Abstract
Introduction
The cognitive safety of monoclonal antibody proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) has been established in clinical trials, but not yet in real-world observational studies. We assessed the cognitive function in patients initiating PCSK9i, and differences in cognitive function domains, to analyze subgroups by the low-density lipoprotein cholesterol (LDL-C) achieved, and differences between alirocumab and evolocumab.
Methods
This has a multicenter, quasi-experimental design carried out in 12 Spanish hospitals from May 2020 to February 2023. Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA).
Results
Among 158 patients followed for a median of 99 weeks, 52% were taking evolocumab and 48% alirocumab; the mean change from baseline in MoCA score at follow-up was + 0.28 [95% CI (− 0.17 to 0.73; p = 0.216)]. There were no significant differences in the secondary endpoints—the visuospatial/executive domain + 0.04 (p = 0.651), naming domain − 0.01 (p = 0.671), attention/memory domain + 0.01 (p = 0.945); language domain − 0.10 (p = 0.145), abstraction domain + 0.03 (p = 0.624), and orientation domain − 0.05 (p = 0.224)—but for delayed recall memory the mean change was statistically significant (improvement) + 0.44 (p = 0.001). Neither were there any differences in the three stratified subgroups according to lowest attained LDL-C level—0–54 mg/dL, 55–69 mg/dL and ≥ 70 mg/dL; p = 0.454—or between alirocumab and evolocumab arms.
Conclusion
We did not find effect of monoclonal antibody PCSK9i on neurocognitive function over 24 months of treatment, either in global MoCA score or different cognitive domains. An improvement in delayed recall memory was shown. The study showed no differences in the cognitive function between the prespecified subgroups, even among patients who achieved very low levels of LDL-C. There were no differences between alirocumab and evolocumab.
Registration
ClinicalTtrials.gov Identifier number NCT04319081.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Monoclonal antibody proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) were not significantly associated with neurocognitive events over 2 years, even in patients with very low serum LDL-C levels. |
There were no significant changes observed in the primary MoCA score or cognitive domains, but significant changes were observed in delayed recall memory. |
No differences in neurocognitive outcomes were found between patients treated with alirocumab or evolocumab. |
This is the first long-term, real world study to assess neurocognitive function in monoclonal antibody PCSK9i recipients. |
1 Introduction
Low-density lipoprotein (LDL) cholesterol (LDL-C) is one of the main risk factors for cardiovascular disease (CVD) [1]. Reduction of LDL-C is highly effective in reducing major cardiovascular events (MACE) [2, 3]. Lipid-lowering drugs are the main tool to prevent atherosclerotic CVD by upregulation of LDL receptors, including statins, ezetimibe, protein convertase subtilisin/kexin type 9 inhibitors (PCSK9i), bempedoic acid, and inclisiran, a small interfering RNA (siRNA) that inhibits the hepatic synthesis of PCSK9 [4,5,6,7,8]. Alirocumab and evolocumab are the first class of PCSK9i that demonstrated in randomized clinical trials the ability to reduce the LDL-C levels by about 60% [9, 10].
The safety of very low LDL-C values, achieved through lipid-lowering therapies, have frequently been questioned due to the possibility of an increased risk of neurocognitive decline. In fact, the US Food and Drug Administration (FDA) instructed the laboratories to assess potential neurocognitive side effects of PCSK9i [11]. Several studies showed that PCSK9i were associated with neurocognitive adverse events: The OSLER1 and OSLER2 studies reported a higher incidence of neurocognitive events in patients treated with evolocumab when compared with standard therapy [9]. In the ODYSSEY LONG TERM study, alirocumab had a higher incidence of neurocognitive events than placebo [12]. Two more meta-analyses described similar results [13, 14]. In contrast, in two multicenter and randomized clinical trials (RCT), FOURIER and ODYSSEY OUTCOMES, there were not significant differences between PCSK9i and placebo [9, 10]. Successively, two meta-analyses were not associated with cognitive impairment [15, 16], and the EBBINGAHUS study (first study assessing cognitive changes as a principal endpoint) did not find an association among adverse cognitive effects and evolocumab [17]; furthermore, alirocumab also did not show effect on neurocognitive function over 96 weeks in the trial from Janik et al. [18]. Recently, in FOURIER-OLE [19], an open-label extension study with evolocumab and with a follow-up of 8.4 years, neurocognitive events with evolocumab in the long term did not exceed those reported for placebo-treated patients.
