Abstract
Combination therapy is now regarded as the standard of care in pulmonary arterial hypertension (PAH) and is becoming widely used in clinical practice. Given the inherent complexities of combining medications, there is a need for practical advice on implementing this treatment strategy in the clinic. Drawing on our experience and expertise, within this review, we discuss some of the challenges associated with administration of combination therapy in PAH and how these can be addressed in the clinic. Despite their differing modes of action, all of the currently available classes of PAH therapy induce systemic vasodilation. In initial combination therapy regimens in particular, this may lead to additive side effects and reduced tolerability compared with monotherapy. However, approaches such as staggered treatment initiation and careful up-titration may reduce the risk of additive side effects and have been used successfully in clinical practice, as well as in clinical trials and registry studies. When combination therapy regimens are initiated, it is important that patients are monitored regularly for the presence of any side effects and that these are then managed promptly and appropriately. For patients to attain the best outcomes, the treatment regimen must be tailored to the individual’s specific needs, including consideration of PAH etiology, the presence of comorbidities and concomitant medications beyond PAH therapy, and patient lifestyle and preference. It is also vital that individuals are managed at expert care centers, where multidisciplinary teams have a wealth of specialist experience in treating PAH patients. Adherence to therapy can be a concern in a chronic disease such as PAH, and as treatment regimens become increasingly complex, maintaining good treatment adherence may become more challenging. It is essential that patients are educated on the importance of treatment adherence, and this is a key role for the PAH nurse specialist. For patients who are managed carefully in expert centers with combination therapy regimens that are tailored to their specific needs, a favorable benefit–risk ratio can be achieved. With individual and carefully managed approaches, the excellent results observed with combination therapy in clinical trials can be obtained by patients in a real-world setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Combination therapy is the standard of care in pulmonary arterial hypertension (PAH); however, there is a need for clear, practical advice for using this approach in the clinic. |
Combination therapy must be carefully managed by an experienced, multidisciplinary team, with particular focus on the timing of treatment initiation, management of side effects and maintaining adherence to potentially complex treatment regimens. |
Combination therapy can be effectively managed in expert centers and provides long-term benefits for PAH patients. |
1 Introduction
Using combination therapy to target multiple pathogenic pathways is now considered the standard of care in pulmonary arterial hypertension (PAH). Strong evidence from randomized controlled trials (RCTs) and observational studies support this approach [1], and both initial and sequential combination therapy have a prominent role in the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines on pulmonary hypertension (PH) [2, 3]. Combination therapy has been administered in clinical practice for > 15 years, and its use is increasing [4,5,6,7]. This is not surprising given that there is a strong scientific rationale for its use in PAH and the clear importance of this option for a disease that is progressive and ultimately fatal [1, 8]. Given the rapid expansion of the evidence in the past few years and the strength of the ESC/ERS guideline recommendations, it is now clear that the benefits of combination therapy should be made available to the majority of PAH patients.
Combination therapy has been shown to be efficacious in the clinical trial setting, but it may also increase the practical challenges in clinical practice, and care must be taken to ensure that the benefit–risk ratio remains favorable for the patient. The robust clinical efficacy data supporting combination therapy and the role of risk stratification to guide clinical decision-making have recently been comprehensively reviewed [9]. However, practical advice for healthcare professionals regarding combination therapy management in the clinic is also required. For this review, we performed a search of the literature using PubMed to identify all published papers in the English language related to the use of combination therapy in PAH. With the help of this literature review, we identified some of the challenges associated with the use of combination therapy and drew on our clinical experience to provide advice on these practical aspects within this review article. This includes tailoring the treatment regimen to the needs of each patient, optimizing the protocol used for treatment initiation and appropriately managing side effects. By implementing these steps into the management of combination therapy, it is possible to strike the right balance between therapeutic safety and efficacy, in order to achieve the best possible outcome for patients.
