Abstract
Extending the operation of proton exchange membrane fuel cells (PEMFCs) at high temperature (i.e., 120 °C) and/or low relative humidity (< 50% RH) remains a significant challenge due to dehydration and subsequent performance failure of the Nafion electrolyte. We approached this problem by integrating the Nafion matrix with a novel hybrid nanofiller, created through direct growth of TiO2 nanoparticles on the surface of carbon nanotubes. This synthetic approach allowed to preserve an effective nanodispersion of Titania particles in the hosting matrix, thereby boosting dimensional stability, hydrophilicity, and physiochemical properties of the Nafion/MWCNTs-TiO2 (NMT-x) nanocomposites compared to parental Nafion. At optimal concentration (i.e., 3 wt% with respect to the polymer), the nanocomposite membrane exhibited high transport characteristics with impressive water retention capabilities, resulting in a proton conductivity of 8.3 mS cm− 1 at 80 °C and 20% RH. The Titania nanoparticles plays a key role in retaining water molecules even under dehydrating conditions, while also directly contributing to proton transport. Additionally, the long carbon nanotubes promote the formation of additional paths for proton conductivity. These combined features enabled the NMT-3 membrane to achieve a maximum power output of 307.7 mW/cm2 in a single H2/air fuel cell (5 cm2 active electrode area and 0.5 mg Pt/cm2 at both electrodes) under very challenging conditions, specifically at 120 °C and 30% RH. This represents a significant advancement towards overcoming the limitations of traditional Nafion membranes and opens up new possibilities for high-temperature, low-humidity H2/air fuel cell applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fuel Cells, especially Proton Exchange Membranes Fuel Cell (PEMFCs), are poised at the forefront of alternative energy generation technologies, driving the so-called “hydrogen revolution” [1]. They boast efficiency up to 60%, rapid start-up and shut-down times [2], reduced noise pollution [3], and emissions that are either undetectable or entirely absent [4]. Nowadays, most of the effort are devoted to development of new proton electrolyte membranes (PEMs) able to operate at elevated temperatures with reduced humidification levels [5]. Operating at temperature above 100 °C would furnish PEMFCs with accelerated electrode kinetics, enhanced tolerance to carbon monoxide in the fuel gas, and easier thermal and water management [6, 7]. Regrettably, the current electrolyte membrane – whether from the Nafion series or other perfluorosulfonic acid (PFSA) membranes - exhibits excessive sensitivity to fluctuations in its internal moisture level. At high temperature or low RH, hydrophilic channels in the Nafion membrane collapse, leading to a substantial surge in membrane resistance and inadequate contact at the membrane-electrode interface [8, 9]. Consequently, device performance experiences a significant decline. In a nutshell, the fabrication of PEMs prone to operate under elevated temperatures and/or low humidification of reactant gases still persists as the major obstacle against large-scale commercialization of PEMFC systems.
The incorporation of hygroscopic metal oxide particles, such as SiO2, ZrO2, TiO2, and zirconium phosphate, in the hydrophilic domains of Nafion is widely recognized as a leading strategy to enhance its operational capabilities. These materials are anticipated to serve as a water reservoir, promoting proton mobility at HT and low RH [10,11,12,13,14,15]. Among ceramic oxides, titanium dioxide (TiO2) nanoparticles possess a blend of desirable traits including a high specific surface area, pronounced hydrophilicity, exceptional thermal and mechanical stability, as well as crucial compatibility with organic solvents and polymers [16]. Consequently, significant attention has been directed towards the development Nafion/TiO2 composite membranes by many research groups. For example, Santiago et al. [17] demonstrated that TiO2 can be easily incorporated inside Nafion by sol-gel method achieving an appreciable improvement in the cell performance above 100 °C. Baglio and coworkers [18] prepared TiO2/Nafion hybrid electrolytes tailored for direct methanol fuel cell applications. The infiltration of ceramic oxide significantly reduced the membrane permeability toward methanol fuel, leading to a notable expansion of the DMFC’s operating temperature range. Similar evidences were collected by Barbora et al. [19], which synthesized Nafion composite membrane at various Titania loading and assessed their suitability in direct ethanol fuel cell. Despite these encouraging findings, the challenge of controlling the structural and morphological characteristics of the TiO2 filler, which crucially defines the performance of the final nanocomposite at high temperature, still persists as a major obstacle [20, 21]. Ceramic oxides tend to lose their nano-dispersion within the hosting polymer matrix even at very low filler content, instead forming micrometric agglomerates with detrimental effect on the transport and mechanical features of the PEMs [22,23,24].