However, real-world data assessing cognitive function as the primary endpoint are necessary. However, there are currently no published data comparing alirocumab and evolocumab.
The MEMOGAL study is a multicenter, non-randomized, open-label trial aiming to assess cognitive function in patients who initiate treatment with PCSK9 inhibitors in a real-world setting.
2 Methods
2.1 Study Design
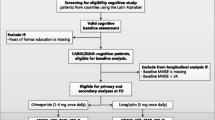
MEMOGAL is a multicenter, prospective study with a quasi-experimental design in patients initiating PCSK9i in 12 Spanish hospitals. The protocol and design were already published [20] and registered in clinicaltrial.gov (NCT05136287). This protocol was approved by the ethics committee and the Agencia Española de Medicamentos y Productos Santitarios. Study design and patient disposition are shown in Fig. 1.
2.2 Population
The inclusion criteria were individuals over 18 years of age with a first prescription of PCSK9 inhibitors evolocumab (140 mg every 2 weeks) or alirocumab (75 mg or 150 mg every 2 weeks), and a diagnosis of established atherosclerotic cardiovascular disease or hypercholesterolemia disease. Established atherosclerotic cardiovascular disease was defined as myocardial infarction, stroke, or peripheral arterial disease and hypercholesterolemia disease as homozygous familial hypercholesterolemia and heterozygous (familial and non-familial) or mixed dyslipidemia. Exclusion criteria were a previous diagnosis of cognitive impairment disease or dementia. All the subjects provided written informed consent, and the study was conducted in accordance with the principles of the Declaration of Helsinki.
2.3 Study Procedures
The study consisted of two study periods (Fig. 1): the inclusion period from May 2020 to May 2022 and the follow-up from May 2020 to February 2023. Montreal Cognitive Assessment (MoCA) tests were assessed at baseline, month 12, month 24, and/or final visit for those patients who were followed more or less than 24 months. The investigators from the different participating centers (Appendix 1) were responsible for administering the MoCA questionnaires to the patients during the various study visits. They received initial training before commencing the study, and they were kept up to date through different meetings throughout the study duration.
2.4 Endpoints
The primary endpoint was cognitive impairment, assessed by the MoCA questionnaire [21]. Briefly, the score ranges from 0 to 30 and normal values ≥ 26 are considered normal. Using this cutoff, the MoCA detects 90% of mild cognitive impairment of subjects [21]. MoCA assesses cognitive impairment across different domains (further details of the MoCA are provided in the Appendix 2). It was performed at baseline visit, at 12 months, at 24 months, and/or at the end of the study. It is important to note that an increase in each domain score over time indicates improvement, while a decrease indicates potential deterioration in cognitive function.
The secondary end points included measures of other components of MoCA test: visuospatial/executive memory, naming, attention, language, abstraction, delayed recall memory, and orientation (Appendix 2).
Another secondary endpoint was changes in the MoCA test by analysis of subgroups. Patients were categorized into three pre-specified subgroups based on their achieved LDL-C values at follow-up—< 55 mg/dL, 55–< 70 mg/dL, and 70 mg/dL or higher—and by the magnitude of LDL reduction. We assessed the differences in the cognitive function between alirocumab and evolocumab.
Patients were included in the trial from May 2020 to May 2022 and 32 months of follow-up. The study was closed in February 2023.