2 Combination Therapy in the Treatment of PAH
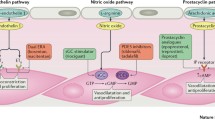
Therapies are available that each target one of the three pathogenic pathways underlying PAH: the endothelin (ET) pathway, targeted by endothelin receptor antagonists (ERAs); the nitric oxide (NO) pathway, targeted by phosphodiesterase type 5 inhibitors (PDE-5is) and soluble guanylate cyclase (sGC) stimulators; and the prostacyclin (PGI2) pathway, targeted by synthetic PGI2, PGI2 analogs and IP prostacyclin receptor agonists. The decision on when and which therapies to combine should be primarily based on the patient’s 1-year mortality risk, as determined by comprehensive, multiparameter risk assessments [2, 3]. For patients considered to be at low or intermediate risk, the ESC/ERS guidelines recommend either initial or sequential double oral combination therapy [2, 3]. For treatment-naïve, high-risk patients, the guidelines provide a clear recommendation for initial combination therapy including an intravenous (IV) PGI2 analog [2, 3].
These recommendations are based on a growing body of evidence that supports the use of double oral combination therapy as an effective treatment strategy in PAH [1], including long-term outcome data from large RCTs [10,11,12]. More recently, the long-term GRIPHON study has, for the first time, provided RCT evidence in support of triple combination therapy, in addition to double combination therapy, including the oral selective IP prostacyclin receptor agonist, selexipag [12].
In our clinical practice, drugs targeting the ET and NO pathways are most commonly used as first-line combination therapy. The majority of patients are treated with an ERA combined with a PDE-5i; however, some receive double oral combination therapy with an ERA and an sGC stimulator. Therapies targeting the PGI2 pathway are also frequently administered in double or triple combination regimens, especially in patients with severe PAH.
As for any disease, the potential for a greater number of side effects when using multiple therapies versus a single therapy should be considered. While ERAs, PDE-5is, sGC stimulators and therapies targeting the PGI2 pathway have distinct modes of action and exert a different range of effects on the pulmonary vasculature [13], all of these agents induce vasodilation. As a result, there is a clear overlap in their safety profiles. For example, all of these drugs are associated with adverse events (AEs) such as headache, hypotension and edema [14,15,16,17,18,19]. With this in mind, treating patients with more than one of these drugs may result in additive side effects and a greater number of AEs compared with monotherapy. This was observed in the AMBITION study, which reported that edema and headache occurred more frequently in patients taking a combination of ambrisentan and tadalafil compared with patients treated with either drug as monotherapy (Table 1) [11]. Despite some differences in the frequency of AEs, the tolerability of initial combination therapy with an ERA and a PDE-5i is supported by the similar rates of discontinuations due to AEs observed with combination therapy and with monotherapy (Table 1) [11]. Similarly, in the long-term GRIPHON study, the frequency with which AEs were reported increased with the number of therapies (Table 2) [20]. However, patients receiving selexipag as part of a sequential double or triple combination therapy regimen had broadly comparable rates of discontinuations due to AEs, albeit in both groups this was higher than in patients receiving monotherapy (Table 2) [20].
Additive side effects are thought to be less likely with sequential than initial combination therapy as patients adapt to the systemic vasodilatory effects of the first drug before another is added. The tolerability of sequential combination therapy is supported by data from the SERAPHIN trial, in which macitentan was generally well-tolerated, despite the majority (64%) of patients receiving a PAH therapy at baseline [10]. Similar results were obtained with riociguat in the PATENT trial, in which 50% of patients were receiving PAH therapies at baseline [21]. Low discontinuation rates due to AEs have also been demonstrated in RCTs where therapies targeting the PGI2 pathway are used in sequential double combination therapy [12, 22,23,24].
PAH therapies are also associated with side effects that are not related to vasodilation. For example, ERAs, PDE-5is and sGC stimulators have been associated with anemia, PDE-5is and sGC stimulators can cause muscle pain, and bosentan has been associated with liver enzyme elevations. However, drug or drug-class–specific side effects are not expected to be additive during combination therapy.
