrospective cohort study showed that the safety and effectiveness of apixaban versus warfarin in Japanese patients with NVAF who were naïve to oral anticoagulants were consistent across different renal function subgroups, and including those with severe renal impairment (CrCl ≥ 15 to < 30 mL/min). Our results also highlight the importance of monitoring the variations in renal function over time in patients with NVAF.
References
Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16:217–21. https://doi.org/10.1177/1747493019897870.
Rehm M, Rothenbacher D, Iacoviello L, Costanzo S, Tunstall-Pedoe H, Fitton CA, et al.; BiomarCaRE Consortium. Chronic kidney disease and risk of atrial fibrillation and heart failure in general population-based cohorts: the BiomarCaRE project. ESC Heart Fail. 2022;9:57–65. https://doi.org/10.1002/ehf2.13699
O'Neal WT, Tanner RM, Efird JT, Baber U, Alonso A, Howard VJ, Howard G, Muntner P, Soliman EZ. Atrial fibrillation and incident end-stage renal disease: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Int J Cardiol. 2015;185:219–23. https://www.internationaljournalofcardiology.com/article/S0167-5273(15)00343-5/fulltext
Ding WY, Gupta D, Wong CF, Lip GYH. Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc Res. 2021;117:1046–59. https://doi.org/10.1093/cvr/cvaa258.
Ocak G, Khairoun M, Khairoun O, Bos WJW, Fu EL, Cramer MJ, et al. Chronic kidney disease and atrial fibrillation: a dangerous combination. PLoS ONE. 2022;17:e0266046. https://doi.org/10.1371/journal.pone.0266046.
Inoue H, Kodani E, Atarashi H, Okumura K, Yamashita T, Origasa H; J-RHYTHM Registry Investigators. Renal dysfunction affects anticoagulation control with warfarin and outcomes in Japanese elderly patients with non-valvular atrial fibrillation. Circ J. 2018;82:2277–83. https://doi.org/10.1253/circj.CJ-18-0242
Yuzawa Y, Kuronuma K, Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, et al. Relationship between the renal function and adverse clinical events in patients with atrial fibrillation: a Japanese multicenter registry substudy. J Clin Med. 2020;9:167. https://doi.org/10.3390/jcm9010167.
Akao M, Ogawa H, Masunaga N, Minami K, Ishigami K, Ikeda S, et al. 10-Year trends of antithrombotic therapy status and outcomes in Japanese atrial fibrillation patients—the Fushimi AF Registry. Circ J. 2022;86:726–36. https://doi.org/10.1253/circj.CJ-22-0023.
Sato T, Aizawa Y, Kitazawa H, Okabe M. The characteristics and clinical outcomes of direct oral anticoagulants in patients with atrial fibrillation and chronic kidney disease: from the database of a single-center registry. J Atr Fibrill. 2020;13:2308. https://doi.org/10.4022/jafib.2308.
Kim ED, Soliman EZ, Coresh J, Matsushita K, Chen LY. Two-week burden of arrhythmias across CKD severity in a large community-based cohort: the ARIC study. J Am Soc Nephrol. 2021;32:629–38. https://journals.lww.com/jasn/fulltext/2021/03000/two_week_burden_of_arrhythmias_across_ckd_severity.14.aspx
Carrero JJ, Trevisan M, Sood MM, Bárány P, Xu H, Evans M, et al. Incident atrial fibrillation and the risk of stroke in adults with chronic kidney disease: The Stockholm CREAtinine Measurements (SCREAM) Project. Clin J Am Soc Nephrol. 2018;13:1314–20. https://doi.org/10.2215/CJN.04060318.
Ono K, Iwasaki YK, Akao M, Ikeda T, Ishii K, Inden Y, et al. JCS/JHRS 2020 guideline on pharmacotherapy of cardiac arrhythmias. Circ J. 2022;86:1790–924. https://doi.org/10.1253/circj.CJ-20-1212.
Malhotra K, Ishfaq MF, Goyal N, Katsanos AH, Parissis J, Alexandrov AW, et al. Oral anticoagulation in patients with chronic kidney disease: a systematic review and meta-analysis. Neurology. 2019;92:e2421–31. https://doi.org/10.1212/WNL.0000000000007534.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. https://doi.org/10.1056/NEJMoa1107039.
Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1:451–60. https://doi.org/10.1001/jamacardio.2016.1170.
Akao M, Yamashita T, Suzuki S, Okumura K; J-ELD AF investigators. Impact of creatinine clearance on clinical outcomes in elderly atrial fibrillation patients receiving apixaban: J-ELD AF Registry subanalysis. Am Heart J. 2020;223:23–33. https://doi.org/10.1016/j.ahj.2020.02.007
Stanton BE, Barasch NS, Tellor KB. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment. Pharmacotherapy. 2017;37:412–9. https://doi.org/10.1002/phar.1905.
Herndon K, Guidry TJ, Wassell K, Elliott W. Characterizing the safety profile of apixaban versus warfarin in moderate to severe chronic kidney disease at a veterans affairs hospital. Ann Pharmacother. 2020;54:554–60. https://doi.org/10.1177/1060028019897053.
Hanni C, Petrovitch E, Ali M, Gibson W, Giuliano C, Holzhausen J, et al. Outcomes associated with apixaban vs warfarin in patients with renal dysfunction. Blood Adv. 2020;4:2366–71. https://doi.org/10.1182/bloodadvances.2019000972.
Fu CM, Li LC, Lee YT, Wang SW, Hsu CN. Apixaban vs. warfarin in atrial fibrillation patients with chronic kidney disease. Front Cardiovasc Med. 2021;8:752468. https://doi.org/10.3389/fcvm.2021.752468
Heleniak Z, Papuga-Szela E, Krzysztof P, Anetta U. Efficacy and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and chronic kidney disease stage G4: a single-center experience. J Cardiovasc Pharmacol. 2020;76:671–7. https://doi.org/10.1097/FJC.0000000000000911.
Koretsune Y, Hoshino H, Matsuo Y, Ibuki T, Morimoto T. Comparative safety and effectiveness of apixaban vs. warfarin in oral anticoagulant-naïve Japanese patients with non-valvular atrial fibrillation—a retrospective chart review study. Circ J. 2022;86:213–21. https://doi.org/10.1253/circj.CJ-21-0682
Becattini C, Giustozzi M, Ranalli MG, Bogliari G, Cianella F, Verso M, et al. Variation of renal function over time is associated with major bleeding in patients treated with direct oral anticoagulants for atrial fibrillation. J Thromb Haemost. 2018;16:833–41. https://doi.org/10.1111/jth.13985.
Yao X, Tangri N, Gersh BJ, Sangaralingham LR, Shah ND, Nath KA, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:2621–32. https://doi.org/10.1016/j.jacc.2017.09.1087.
Miyamoto K, Aiba T, Arihiro S, Watanabe M, Kokubo Y, Ishibashi K, et al. Impact of renal function deterioration on adverse events during anticoagulation therapy using non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Heart Vessels. 2016;31:1327–36. https://doi.org/10.1007/s00380-015-0725-6.
Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. https://doi.org/10.1111/j.1538-7836.2005.01204.x
Kubota K, Ooba N. Effectiveness and safety of reduced and standard daily doses of direct oral anticoagulants in patients with nonvalvular atrial fibrillation: a cohort study using national database representing the Japanese population. Clin Epidemiol. 2022;14:623‒39. https://www.dovepress.com/effectiveness-and-safety-of-reduced-and-standard-daily-doses-of-direct-peer-reviewed-fulltext-article-CLEP
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al.; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373‒498. https://academic.oup.com/eurheartj/article/42/5/373/5899003?login=false
Kohsaka S, Katada J, Saito K, Jenkins A, Li B, Mardekian J, et al. Safety and effectiveness of non-vitamin K oral anticoagulants versus warfarin in real-world patients with non-valvular atrial fibrillation: a retrospective analysis of contemporary Japanese administrative claims data. Open Heart. 2020;7:e001232. https://openheart.bmj.com/content/7/1/e001232.long
Kohsaka S, Katada J, Saito K, Terayama Y. Safety and effectiveness of apixaban in comparison to warfarin in patients with nonvalvular atrial fibrillation: a propensity-matched analysis from Japanese administrative claims data. Curr Med Res Opin. 2018;34:1627–34. https://doi.org/10.1080/03007995.2018.1478282?journalCode=icmo20.