2.5 Statistical Analysis
For the primary and secondary endpoints related to MoCA domains, statistical analysis was performed following the criteria of MoCA validation [21]. Thus, the means along with their 95% confidence intervals (CI) and standard deviations (SD) were calculated at different points during the follow-up. Differences were assessed using the paired student t-test. Additionally, we conducted an analysis of the cognitive test, stratifying by LDL-C levels on one hand, and comparing cognitive function between the two PCSK9 inhibitors on the other. Student t-tests for independent samples were carried out to establish if there were differences in each case. Statistical significance was defined as p < 0.05. All analyses were performed using SPSS 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.).
3 Results
3.1 Patient Disposition
A total of 158 patients were recruited in the study from 25 May 2020 (first patient) to 6 April 2022 (last patient included), and 143 (90.50%) patients completed the final follow-up MoCA test (Fig. 1).
3.2 Baseline Characteristics and Concomitant Medication
The baseline characteristics are shown in Table 1. The mean (SD) age of the patients was 6 years (10 years), 66.5% were male, the mean (SD) body weight was 81 kg (16 kg), and mean (SD) body mass index (BMI) was 29 kg/m2 (5 kg/m2). Overall, regarding concomitant diseases, 85% had cardiovascular ischemic disease (CVD), 25% familiar hypercholesterolemia (FH), 55% hypertension, 22% type 2 diabetes (T2D), and 17% heart failure. Regarding the comorbidities, 20% had family history of dementia, 11% were smokers, and 72% were adherent to diet.
Among 158 patients, 75 patients were taking evolocumab 140 mg every 2 weeks (47.46%), 65 patients were on alirocumab 150 mg every 2 weeks (41.14%), and 18 patients were taking alirocumab 300 mg every 2 weeks (11.40%). Regarding additional lipid-lowering therapy, 33.5% of them were using rosuvastatin, 18.4% atorvastatin, 3.2% pitavastatin, 1.2% other statins, and 58.6% ezetimibe, and 43.7% of the sample were not taking any statin. Of all patients, 43.7% had statins intolerance (Table 1).
3.3 LDL-C Reduction
The median duration of follow-up was 99.57 weeks (74.53–104.29 weeks). During this period, the median LDL-C (SD) level at baseline was 145.18 mg/dL (43.43 mg/dL) and the median LDL-C (SD) at follow-up was 62.11 mg/dL (33.98 mg/dL). The reduction in LDL-C was maintained over time, and the percentage of reduction was 55.6% (95% CI 51.04–60.23; p < 0.001; Table 1).
3.4 Outcomes
3.4.1 Primary Endpoint: Neurocognitive Function
At follow-up, data were missing for 15 patients (9.50%), and the rest of patients presented a MoCA test at baseline and during the follow-up study. The median MoCA score (SD) at baseline was 24.22 (3.98) [95% CI (23.52–24.84)], and the median MoCA score (SD) at follow-up was 24.50 (4.14) [95% CI (23.72–25.09)]. The primary endpoint mean change from baseline in MoCA score at follow-up was + 0.28 [95% CI (− 0.17 to 0.73); p = 0.216]; there was no significant change in the MoCA total score from baseline to follow-up. See Fig. 2.
3.4.2 Secondary Endpoints: Changes in MoCA Cognitive Domains; Subgroups and PCSK9 Analysis
The reported scores in the different cognitive domains of the MoCA test had a similar trend; there were no significant changes. For the visuospatial/executive domain the mean change from baseline to follow-up was + 0.04 [95% CI (− 0.14 to 0.23); p = 0.651]; for the naming domain the mean change from baseline to follow-up was − 0.01 [95% CI (− 0.08 to 0.52); p = 0.671]; for attention/memory domain the mean change from baseline to follow-up was + 0.01 [95% CI (− 0.20 to 0.21); p = 0.945]; for language domain the mean change from baseline to follow-up was − 0.10 (95% CI (− 0.23 to 0.03); p = 0.145]; for abstraction domain the mean change from baseline to follow-up was + 0.03 (95% CI (− 0.08 to 0.14); p = 0.62]; and for orientation domain the mean change from baseline to follow-up was − 0.05 [95% CI (− 0.13 to 0.03); p = 0.224), but for delayed recall memory domain the mean change from baseline to follow-up was statistically significant, indicating a meaningful improvement of + 0.44 [95% CI (0.18–0.70); p = 0.001]. See Fig. 2.