3 Practical Recommendations for Clinical Practice
When patients commence combination therapy, a number of practical measures can be taken to minimize the risk of side effects, monitor safety and tolerability, and effectively manage any side effects that occur.
3.1 Initial Combination Therapy
3.1.1 Staggered Initiation
In an initial combination therapy regimen, patients may be more likely to experience side effects if therapies are introduced in close succession. In addition, the concomitant introduction of multiple drugs can make it difficult to identify which drug is causing the side effect [25]. A short delay between initiation of therapies may allow patients to adapt to the vasodilator effects of one drug before another is added, and would make determination of which agent is responsible for the side effect in question more likely. In our European and US expert centers, double initial combination therapy with an ERA and a PDE-5i involves starting the two therapies a few weeks apart, with the aim of establishing patients on the full dose of both treatments within 1 month. This approach is also applied in combination regimens that include a therapy targeting the PGI2 pathway. For high-risk patients who require a parenteral PGI2 therapy immediately after diagnosis, the parenteral drug is initiated as a first step, and an ERA and/or a PDE-5i initiated a few days later.
3.1.2 Up-Titration
Another approach to improve tolerability is to initiate one or both therapies at a lower than recommended dose. For example, in our clinical experience, administration of tadalafil at 20 mg once daily and then increasing after 1 to 2 weeks to 40 mg once daily is often more tolerable than starting at 40 mg once daily as recommended in the summary of product characteristics [14]. Similarly, for patients who experience headaches when starting a PDE-5i shortly after initiation of an IV PGI2 therapy, up-titration of the IV therapy can be delayed until the headache becomes more manageable.
Such an up-titration approach to combination therapy is supported by data from a number of studies. In the AMBITION trial, ambrisentan and tadalafil were started together at low doses (5 and 20 mg, respectively) and up-titrated to 10 and 40 mg over an 8-week period [11]. While the number of AEs was increased in the combination therapy arm, no differences were observed in the rates of discontinuations due to AEs between the combination therapy arm (12%) and the pooled monotherapy arms using this approach (11%).
Similarly, in a retrospective analysis of newly diagnosed patients admitted to expert centers in the French PH Network [7], patients were initiated on an ERA and a PDE-5i on the same day and then up-titrated according to clinical need and tolerability. However, this study utilized a faster up-titration schedule than AMBITION, with patients achieving their maximal dose within 4 weeks. This treatment regimen was well-tolerated: over a median follow-up of 30 months, only one patient out of 97 discontinued double combination therapy [7]. Further changes to the up-titration schedule have been made in the ongoing OPTIMA study, where patients receive macitentan 10 mg once daily and tadalafil 20 mg once daily on day 1, followed by a step up to tadalafil 40 mg once daily at day 8 (± 3 days) [26].
3.1.3 Need for Regular Patient Monitoring
After starting initial combination therapy, it is essential that patients are followed up on a regular basis to monitor the safety and tolerability of the treatment regimen. For initial double combination therapy with an ERA and a PDE-5i, nurses typically perform a telephone check-up 1 week after initiation of the second drug and an assessment at the clinic 4–6 weeks later. More frequent check-ups are necessary if riociguat is used, as this drug requires careful up-titration to avoid hypotension (systolic blood pressure < 95 mmHg) [19]. For example, at several of our centers, nurses perform a weekly telephone check-up and assess the patient in the clinic after 1 month. For all combinations, home visits by a nurse may be required instead of telephone check-ups if there are any problems with the initiation of therapies.
In addition to follow-up assessments performed by healthcare professionals, patients are advised to self-monitor for the development of certain side effects. An example of this is edema, which is a side effect of vasodilator therapies [14,15,16,17,18,19], but also a complication of PAH as a result of right heart failure [2, 3]. When initiating combination therapy, patients should check their weight daily and report any rapid and significant gain of over 2 kg promptly to their expert center. Such monitoring will ensure that the development of edema is detected at an early stage.