To address this crucial challenge, we have recently developed a hybrid filler comprising Multi Walled Carbon Nanotubes (MWCNTs) decorated with a large number of TiO2 nanoparticles. The MWCNTs-TiO2 material was synthesized by an easy and scalable one-pot hydrothermal method, with Titanium butoxide (Ti(OBu)4) serving as precursor for Titania. Worth to note, this approach enabled fine and nanometric dispersion of the hybrid material within various polymer matrices, effectively preventing filler agglomeration [25,26,27,28].
In the present study, Nafion/MWCNTs-TiO2 nanocomposite membranes were produced with varying doping levels of hybrid particles using a simple solution casting method. The resulting nanocomposite membranes were then evaluated for high-temperature PEMFCs application. Membranes’ hydrophilicity and dimensional stability were evaluated by swelling tests, whereas NMR methods were used to clarify the effect of filler introduction on the transport properties of Nafion. Proton conductivity of NMT nanocomposites was investigated by Electrochemical Impedance Spectroscopy under different temperature and humidification conditions. Lastly, the single H2/air fuel cell performance of the electrolytes was also investigated. The incorporation of MWCNTs-TiO2 nanomaterial has proven to be a highly promising approach for effectively extending the operating conditions of Nafion-based PEMs, showcasing significant potential for advancing the field of fuel cell technology.
Experimental
Materials
Nafion (20wt% dispersion in water and lower aliphatic alcohols), MWCNTs (particle size < 20 nm), titanium (IV) butoxide (reagent grade of 97%), and all the solvents were supplied by Sigma-Aldrich and used as received.
Synthesis of the MWCNTs/TiO2 nanohybrid
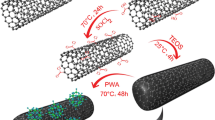
Commercial MWCNTs were first oxidized by treatment with a mixture of H2SO4/HNO3 (3:1 volume ratio) while sonicating for 3 h at room temperature. The oxidized MWCNTs (oxMWCNTs) were recovered by washing several times with distilled water and drying in the oven at 60ºC overnight. Thereafter, the oxMWCNTs were directly used for synthesis of the MWCNTs-TiO2 hybrid material though a one-pot hydrothermal method [27], as schematized in Fig. 1. Briefly, a mixture of isopropanol (C3H8O), water, and nitric acid (HNO3) was added to Titanium butoxide (Ti(OBu)4) as Titania precursor, and the reaction batch was heated at 60 °C and left under mechanical stirring for 1 h. During this time, the appropriate amount of oxMWCNTs was dispersed into water by sonication and the resulting homogeneous dispersion was then slowly added to the Ti4+ solution. The molar ratio between the Ti(OBu)4 and MWCNTs was set at 14:1 (w/w). This ratio was determined to strike the optimal balance between titania loading and TiO2 particle size. Mixture was left under stirring at 60 °C for additional 18 h before quenching with cold ethanol. Subsequently, the MWCNTs-TiO2 hybrid material was recovered as a fine black powder through centrifugation, rising several times with deionized water and vacuum drying at 60 °C overnight. TiO2 content was 47.6 wt% calculated by TGA while average grain size for TiO2 particles was of ca. 80 nm on the basis of the broadening of the (101) anatase peak in the XRD pattern [27].
Membrane preparation
For Nafion recast membranes, 1 g of commercial Nafion solution was completely dried at 60–65 ºC and then re-dissolved with 25 ml of Dimethylformamide (DMF) by heating at 80 ºC. Complete solubilization of Nafion polymer was achieved after almost 2 h. Finally, the polymer solution was cast onto a Petri dish and heated at 60 ºC till dryness. In the case of nanocomposite membranes, the optimal amount of MWCNTs, oxMWCNTs, and MWCNTs-TiO2 powder was directly added to the commercial Nafion solution. The mixture was sonicated for 4 h and left under vigorous mechanical stirring at RT for additional 4 h. After a macroscopically homogeneous dispersion was obtained, the mixture was cast on a petri dish and placed in an oven at 45 ºC for 24 h followed by 1 h at 100 ºC. The filler loading ranged from 1 to 5 wt% with respect to the polymer, and Nafion/MWCNT-TiO2 were referenced to be NMT-x, where x indicates the amount of filler. Following this procedure, macroscopically homogeneous membranes were obtained, as illustrated in Fig. 2.