The cognitive test results were also stratified according to lowest attained LDL-C level in the three groups: from 0–54 mg/dL, from 55–69 mg/dL, and ≥ 70 mg/dL. The results are shown in Fig. 3. For the first group (0–54 mg/dL), the mean MoCA score (SD) at follow-up was 24.06 (4.23) [95% CI (22.90–025.21)]; for the second group (55–69 mg/dL), the mean MoCA score (SD) at follow-up was 25.33 (3.70) [95% CI (23.77–26.90)]; and for the third group (≥ 70 mg/dL), the mean MoCA score (SD) at follow-up was 24.18 (4.63) [95% CI (22.54–25.82)]. There were not significant differences in the different cognitive domains of MoCA test scores between the three groups of subanalysis (p = 0.454; Table 2). Additionally, even for patients who achieved a final LDL value ≤ 40 mg/dL (n = 36), the results did not show statistical significance (p = 0.118).
An additional stratified analysis was performed according to magnitude of total LDL-C reduction and MoCA score. We divided the patients into three subgroups: mild reduction (< 30%; n = 15), moderate reduction (30–60%; n = 64), and intense reduction (> 60%; n = 64) [3]. These results did not show significant differences among the different groups (p = 0.095).
Regarding sex, there were no significant differences between sex and the final MoCA score value (p = 0.221). However, there were significant differences between patients aged ≥ 65 years and those younger than 65 years (p < 0.001).
We also compared the differences between changes in cognitive function for the two PCSK9i: For alirocumab the mean MoCA score (SD) at follow-up was 24.82 (3.8) [95% CI (23.94–25.70)], and the mean change from baseline to follow-up was + 0.31 [95% CI (− 0.32 to 0.94)], whereas for evolocumab the mean MoCA score (SD) at follow-up was 23.96 (4.4) [95% CI (22.88–25.03); p = 0.212], and the mean change from baseline to follow-up was + 0.13 [95% CI (− 0.51 to 0.77); p = 0.690]. There were no differences in change in cognitive function between alirocumab and evolocumab. See Table 2 and Fig. 4.
4 Discussion
The analyses on this observational and prospective study involving real-world patients treated with PCSK9 inhibitors demonstrates the absence of effect on cognitive function, measured by MoCA test after 24-month of follow-up. Additionally, we also explored all the different cognitive domains without differences in visuospatial/executive memory, naming, attention, language, and orientation domains; moreover, a significant improvement in delayed recall cognitive domain was observed. Our results showed no statistically significant differences between the three prespecified subgroups stratified according to the LDL-C target reached at follow-up for ≥ 70 mg/dL or 55–69 mg/dL, or even among the patients who achieved LDL-C concentrations ≤ 55 mg/dL and by the magnitude of LDL reduction.
Among the 158 patients included in this study, the groups taking alirocumab and evolocumab were balanced (48% and 52%), and 143 patients filled out the MoCA test at follow-up (90%; Fig. 1). The main reduction of LDL-C was 55.6% (from 145.18 mg/dL to 62.11 mg/dL), very similar results to those reported in the pivotal RCT [9, 10]. Half of the population achieved the target level recommended by current guidelines [3], in line with recent registries performed in our environment [22].