3.1.4 Management of Side Effects
When side effects occur, it is important that they are managed appropriately. For example, therapies that target the ET and NO pathways are associated with headaches and muscle pain. Temporary dose reduction, in particular for PDE-5is and sGC stimulators, may effectively manage these side effects. It is also important to advise patients that some side effects are temporary. Patients should be encouraged to persist with their treatment regimen beyond the first week, as side effects, such as headache and muscle pain, will likely dissipate over time. In the case of development of edema, clarification of whether this phenomenon is due to heart failure or to systemic vasodilation is required. In the first case, edema associated with weight gain should prompt diuretic dose increase and PAH treatment escalation. Of note, many PAH patients have edema at diagnosis as a manifestation of right heart failure. For these patients, diuretic therapy should be prescribed from the outset. In cases of edema as a result of systemic vasodilation, usually no specific measure is necessary beyond reassurance.
These practical recommendations, particularly the benefits associated with staggered initiation of initial combination therapy, are highlighted in a recent case from one of our centers (Fig. 1).
Patient case illustrating the benefits of initial combination therapy for PAH. A 34-year-old female presented with evidence of severe pulmonary hypertension. After being diagnosed with PAH, the patient received initial triple combination therapy with a PDE-5i, an ERA and an intravenous PGI2 analog using a staggered approach to treatment initiation. Significant functional and symptomatic improvements were reported within 2 months following diagnosis. 6MWD 6-min walk distance, ERA endothelin receptor antagonist, FC functional class, I.v. intravenous, PAH pulmonary arterial hypertension, PDE-5i phosphodiesterase type 5 inhibitor, PGI2 prostacyclin, RHC right heart catheterization. Asterisk indicates an intravenous PGI2 analog was initially up-titrated to 8 ng/kg/min in hospital and then further up-titrated by 1 ng/kg/min per week at home until a target dose of 20–25 ng/kg/min was reached. For this patient, an oral therapy targeting the PGI2 pathway could be an alternative option to an intravenous therapy
3.2 Sequential Combination Therapy
In clinical practice, many patients receive sequential combination therapy. This typically includes patients who have maintained a low risk status for months or even years on monotherapy or double combination therapy, but who now need to escalate their treatment with the addition of a second or third therapy. For this type of treatment regimen, patients will have adapted to the systemic vasodilator effects of one drug before starting another. As a result, additive side effects are thought to be less likely than with initial combination therapy. In our clinical experience, adding a therapy to an existing treatment regimen generally results in the same side effect profile as when the drug is given to a treatment-naive patient. Consequently, we manage and monitor the introduction of the new therapy in the same way as if it were given as a monotherapy.
4 Other Considerations for the Use of Combination Therapy
In order to obtain the maximal benefit from combination therapy, the treatment regimen must be tailored to each patient’s specific clinical needs, lifestyle and preference. To achieve this, the following general recommendations should be considered when selecting a combination therapy regimen.