Before characterization, all the membranes were subjected to thermal and chemical activation according to a standard procedure [29]. For thermal activation, the membranes were sandwiched between two Teflon plates, subjected to a pressure of 2 ksc, and placed in the oven at 155 °C for 20 min under pressure. This general procedure allowed to increase the mechanical robustness of the Nafion-based PEMs. Chemical activation was instead carried out by soaking the membranes in: (i) HNO3/H2O solution (1:1 volume ratio) at 90 °C (boiling) for 1 h; (ii) H2O2 solution 30% at 60 °C for 1 h to remove organic impurities; (iii) 1M H2SO4 solution at 80 °C for 1 h to remove inorganic impurities (trace metal); (iv) 0,01 M EDTA solution for 24 h; (v) 2 M HCl solution at 80 °C for 2 h; and (vi) 0,01 M EDTA solution for additional 24 h. After each step, membranes were rinsed at least three times in warm distilled water for 15 min. Finally, the membranes were dried into the oven at 60ºC for 24 h. The prepared membranes had dry thickness 50 ± 5 μm.
Ion Exchange Capacity (IEC), Water Uptake, and Hydrolytic Stability
The ion exchange capacity (IEC), which indicates the number of exchangeable protons (meq) per gram of sample, was determined by an acid-base titration method. The samples were first dried in an oven at 60ºC for 3 h, their dry mass (Mdry) weighted, and then immersed in 2 M NaCl aqueous solution for 24 h. The H+ ions released during this stage, due to the exchange with Na+ ions, were then titrated with 0.01 M KOH solution with phenolphthalein as an indicator. The IEC values (meq/g) were calculated considering the volume (VKOH, mL) and the concentration (CKOH, mol/L) of the NaOH solution consumed to neutralize the H+ ions according to Eq. 1:
Water uptake was determined from the mass difference between the wet (Mwet) and dry (Mdry) states of the samples. For the wet state, each sample was immersed into deionized water for 24 h at RT. Once removed, the surface was rapidly dried with the filtering paper and the wet mass was measured. Water uptake (wu) was then calculated according to Eq. 2.
Hydrolytic stability for the Nafion/MWCNT-TiO2 membranes were determined by considering the water uptake variation (Δwu) in the temperature range of 30–80 ºC, each 10 °C. For each temperature, the sample was immersed into a water bath previously equilibrated at the target temperature. After 2 h, the membranes were removed, their surface rapidly dried with the filtering paper and the water uptake calculated. The Δwu was calculated using Eq. 3.
Where Wi is the water uptake at room temperature and Wf is the water uptake at each temperature.
NMR spectroscopy (Diffusometry & Relaxometry)
The 1H NMR measurements were conducted using a Bruker AVANCE 300 wide bore spectrometer operating at 300 MHz for 1H and equipped with a Diff30 Z-diffusion 30 G/cm/A multinuclear probe with interchangeable RF inserts. The 1H spectra were obtained by processing the Free Induction Decay (FID) from single π/2 pulse sequences.
The self-diffusion coefficients of water molecules absorbed within the hydrophilic domains of the membranes were determined using the Pulsed Field Gradient Stimulated-Echo (PFG-STE) method. This specific sequence is designed for materials with transverse relaxation time (T2) significantly shorter than the longitudinal relaxation time (T1). It involves three 90° RF pulses (π/2-τ1-π/2-τm-π/2) with two gradient pulses applied after the first and third RF pulses. The echo is detected at time τ = 2τ1 + τm. The fundamental principle of this technique relies on observing the behavior of a diffusing nucleus when subjected to magnetic field gradients. These gradients induce changes in both the magnetic field and phase of the diffusing nucleus. This phase change results in a reduction in magnetization M, which is quantified by an attenuation factor described in Eq. 4 [30]:
Here, M0 represents the initial magnetization, γ is the gyromagnetic ratio, g is the gradient strength,
δ is the duration of the gradient pulse, and Δ is the diffusion time. This equation characterizes the attenuation of magnetization due to diffusion effects in the PFG-STE method. The experimental parameters used in this investigation were gradient pulse length δ = 0.8 ms, time delay Δ = 8 ms, and the gradient amplitude varied from 80 to 800 G cm− 1. To measure the longitudinal relaxation time (T1), an inversion recovery sequence was employed. This sequence consists of two RF pulses: π (180º) and π/2 (90º), separated by a relaxation time τ. The longitudinal relaxation times (T1) of water were measured by varying the τ values from 0.0001 to 10 s. Details regarding the preparation of NMR samples can be found elsewhere [31]. Spectra, self-diffusion coefficients, and T1 were analyzed within a temperature range of 20 to 130 ºC, with 20 ºC increments. The membranes were allowed to equilibrate for 15 min at each temperature before measurements were taken [32].