4.1 Outcomes
Regarding the primary endpoint, the cognitive function assessed by the MoCA test score, our results coincide with the observations from the EBBINGHAUS trial [17] and alirocumab neurocognitive trial [18], which revealed no significant differences between the cognitive function from baseline visit to follow-up. In relation to secondary endpoints, we also did not find differences in the different domains compared with the main RCT, nor in the subgroup analysis [17, 18, 31], with the exception of the delayed recall cognitive domain.
4.1.1 Background of Neurocognitive Function with Lipid-Lowering Therapy
Since the FDA instructed laboratories to study the possibility of neurocognitive impairment with any lipid-lowering therapy [23], numerous studies about cognition with different methodologies have been published. In the main RCT with evolocumab and alirocumab (the EBBINGHAUS trial [17] and the trial from Matthew et al. [18]), the primary endpoint was to assess the cognitive function through the CANTAB questionnaire; the results showed no differences between LDL-C levels and cognitive changes [9, 10]. Conversely, other important RCT with alirocumab showed contradictory results. In the OYSSEY LONG TERM study, alirocumab presented a higher incidence of neurocognitive events compared with placebo [24], but in the ODYSSEY COMBO I [25] no patients in the alirocumab arm reported neurocognitive decline (0.9%). In relation to evolocumab, the OSLER 1 and OSLER 2 trials did not show differences in minimum post-baseline LDL-C levels [9], but in the OSLER-1 Extension study, 0.4% of patients receiving evolocumab (n = 1255) experienced neurocognitive events compared with 0 patients receiving standard therapy [26]. Two meta-analyses were also published, concluding that contemporary lipid-lowering drugs were not associated with cognitive impairment in RCT [15, 16]. However, a meta-analysis by Lipinski et al. reported an increased incidence of neurocognitive adverse events associated with PCSK9 inhibitors compared with placebo [odds ratio (OR) 2.34; p = 0.02] [27]. Regarding real-world studies, a hospital registry from Gürgöze et al. [28] outlined higher cognitive disorders attributed to PCSK9i than in RCT; in contrast, another real-world study showed a favorable safety profile in cognitive impairment [29].
4.1.2 Primary and Secondary Neurocognitive Endpoints
Taking all the results into consideration, there is a paucity of prospective studies evaluating the association of PCSK9 inhibitors with neurocognitive events. To our knowledge, our study is the first prospective real-world study that assess the cognitive function as primary endpoint, measuring the scores through a validated and specific tool (MoCA questionnaire). Cognition is a very wide and complex concept involving mainly four domains: executive function, memory, language, and visuospatial memory. Cognitive dysfunction can be an impairment in any of these domains [30]. The mini-mental status examination (MMSE) was one of the initial tests used to assess cognition; however, it does not evaluate executive function. Therefore, in the current study, the Montreal Cognitive Assessment (MoCA) was utilized. From our point of view, although more time-consuming, including seven different domains could obtain more accurate conclusions. Importantly, the present study is an independent study, representing the first non-commercial project aimed at analyzing the cognitive function test scores, in contrast with the other real-world studies, where the events were self-reported by the patients without an objective assessment of cognitive status. Another strength of the MEMOGAL study is the standardized follow-up, in which subjects were followed for 2 years, with only 10% of the patients dropping out. Our results are in line with the main RCT published [17, 18, 31], where no neurocognitive impairment was observed, not only with the principal variable (MoCA global score) but also in addition to the rest of domains of the assessment. After a 24-month follow-up we did not find differences in the visuospatial/executive, naming, attention, language, abstraction, or orientation domains. However, we did observe a significant improvement in the delayed recall memory domain. To our knowledge, this is the first study to report such results, and a possible mechanism that may explain this improvement is that, in this domain, patients were required to memorize and repeat specific words (“face,” “velvet,” “church,” “daisy,” and “red”) during the questionnaire. The repetition of the same test in successive visits (at 0, 12, and 24 months) could have led the patients to learn or remember the words from one visit to another. Future studies incorporating different words in each test may be necessary to further investigate this phenomenon.