4.1 Underlying Disease and Comorbid Conditions
The decision on which treatment strategy is most appropriate for each patient is at the discretion of the treating physician and involves the consideration of several factors, including comorbidities and PAH etiology. Patients seen in clinical practice tend to be older, with more comorbid conditions than historical PAH populations [27]. The administration of combination therapy regimens in these patients may be more challenging and should include assessment of potential interactions with existing medications. For example, patients with coronary artery disease (CAD) may be treated with nitrates to relieve the symptoms of angina. As nitrates, PDE-5is and sGC stimulators all induce vasodilation by increasing the endothelial production of NO, co-administration of these drugs can have serious additive hypotensive effects and is formally contraindicated [28]. Despite this, for a PAH patient with co-existing CAD, the benefits of using a PDE-5i or sGC stimulator may outweigh the risk of eliminating nitrates from the patient’s treatment options. However, it is essential that this is carefully managed. Patients should be provided with written guidance as to why they cannot receive nitrates, and this information should be clearly documented in the patient’s electronic medical record and communicated to cardiologists and referring doctors. Another comorbidity prevalent in older patients is diabetes [4, 6]. Many patients with type 2 diabetes are treated with the glucose-lowering drug glyburide [international nonproprietary name (INN), glibenclamide]. However, a drug–drug interaction between glyburide and the ERA bosentan has been reported [29]. Concomitant administration of bosentan and glyburide resulted in reduced plasma levels of both drugs compared with the levels after a single drug was administered [29]. Furthermore, in the REACH-1 trial of patients with chronic heart failure, an increased incidence of elevated liver enzymes was observed in patients receiving both bosentan and glyburide compared with patients receiving bosentan monotherapy [30]. As a result, the co-administration of bosentan and glyburide is contraindicated [16] and diabetic patients treated with glyburide should receive an alternative ERA as part of their PAH combination therapy regimen.
Disease etiology may also influence the selection of drugs used in each patient’s treatment regimen. For example, caution must be applied for human immunodeficiency virus patients treated with anti-retroviral drugs that are strong CYP3A4 inhibitors, such as ritonavir and saquinavir, as these can interfere with the metabolism of ERAs and PDE-5is [2, 3, 31]. Care is needed when managing PAH patients with associated connective tissue disease, who may be receiving immunosuppressive therapies to treat their underlying condition. For example, co-administration of bosentan with the immunosuppressant cyclosporine is contraindicated [32]. It is also important to be mindful that PAH therapies can exacerbate symptoms in certain etiologies. For instance, the use of vasodilators may lead to a greater degree of hypoxia in patients with systemic sclerosis complicated by interstitial lung disease [33].
4.2 Expert Centers and Patient Support Groups
Due to the challenges in administering combination therapy, we recommend that all PAH patients are managed in expert centers. We are aware that some patients may have to travel considerable distances to reach their nearest expert center, which often poses a logistical challenge. However, managing PAH in a specialist setting provides considerable benefit, which is not always achievable with local care. In expert centers, patients receive care from a multidisciplinary team, including specialist healthcare professionals who see similar patients on a daily basis and thus have considerable experience in recognizing and managing side effects, and can ensure optimal timing when initiating PAH therapies in a combination therapy regimen. Specialist nurses should pro-actively contact patients after the initiation of therapies to provide support and to monitor for side effects, particularly during the early stages of treatment when patients are more likely to be experiencing symptoms. In addition, nurses play a key role in managing patients’ expectations of their treatment and educating patients and their carers and families to ensure they are fully informed of the benefits and potential challenges associated with combination therapy. In addition to the availability of specialist staff, expert centers are more likely to be associated with, and to advocate for, patient support groups. Such groups are vital as they provide reassurance and first-hand experience of combination therapy from other patients. Initiatives such as these can engage patients and lead to patient activation, which in turn improves patient confidence and therapy adherence [34].
4.3 Adherence to Therapy
Adherence to therapy can be an issue in many chronic diseases that require long-term pharmacological treatment. Our clinical experience suggests that PAH patients are generally adherent with their combination therapy regimen; they understand the severity of their disease and, as a result, are motivated to take their medication. Nevertheless, PAH specialists must be aware that adherence may be reduced as the number of medications to be taken each day increases, particularly if the treatment regimen requires administration of different drugs at different times of the day. This can be due to patient forgetfulness or perhaps because the treatment schedule does not fit with the patient’s lifestyle or other commitments. Another reason for reduced adherence could be that patients may not notice short-term effects of occasionally missing a dose of one out a number of drugs and therefore may not appreciate the importance of taking all therapies regularly. The impact of increasing treatment complexity on adherence has been demonstrated in a number of chronic diseases, where the more tablets that are required each day, the lower the adherence [35, 36]. However, increasing the complexity of the treatment regimen can also have the opposite effect and can lead to increased adherence. This trend was observed in a study of congestive heart failure patients, which demonstrated that taking medications twice daily or less was associated with reduced adherence compared with more complex treatment regimens [37]. This might be related to a higher level of attention to routine; indeed, having a highly structured daily routine is a strong independent predictor of adherence [38]. In order for PAH patients to receive the maximal benefit from their combination therapy regimen, it is essential that they take their drugs as prescribed. Although the impact of combination therapy on adherence in PAH is unknown, the necessity of educating patients on the importance of adherence to therapy cannot be stressed enough and is a vital role for the specialist nurse. In addition, PAH specialists can provide useful tools and tips to aid adherence, including setting alarms as reminders for taking medication and preparing weekly pillboxes.