The Activation Energy (Ea), which represents the energy barrier for carrier transfer from one free site to another in the diffusion process, was calculated using the Arrhenius equation (Eq. 5)
Where D is our diffusion coefficient, D0 is the preexponential factor, R is the gas constant equal 8,31 J/mol·K and T is the temperature in Kelvin.
Electrochemical Impedance Spectroscopy (EIS)
The though-plane proton conductivity of the PEMs was assessed through Electrochemical Impedance Spectroscopy. Circular samples were cut from the membranes, sandwiched between two carbon cloths electrodes, and placed in a custom-made two-electrode cell. The cell was fitted between the anode and cathode flow fields of a fuel cell test hardware (850 C, Scribner Associates Inc.). The AC impedance response of the cell was recorded using a multichannel potentiostat galvanostat impedance analyzer (PGSTAT 30 potentiostat/galvanostat from Metrohm Autolab) with an FRA module. Impedance response was collected at Open Circuit Voltage (OCV) within the frequency range of 1 Hz to 1 MHz, using an oscillating potential of approximately 10 mV. The membranes underwent conditioning at two temperatures: 30 ºC and 80 ºC, under varying RH conditions. Membrane resistance (R, Ω) was determined by extrapolating the high-frequency intercept with the real axis (Z’) in the Nyquist plot. Subsequently, proton conductivity (σ) was calculated using Eq. 6, taking into account the distance between the electrodes (l, cm) and the active area (A, cm2) according to Eq. 6:
H2/air fuel cell tests
The electrodes for H2/air fuel cell tests were fabricated using the casting knife technique, as described in detail elsewhere [33]. A catalytic ink was prepared by sonication, combining commercial platinum (Pt/C, 40% supported on carbon - Alfa Aesar) with 33% Nafion ionomer (20% ionomer solution – Ion Power). Once the proper dispersion was achieved, this catalytic ink was applied onto the backing layer of a commercial Sigracet 25-BC Gas Diffusion Layer (SGL). A Pt loading of 0.5 mg cm-2 was used for both the anode and cathode sides. Membrane Electrode Assemblies (MEAs) were created by hot pressing the electrodes onto two membranes, i.e., Nafion 212 benchmark and NMT-3 membranes, at 130 °C and 30 kgN/cm2 for 2 minutes. The two membranes had comparable thicknesses, approximately 50 μm, which ensured a more accurate comparison of resulting cell performances. Galvanostatic measurements were conducted under steady-state conditions for the polarization experiments. The cell was connected to a PEMFC test station equipped with an electric load (Fideris, 125 W, 20 V, 5 A). The MEAs were tested in a 5 cm2 single cell operating at 120 °C and 30% RH. Hydrogen and air gas fluxes were set at 1.5 and 2 times the stoichiometry, respectively, at a current density of 0.25 A/cm. Mass flow controllers from Brooks Instruments were employed to regulate the feed flow rate on both sides of the cell, while appropriate sensors measured the cell’s temperature and RH.