4.1.3 Subgroup Analysis
The cognitive results in prespecified subgroups were consistent with the results in the overall population and in the published RCT [17, 18, 31]. We did not find differences between the three groups for ≥ 70 mg/dL or 55–69 mg/dL or even among the patients who achieved LDL-C concentrations ≤ 55 mg/dL. Furthermore, there were no observed differences among the different subgroups stratified based on the magnitude of LDL reduction. Our results have shown that PCSK9i are safe in those patients achieving current targets from dyslipidemia guidelines [2]. In our study, we opted to categorize the population into three groups, with the lowest group range being ≤ 55 mg/dL instead of ≤ 25 mg/dL, as in the EBBINGHAUS study [17]. This decision was influenced by the current funding constraints for these drugs in the public health system (12 hospitals participated in patient recruitment for this study). The current Spanish funding for PCSK9 inhibitors is applicable to patients with LDL-C levels over 100 mg/dL [31], resulting in higher baseline values of LDL-C and consequently higher values of LDL-C at follow-up. Nevertheless, we defined a cutoff point at ≤ 40 mg/dL, and our analysis revealed no statistically significant differences when compared with the rest of the patients in the final MoCA score outcome. For this reason, we think that our study has shown that PCSK9i are safe from a neurological standpoint, not only because of the results but also because it represents a more realistic cohort of patients. For these reasons, we conclude that PCSK9i seems to be safe in the overall population but even in patients with very low values of LDL-C.
4.1.4 Differences by Sex
There were no statistically significant differences when adjusting the final MoCA score value for sex (p = 0.221), but we did find statistically significant differences when adjusting for age, revealing disparities in the final MoCA outcome between patients aged 65 years and older and those younger than 65 years (p < 0.001). Since we also lack data from clinical trials due to the underrepresentation of elderly patients, these results could be interpreted as suggesting that cognitive functions tend to diminish overall as age advances.
4.1.5 Differences Between Alirocumab and Evolocumab
To the best of our knowledge, to date, no studies have compared neurocognitive decline between alirocumab and evolocumab. Some RCT were published with PCSK9i, but they were carried out with only one PCSK9i [17, 18, 31]. Our results indicate that there are no apparent differences in terms of cognitive function between alirocumab and evolocumab.
4.2 Limitations
Our study has several limitations. The sample size is smaller than RCT where more subjects were included. This is a consequence of a prospective real-word study and the fact of recruitment started at the beginning of the COVID-19 outbreak (April 2020). In contrast, we would like to highlight that all of the population was from 12 public hospitals in a region in the northwest of Spain (population of 3 million), where 99% of the population is covered by the National Health Service, which ends up being a real and very complete analysis of the situation of PCSK9i in the real world. Another possible limitation is that the follow-up is not long term, because it took over 2 years, very in line with main RCT, and even longer than some of them [17, 18, 31]. Subsequently, an extended long-term study will be carried out.
5 Conclusions
In conclusion, among patients who received PCSK9 inhibitors in the real world in addition to other lipid-lowering therapies, we did not find any effect on neurocognitive function over 24 months’ treatment, either by global MoCA score or different cognitive domains. We only detected an improvement in delayed recall memory, probably as consequence of the methodology test. The study showed no differences in the cognitive function between the prespecified subgroups, even among patients who achieved very low levels of LDL-C. There were no differences in cognitive function between alirocumab and evolocumab. As a result of these real-world findings, it has become evident that the use of PCSK9 inhibitors is safe from a neurocognitive standpoint. These findings contribute to the growing body of evidence supporting the safety profile of these drugs in relation to neurocognitive function.
References
Silverman MG, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. J Am Med Assoc. 2016;316(12):1289–97.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–350.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455.
Jellinger PS, Handelsman Y, Rosenblit P, Bloomgarden ZT. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(April):1–24.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/ AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol. 2019;73(24):e285–350. https://doi.org/10.1016/j.jacc.2018.11.003.