4.4 Management of Long-Term Combination Therapy
PAH patients are surviving longer than ever and may maintain good functional capacity for years, albeit with a continued risk of rapid deterioration and morbidity [39]. This is supported by three recent registry studies, which reported that more than three-quarters of PAH patients with a low-risk status will still be alive 5 years after enrollment [40,41,42]. As a result, many patients may receive double or triple combination therapy for years. In our opinion, there are no additional considerations that are specific to the longer-term use of PAH therapies in combination, compared with their short-term use. After successful initiation of combination therapy and resolution of any side effects, patients can be managed in the same way as if they were receiving each agent as a monotherapy. This includes assessments every 3–6 months at an expert center with the overall therapeutic aim of achieving a low mortality risk status [2, 3].
5 Conclusion
As supported by long-term efficacy data [10,11,12] and the latest ESC/ERS guidelines [2, 3], combination therapy is now the mainstay of PAH management. Although combination therapy has the potential to be associated with practical challenges and additive side effects, these can be successfully managed in most cases. Specialist healthcare professionals in expert PAH centers possess the experience and expertise to allow the benefits of combination therapy to be maximized whilst reducing the risk to the patient.
References
Sitbon O, Gaine S. Beyond a single pathway: combination therapy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:408–17.
Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903–75.
Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119.
Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–87.
Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168:871–80.
Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–6.
Sitbon O, Sattler C, Bertoletti L, et al. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J. 2016;47:1727–36.
Channick RN. Combination therapy in pulmonary arterial hypertension. Am J Cardiol. 2013;111:16C–20C.
Gaine S, McLaughin VV. Pulmonary arterial hypertension: tailoring treatment to risk in the current era. Eur Respir Rev. 2017;26:170095.
Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–18.
Galiè N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–44.
Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–33.
Duarte JD, Hanson RL, Machado RF. Pharmacologic treatments for pulmonary hypertension: exploring pharmacogenomics. Future Cardiol. 2013;9:335–49.
Adcirca (tadalafil). Summary of product characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001021/WC500032789.pdf. Accessed Dec 2017.
Revatio (sildenafil). Summary of product characteristics. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000638/WC500055840.pdf. Accessed Dec 2017.
Tracleer® (bosentan). Summary of product characteristics. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000401/WC500041597.pdf. Accessed Dec 2017.
Opsumit® (macitentan). Summary of product characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002697/WC500160899.pdf. Accessed Dec 2017.
Volibris (ambrisentan). Summary of product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000839/WC500053065.pdf. Accessed Dec 2017.
Adempas (riociguat). Summary of product characteristics. 2017. https://ec.europa.eu/health/documents/community-register/2014/20140327128191/anx_128191_en.pdf. Accessed Dec 2017.
Lang I, Gaine S, Galiè N, et al. Effect of selexipag on long-term outcomes in patients with pulmonary arterial hypertension (PAH) receiving one, two or no PAH therapies at baseline: results from the GRIPHON study. In: Poster presented at European Society of Cardiology. 2015. p. P2365.
Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–40.
McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–22.
McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257–63.
Hoeper MM, Leuchte H, Halank M, et al. Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;28:691–4.