Results and discussion
Ion exchange capacity, water uptake and hydrolytic stability of nanocomposite membranes
Table 1 reports the Ion Exchange Capacity (IEC) values of the Nafion/MWCNTs-TiO2 nanocomposite membranes at varying filler loadings, alongside values for pristine Nafion, Nafion/MWCNTs, and Nafion/oxMWCNTs for comparison. The IEC is a crucial parameter that reflects the number of exchangeable functional groups and available protons, profoundly influencing proton transfer mechanisms within PEMs, particularly the Grotthuss mechanism. It is evident that the incorporation of MWCNTs does not significantly alter the IEC of Nafion, (0.87 meq/g vs. 0.88 meq/g) owing to the neutral nature of the CNTs filler. In contrast, the presence of oxygen-containing groups (-OH and -COOH) on the surface of the oxidized CNTs results in an increase in IEC to 0.93 meq/g for Nafion-oxMWCNTs. Notably, the IEC sees a further increase with the incorporation of MWCNTs/TiO2 nanofiller, attributed to the presence of charged TiO2 nanoparticles. It is essential to highlight that the IEC rises with increasing filler loading up to 3 wt%, reaching 0.97 meq/g, before decreasing for higher content (i.e., 0.92 meq/g at 5 wt%). This decline is likely due to particle agglomeration, which reduces the number of exchangeable groups available.
The variation in IEC is generally expected to impact the membrane’s hydrophilicity, thereby affecting its water uptake capacity (wu). However, the introduction of filler can also influence the microstructure, including size and shape, of the hydrophilic domains within a PEM, thereby playing a crucial role in altering its maximum adsorption capability. In Fig. 3, the wu at 25 °C is illustrated for the different membranes. Despite having a similar IEC to Nafion, the water uptake of Nafion/MWCNTs is notably lower, namely 20.1 wt%, due to the hydrophobic nature of the unmodified carbon nanotubes. Contrariwise, it increases to 29.5 wt% upon incorporation of oxMWCNTs, attributed to the hydrophilic nature of -OH and -COOH functional groups linked to the nanotubes, rendering the surface more hydrophilic [34]. Furthermore, the water uptake for PEMs containing MWCNTs-TiO2 material remains always lower than pristine Nafion, regardless of the filler loading, and although having higher IEC. It can be hypothesized that the synergy between long MWCNTs and TiO2 nanoparticles allows a physically interconnected network at the microscale, reducing the dimensional fluctuations of the hydrophilic clusters within the nanocomposite membranes. This phenomenon, potentially restricts the swelling capability of the ionic domains in Nafion, consequently limiting the absorption of water molecules [33].
It is important to clarify that while an adequate water content is crucial for efficient proton conduction, excessive swelling of the PEM is often associated with MEA deterioration and, in some instances, mechanical failure. In this regard, the hydrolytic stability of the Nafion-based electrolytes was assessed by examining the variation in water uptake (Δwu) as temperature increases. Figure 4 illustrates the temperature evolution of Δwu for the PEMs in the range of 20–80 °C. As expected, water uptake increases with heating. Thermal energy softens the polymer chains, resulting in a larger free volume and consequently a greater amount of absorbed water. However, the volume variation is substantial in the case of pristine Nafion and Nafion/MWCNTs, both reaching over 70% of Δwu. Significantly, upon the introduction of MWCNTs-TiO2 hybrid nanoparticles, the volume variation becomes markedly lower and below 30 wt%. This demonstrates that the filler particles notably enhance the hydrolytic stability of the resulting PEM, a crucial improvement in ensuring the membrane’s longevity and performance.
NMR investigation (self-diffusion coefficient and T1-relaxation time)
NMR techniques, including diffusometry and relaxometry, were used to investigate the molecular mobility of water molecules confined within the hydrophilic clusters of the nanocomposite membranes [35]. Specifically, the long-range mobility of water was probed through direct measurements of its self-diffusion coefficient (D) using the Pulse Field Gradient technique, while short-range movements were examined using the Inversion Recovery sequence to measure T1-relaxation times [36]. Figure 5 illustrates the temperature evolution, ranging from 20 to 130 °C, of the diffusivity of the various membranes. A strong correlation between water uptake and the self-diffusion coefficient of the PEMs exists, with higher water uptake leading to faster diffusion [37, 38]. Consequently, Nafion/MWCNTs exhibit the lowest diffusivity, indicating that the presence of unmodified MWCNTs as fillers in the polymer matrix deteriorates the membrane’s performance. Notably, in the low-temperature range (20–80 °C), D increases with temperature for all other Nafion-based membranes, attributed to the thermal energy absorbed by the water molecules. However, it begins to decrease as the temperature reaches 80–100 °C, primarily due to significant water evaporation from the membranes. Unlike conductivity measurements, where relative humidity is kept constant, in NMR measurements water evaporates as temperature increases. It is evident that the diffusion coefficient experiences a sharp drop above 80 °C in the case of pristine Nafion but decreases moderately for most of the nanocomposite membranes. More importantly, for NMT-3, no decrease but rather a plateau is observed, with the membrane retaining a diffusivity of about 1.6 х 10− 5 cm2 s− 1 at 130 °C. This value is almost one order of magnitude higher than that of pristine Nafion. This crucial feature may be attributed not only to the increased number of polar groups provided by the functionalized particles but also to the presence of TiO2 nanoparticles, which significantly enhances the water retention capability of the PEM. As a result, the membranes are able to maintain high diffusion values at elevated temperatures. In other words, they can retain a specific amount of hydration, which holds promise for enhancing proton transport even under dehydrating environment [39].