Lloyd-Jones DM, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk a report of the American College of Cardiology Task Force on Clinical Expert Consensus Decision Pathways. J Am Coll Cardiol. 2016;68(1):92–125.
Lin Y, Parco C, Karathanos A, Krieger T, Schulze V, Chernyak N, Icks A, Kelm M, Brockmeyer M, Wolff G. Clinical efficacy and safety outcomes of bempedoic acid for LDL-C lowering therapy in patients at high cardiovascular risk: a systematic review and meta-analysis. BMJ Open. 2022;12(2): e048893. https://doi.org/10.1136/bmjopen-2021-048893.
Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, Kastelein JJP, ORION-10 and ORION-11 Investigators. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19. https://doi.org/10.1056/NEJMoa1912387.
Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–9.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Swiger KJ, Martin SS. PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N-of-1n trials. Drug Saf. 2015;38(6):519–26.
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99.
Khan AR, Bavishi C, Riaz H, et al. Increased risk of adverse neurocognitive outcomes with proprotein convertase subtilisin-kexin type 9 inhibitors. Circ Cardiovasc Qual Outcomes. 2017;10(1): e003153.
Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J. 2016;37(6):536–45.
Hirsh Raccah B, Yanovsky A, Treves N, Rotshild V, Renoux C, Danenberg H, Eliaz R, Matok I. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and the risk for neurocognitive adverse events: a systematic review, meta-analysis and meta-regression. Int J Cardiol. 2021;15(335):7–14. https://doi.org/10.1016/j.ijcard.2021.04.025.
Ying H, Wang J, Shen Z, Wang M, Zhou B. Impact of lowering low-density lipoprotein cholesterol with contemporary lipid-lowering medicines on cognitive function: a systematic review and meta-analysis. Cardiovasc Drugs Ther. 2021;35(1):153–66. https://doi.org/10.1007/s10557-020-07045-2.
Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, Sabatine MS, Sever PS, Robinson JG, Honarpour N, Wasserman SM, Ott BR, EBBINGHAUS Investigators. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377(7):633–43. https://doi.org/10.1056/NEJMoa1701131.
Janik MJ, Urbach DV, van Nieuwenhuizen E, Zhao J, Yellin O, Baccara-Dinet MT, Pordy R, Manvelian G. Alirocumab treatment and neurocognitive function according to the CANTAB scale in patients at increased cardiovascular risk: a prospective, randomized, placebo-controlled study. Atherosclerosis. 2021;331:20–7. https://doi.org/10.1016/j.atherosclerosis.2021.06.913.
O’Donoghue ML, Giugliano RP, Wiviott SD, Atar D, Keech A, Kuder JF, Im K, Murphy SA, Flores-Arredondo JH, López JAG, Elliott-Davey M, Wang B, Monsalvo ML, Abbasi S, Sabatine MS. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation. 2022;146(15):1109–19. https://doi.org/10.1161/CIRCULATIONAHA.122.061620.
Seijas-Amigo J, Rodríguez-Penas D, Estany-Gestal A, Suárez-Artime P, Santamaría-Cadavid M, González-Juanatey JR. Observational multicentre prospective study to assess changes in cognitive function in patients treated with PCSK9i. Study protocol. Farm Hosp. 2021;45(3):150–4. https://doi.org/10.7399/fh.11569(English).
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. https://doi.org/10.1111/j.1532-5415.2005.53221.x(Erratum in: J Am Geriatr Soc. 2019 Sep;67(9):1991).