Andrade JG, Hawkins NM, Fordyce CB, Deyell MW, Er L, Djurdjev O, et al. Variability in non-vitamin K antagonist oral anticoagulants dose adjustment in atrial fibrillation patients with renal dysfunction: the influence of renal function estimation formulae. Can J Cardiol. 2018;34:1010–8. https://doi.org/10.1016/j.cjca.2018.04.019.
Kuronuma K, Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Oiwa K, et al. Worsening renal function, adverse clinical events and major determinants for changes of renal function in patients with atrial fibrillation: a Japanese multicenter registry substudy. Curr Med Res Opin. 2019;35:2007–13. https://doi.org/10.1080/03007995.2019.1631597.
Trevisan M, Hjemdahl P, Clase CM, de Jong Y, Evans M, Bellocco R, et al. Cardiorenal outcomes among patients with atrial fibrillation treated with oral anticoagulants. Am J Kidney Dis. 2023;81:307-317.e1. https://doi.org/10.1053/j.ajkd.2022.07.017.
Weber J, Olyaei A, Shatzel J. The efficacy and safety of direct oral anticoagulants in patients with chronic renal insufficiency: a review of the literature. Eur J Haematol. 2019;102:312–8. https://doi.org/10.1111/ejh.13208.
Yao X, Inselman JW, Ross JS, Izem R, Graham DJ, Martin DB, et al. Comparative effectiveness and safety of oral anticoagulants across kidney function in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2020;13:e006515. https://doi.org/10.1161/CIRCOUTCOMES.120.006515.
Acknowledgements
The authors thank all the medical institutions and physicians who participated in this retrospective cohort study for their cooperation. Medical writing and editorial assistance were provided by Sarayu Pai, PhD, CMPP, and Mami Hirano, MS, of Cactus Life Sciences (part of Cactus Communications) and funded by Bristol Myers Squibb K.K. and Pfizer Japan Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded and conducted by Bristol Myers Squibb K.K. and Pfizer Japan Inc.
Conflicts of interest
Takeshi Morimoto reports lecture fees from AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Japan Lifeline, Kowa, Toray, and Tsumura; manuscript fees from Bristol Myers Squibb and Kowa; and is a member of the advisory board for Novartis and Teijin. Haruhiko Hoshino reports remuneration (lecture fees) from Bristol Myers Squibb, Daiichi Sankyo, Pfizer, and Bayer, outside the submitted work. Yukako Matsuo and Kayoko Miyata are employees of Bristol Myers Squibb K.K. Tatsuki Ibuki was an employee of Pfizer Japan Inc. during the conduct of this study and a former employee of Otsuka Pharmaceutical. Yukihiro Koretsune reports remuneration (lecture fees) from Daiichi Sankyo outside of the submitted work.
Availability of data and material
All data generated or analyzed during this study are included in this published article (and its supplementary information files). BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.
Ethics approval
This study was reviewed and approved by the Ethics Committee or Independent Review Committee of each participating site (representative facility: Saga Memorial Hospital Ethics Committee, reference number 16000061).
Consent to participate
Obtaining informed consent from patients was not applicable to this study, and an opt-out approach was adopted to provide patients an opportunity to request exclusion from the study.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed to the study conception and design and data interpretation. Material preparation, data collection, and analysis were performed by Yukako Matsuo, Tatsuki Ibuki, and Kayoko Miyata. The first draft of the manuscript was written by Takeshi Morimoto, and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Morimoto, T., Hoshino, H., Matsuo, Y. et al. Safety and Effectiveness of Apixaban Versus Warfarin in Japanese Patients with Nonvalvular Atrial Fibrillation Stratified by Renal Function: A Retrospective Cohort Study. Am J Cardiovasc Drugs 23, 721–733 (2023). https://doi.org/10.1007/s40256-023-00611-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00611-7