For each membrane, the Activation Energy (Ea), relating to the energy barrier for carrier transfer from one free site to another in the diffusion process, was calculated based on the Arrhenius plots (refer to Supplementary Figure S1) and is presented in Table 2. The incorporation of MWCNTs-TiO2 nanoparticles up to 3 wt% of filler loading reduces the Ea value from 14.4 kJ/mol for pristine Nafion to 11.5 kJ/mol for the nanocomposite membranes. This indicates that the presence of the hybrid nanomaterial enhances the efficiency of the diffusion process. However, higher filler content has detrimental effects on water mobility, as evidenced by the notable increase in the activation energy associated with water mobility inside NMT-5, which exhibits the highest Ea of 19.32 kJ/mol. It is likely that excessive loading with the hybrid nanoparticles partially obstructs the hydrophilic channels in such nanocomposite membrane.
The investigation of Spin-lattice relaxation times (T1) provides additional insights into water mobility within the nanocomposite membranes. T1 reflects the rate of energy transfer from the nuclear spin system to the lattice (surrounding molecules). The movement of nuclei in the lattice generates a fluctuating magnetic field (commonly dipole-dipole interaction) at the location of the nuclear spin. The efficiency of molecular rotational and translational movement is determined by the strength of this interaction. Consequently, higher T1 values indicate more efficient movement or easier molecular motion, with T1 typically increasing as the temperature rises [40]. Figure 6 illustrates the temperature dependence of T1 for water confined in both pristine Nafion and nanohybrid membranes. Overall, the T1 values are significantly longer in the nanocomposite membranes compared to the recast Nafion, except for the Nafion/MWCNTs nanocomposite. This suggests that the presence of MWCNTs-TiO2 nanohybrid promotes higher mobility even at a molecular scale. Among these membranes, the highest values were observed in the membrane with 3 wt% loading, indicating that at this filler content, the long carbon nanotubes decorated with titania nanoparticles facilitate.
Proton conductivity of the NMTx membranes
The proton conductivity (σ) of both pristine Nafion and composite membranes was measured under varying Relative Humidity percentages (from 20 to 100% RH) at 30 °C and 80 °C, as shown in Fig. 7(a) and Fig. 7(b), respectively. At 30 °C, the pristine Nafion membrane exhibits proton conductivity ranging from 0.78 mS cm− 1 under 20% RH to 32.5 mS cm− 1 at 100% RH. This conductivity increases significantly to 87.14 mS cm− 1 at 80 °C and 100% RH. For both temperatures, all nanocomposite membranes demonstrate higher proton conductivity compared to Nafion across the entire range of RHs investigated, indicating that the MWCNTs-TiO2 nanohybrid plays a direct role in proton conduction, providing superior conductivity performance. Among the nanohybrid membranes, NMT-3 yields the highest conductivity, reaching a value of 118.3 mS cm− 1 at 80 °C and fully humidified conditions. However, the performance of this hybrid membrane becomes particularly interesting when the relative humidity is extremely low, which is a crucial requirement for the development of PEMFCs able to operate under harsh conditions, such as high temperature and low humidity. Indeed, this nanocomposite membrane demonstrates a proton conductivity of 9.3 mS cm− 1 under 20% RH, which is seven times higher than the Nafion benchmark (1.6 mS cm− 1). This remarkable result can be attributed to the aforementioned combination of very long nanotubes decorated with titania nanoparticles, which have the ability to retain a large amount of water molecules. Furthermore, nanosized TiO2 particles provide additional proton conducting sites. This, in turn, facilitates the formation of additional pathways for proton conduction through the Grotthuss mechanism, as illustrated in Fig. 8.