Seijas-Amigo J, Cordero A, Olmo RF, Cortez Quiroga GA, Fácila L, Salgado-Barreira Á, Reyes-Santías F, Romero-Menor C, Murillo JR, Rodríguez-Mañero M, Bello Mora MC, Valle A, Sandin M, Pamias RF, Bañeras J, García PB, Lorenzo MC, Sánchez-Alvarez S, López-Rodríguez L, González-Juanatey JR. Patients with high cardiovascular risk as candidates to bempedoic acid, after treatment with statins, ezetimibe and pcsk9 inhibitors: an estimation and cost-effectiveness analysis. J Cardiovasc Pharmacol. 2023;81(1):70–5. https://doi.org/10.1097/FJC.0000000000001365.
FDA drug safety communication [Internet] [cited 2012 Aug 7]. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Accessed 11 Feb 2023.
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99.
Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169(6):906-915.e13. https://doi.org/10.1016/j.ahj.2015.03.004.
Koren MJ, Sabatine MS, Giugliano RP, Langslet G, Wiviott SD, Kassahun H, Ruzza A, Ma Y, Somaratne R, Raal FJ. Long-term low-density lipoprotein cholesterol-lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open-label OSLER-1 Extension Study. JAMA Cardiol. 2017;2(6):598–607. https://doi.org/10.1001/jamacardio.2017.0747.
Lipinski MJ, Benedetto U, Escarcega RO, Biondi-Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB Jr, Waksman R. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J. 2016;37(6):536–45. https://doi.org/10.1093/eurheartj/ehv563.
Gürgöze MT, Muller-Hansma AHG, Schreuder MM, Galema-Boers AMH, Boersma E, Roeters van Lennep JE. Adverse events associated with pcsk9 inhibitors: a real-world experience. Clin Pharmacol Ther. 2019;105(2):496–504. https://doi.org/10.1002/cpt.1193.
Feng Z, Li X, Tong WK, He Q, Zhu X, Xiang X, Tang Z. Real-world safety of PCSK9 inhibitors: a pharmacovigilance study based on spontaneous reports in FAERS. Front Pharmacol. 2022;13: 894685. https://doi.org/10.3389/fphar.2022.894685.
Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15(12):757–69. https://doi.org/10.1038/s41569-018-0098-5.
Agencia Española de Medicamentos y Productos Sanitarios [Internet] [cited 2022 Oct 21]. https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT_12-2020-evolocumab-Repatha.pdf. Accessed 28 Feb 2023.
Acknowledgements
Investigators received the support of the Fundación Instituto de Investigación Sanitaria de Santiago de Compostela (FIDIS) and National Network for Biomedical Cardiovascular Research of Cardiovascular Disease [Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV)]. Bonnie Dyer contributed to the English writing assistance.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose. Jose Seijas Amigo reports consulting fees from AstraZeneca.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Servizo Galego de Saúde (Date 29-JAN-2020 /No. 2019/653) and Agencia Española de Medicamentos y Productos Santiarios (Date 28-JAN-2020).
Consent to Participate
Informed consent was obtained from all individual participants included in the study. Informed consent was approved by the Ethics Committee of Servizo Galego de Saúde (Date 29-JAN-2020 /No. 2019/653).
Consent to Publish
Not applicable.
Data Availability
In this article, we are committed to data transparency and the availability of materials used in our research. The data and materials will be available on demand for readers interested in replicating or building upon our study. Interested parties can contact the corresponding author to request access to the data and materials. We will ensure that the requested information and materials are provided in a timely and complete manner.
Code Availability
Not applicable.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jose Seijas-Amigo, Ana Estany, and Pedro Suarez-Artime. The first draft of the manuscript was written by Jose Seijas-Amigo, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article.
Additional information
The members of the Investigadores MEMOGAL group are listed in Supplementary Appendix 1.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Seijas-Amigo, J., Mauriz-Montero, M.J., Suarez-Artime, P. et al. Cognitive Function with PCSK9 Inhibitors: A 24-Month Follow-Up Observational Prospective Study in the Real World—MEMOGAL Study. Am J Cardiovasc Drugs 23, 583–593 (2023). https://doi.org/10.1007/s40256-023-00604-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00604-6