Cahn A, Cefalu WT. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care. 2016;39(Suppl 2):S137–45.
Sitbon O, Canuet M, Picard F, et al. Initial combination therapy with macitentan and tadalafil in newly diagnosed patients with pulmonary arterial hypertension: results from the OPTIMA trial. Am J Resp Crit Care Med. 2017;195:A2297.
McGoon MD, Benza RL, Escribano Subias P. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62:D51–9.
Chrysant SG, Chrysant GS. The pleiotropic effects of phosphodiesterase 5 inhibitors on function and safety in patients with cardiovascular disease and hypertension. J Clin Hypertens (Greenwich). 2012;14:644–9.
van Giersbergen PL, Treiber A, Clozel M, et al. In vivo and in vitro studies exploring the pharmacokinetic interaction between bosentan, a dual endothelin receptor antagonist, and glyburide. Clin Pharmacol Ther. 2002;71:253–62.
Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69:223–31.
Ghofrani A, Schermuly R, Weissmann N, et al. Drug Interactions in pulmonary arterial hypertension and their implications. US Cardiol. 2009;6:105–6.
Venitz J, Zack J, Gillies H, et al. Clinical pharmacokinetics and drug-drug interactions of endothelin receptor antagonists in pulmonary arterial hypertension. J Clin Pharmacol. 2012;52:1784–805.
Le PJ, Girgis RE, Lechtzin N, et al. Systemic sclerosis-related pulmonary hypertension associated with interstitial lung disease: impact of pulmonary arterial hypertension therapies. Arthritis Rheum. 2011;63:2456–64.
Graarup J, Ferrari P, Howard LS. Patient engagement and self-management in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:399–407.
Sax PE, Meyers JL, Mugavero M, et al. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7:e31591.
Gupta P, Patel P, Strauch B, et al. Risk factors for nonadherence to antihypertensive treatment. Hypertension. 2017;69:1113–20.
George J, Shalansky SJ. Predictors of refill non-adherence in patients with heart failure. Br J Clin Pharmacol. 2007;63:488–93.
Wagner GJ, Ryan GW. Relationship between routinization of daily behaviors and medication adherence in HIV-positive drug users. AIDS Patient Care STDs. 2004;18:385–93.
Chakinala MM, Barst R. From short-term benefits to long-term outcomes: the evolution of clinical trials in pulmonary arterial hypertension. Pulm Circ. 2013;3:507–22.
Kylhammar D, Kjellstrom B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2017. https://doi.org/10.1093/eurheartj/ehx257.
Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50. https://doi.org/10.1183/13993003.00889-2017.
Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50. https://doi.org/10.1183/13993003.00740-2017.
Acknowledgements
The authors would like to thank James Glasper from nspm ltd (Meggen, Switzerland) for medical writing assistance, funded by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This manuscript was supported by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland).
Conflict of interest
Ms. Burks has received non-financial medical writing support in the preparation of the submitted work from Actelion Pharmaceuticals Ltd and consulting fees from Actelion Pharmaceuticals Ltd, Bayer Healthcare, GlaxoSmithKline, and Pfizer outside the submitted work. Ms. Stickel has received non-financial medical writing support in the preparation of the submitted work from Actelion Pharmaceuticals Ltd and consulting fees from Actelion Pharmaceuticals Ltd, Bayer Healthcare, and United Therapeutics Europe outside the submitted work. Prof. Gailè has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received research grants and consultancy fees from Actelion Pharmaceuticals Ltd, Bayer Healthcare, GlaxoSmithKline, and Pfizer; has received speaker fees from Merck Sharpe & Dohme; and has received non-financial medical writing support in the preparation of the submitted work from Actelion Pharmaceuticals Ltd.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Burks, M., Stickel, S. & Galiè, N. Pulmonary Arterial Hypertension: Combination Therapy in Practice. Am J Cardiovasc Drugs 18, 249–257 (2018). https://doi.org/10.1007/s40256-018-0272-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-018-0272-5