H2/air fuel cell tests under low-RH condition
Based on the conductivity results, NMT-3 was tested in a single H2/air PEMFC, with active electrode area of 5 cm2 and 0.5 mg Pt/cm2 loading at each electrode. Figure 9 illustrates the resulting polarization and power density curves at 120 °C and 30% RH, in comparison with Nafion 212 as benchmark (due to similar thickness, i.e., 50 μm). The harsh operating conditions were selected to evaluate the potential of NMT-3 to meet the US DOE (Department of Energy) targets for high temperature PEMFCs. A comparison of the open circuit voltages (OCV) shows that NMT-3 has higher OCV (0.977 V) than the cell based on commercial Nafion 212 (0.908 V). This indicates the incorporation of MWCNTs-TiO2 inside the Nafion matrix decreases fuel permeability which hold promise for enhanced fuel utilization and lower peroxide formation. At 0.7 V, the Nafion benchmark yields a current density of 63.7 mA/cm2, and the value is in a good agreement with literature data [41,42,43]. Noteworthy, the presence of hybrid nanoparticles in the PFSA membrane retains its hydration resulting in higher performance at elevated temperature and low humidification. De facto, at the same potential, NMR-3 exhibited remarkably higher current density reaching a value of 392.2 mA/cm2. As concern the power density, the higher proton conductivity and thus the lower ohmic resistance enable the cell based on NMT-3 to yield a peak power density of 307.7 mW/cm2, which is more than 3-fold higher than the Nafion 212 benchmark cell (92.2 mW/cm2). The evidences above, clearly demonstrated the huge potential of MWCNTs-TiO2 nanoparticles to extend the operating temperature of Nafion.
Conclusions
Hygroscopic TiO2 nanoparticles were directly grown onto commercial MWCNTs and the resulting hybrid nanomaterial successfully doped inside the Nafion matrix to prepare novel nanocomposite membranes for high temperature/low humidity fuel cell. At optimal loadings, incorporation of MWCNTs-TiO2 nanohybrid resulted in increased hydrolytic stability while promoting higher water self-diffusion coefficient and T1 relaxation times indicating an overall improvement of the transport properties. The features facilitated an impressive enhancement of proton conductivity in the composite membrane under both fully humid and dry conditions. Compared to recast Nafion, the NMT-3 nanocomposite yielded 7 times higher proton conductivity at 80 °C and 20% RH (i.e., 9.3 mS cm− 1 and 1.6 mS cm− 1, respectively) and 3-fold higher the peak power density of commercial Nafion 212 used as benchmark (307.7 mW/cm2 vs. 92.2 mW/cm2 for single H2/air fuel cell operating at 120 °C and 30% RH). The nanosized TiO2 particles decorating CNTs acted as a water reservoir under dehydrating environment while simultaneously providing additional proton conductive paths even under high temperature and low humidification, successfully extending the operating conditions of Nafion.
Data availability
Data are available on request.
References
Di Noto, V., Zawodzinski, T.A., Herring, A.M., Giffin, G.A., Negro, E., Lavina, S.: Polymer electrolytes for a hydrogen economy. Int. J. Hydrogen Energy. 37, 6120–6131 (2012). https://doi.org/10.1016/j.ijhydene.2012.01.080
Andújar, J.M., Segura, F.: Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 13, 2309–2322 (2009). https://doi.org/10.1016/j.rser.2009.03.015
Astafev, E.A., Ukshe, A.E., Manzhos, R.A., Dobrovolsky, Y.A., Lakeev, S.G., Timashev, S.F.: Flicker noise spectroscopy in the analysis of electrochemical noise of hydrogen-air PEM fuel cell during its degradation. Int. J. Electrochem. Sci. 12, 1742–1754 (2017). https://doi.org/10.20964/2017.03.56
Dijoux, E., Steiner, N.Y., Benne, M., Péra, M.C., Pérez, B.G.: A review of fault tolerant control strategies applied to proton exchange membrane fuel cell systems. J. Power Sources. 359, 119–133 (2017). https://doi.org/10.1016/j.jpowsour.2017.05.058
Rosli, R.E., Sulong, A.B., Daud, W.R.W., Zulkifley, M.A., Husaini, T., Rosli, M.I., Majlan, E.H., Haque, M.A.: A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy. 42, 9293–9314 (2017). https://doi.org/10.1016/j.ijhydene.2016.06.211
Yang, C., Costamagna, P., Srinivasan, S., Benziger, J., Bocarsly, A.B.: Approaches and technical challenges to high temperature operation of proton exchange membrane fuel cells. J. Power Sources. 103, 1–9 (2001). https://doi.org/10.1016/S0378-7753(01)00812-6
Zhang, J., Tang, Y., Song, C., Cheng, X., Zhang, J., Wang, H.: PEM fuel cells operated at 0% relative humidity in the temperature range of 23–120°C. Electrochim. Acta. 52, 5095–5101 (2007). https://doi.org/10.1016/j.electacta.2007.02.002
Shao, Z.G., Joghee, P., Hsing, I.M.: Preparation and characterization of hybrid nafion-silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells. J. Memb. Sci. 229, 43–51 (2004). https://doi.org/10.1016/j.memsci.2003.09.014
He, X., He, G., Zhao, A., Wang, F., Mao, X., Yin, Y., Cao, L., Zhang, B., Wu, H., Jiang, Z.: Facilitating Proton Transport in Nafion-based membranes at low humidity by incorporating multifunctional Graphene Oxide nanosheets. ACS Appl. Mater. Interfaces. 9, 27676–27687 (2017). https://doi.org/10.1021/acsami.7b06424
Mabrouk, W., Charradi, K., Kacem, I., Ben, Lafi, R., Bellakhal, N., Marzouki, R., Keshk, S., M.A.S: Significant augmentation of proton conductivity in low sulfonated polyether sulfone octyl sulfonamide membranes through the incorporation of hectorite clay. Mater. Renew. Sustain. Energy. (2024). https://doi.org/10.1007/s40243-023-00251-6
Charradi, Mabrouk, W., Kacem, I., Ben, Bellakhal, N., Al-Ghamdi, Y.O., Marzouki, R., Keshk, S.M.A.S.: Incorporation of multilayered double hydroxides/sepiolite augments proton conductivity performance in low sulfonated polyether sulfone octyl sulfonamide. Mater. Renew. Sustain. Energy. (2024). https://doi.org/10.1007/s40243-024-00256-9
Ramadan, M., Elnouby, M.S., El-Shazly, O., El-Wahidy, E.F., Farag, A.A.M., Roushdy, N.: Facile fabrication, structural and electrical investigations of cadmium sulfide nanoparticles for fuel cell performance. Mater. Renew. Sustain. Energy. 11, 277–286 (2022). https://doi.org/10.1007/s40243-022-00220-5
Vinothkannan, M., Hariprasad, R., Ramakrishnan, S., Kim, A.R., Yoo, D.J.: Potential bifunctional filler (CeO2-ACNTs) for nafion matrix toward extended electrochemical power density and durability in proton-exchange membrane fuel cells operating at reduced relative humidity. ACS Sustain. Chem. Eng. 7, 12847–12857 (2019). https://doi.org/10.1021/acssuschemeng.9b01757
Gerasimova, E., Safronova, E., Ukshe, A., Dobrovolsky, Y., Yaroslavtsev, A.: Electrocatalytic and transport properties of hybrid Nafion® membranes doped with silica and cesium acid salt of phosphotungstic acid in hydrogen fuel cells. Chem. Eng. J. 305, 121–128 (2016). https://doi.org/10.1016/j.cej.2015.11.079
Ketpang, K., Lee, K., Shanmugam, S.: Facile synthesis of porous metal oxide nanotubes and modified nafion composite membranes for polymer electrolyte fuel cells operated under low relative humidity. ACS Appl. Mater. Interfaces. 6, 16734–16744 (2014). https://doi.org/10.1021/am503789d
Lanin, S.N., Vlasenko, E.V., Kovaleva, N.V., Zung, F.T.: The adsorption properties of titanium dioxide. Russ J. Phys. Chem. A. 82, 2152–2155 (2008). https://doi.org/10.1134/S0036024408120315
Santiago, E.I., Isidoro, R.A., Dresch, M.A., Matos, B.R., Linardi, M., Fonseca, F.C.: Nafion-TiO2 hybrid electrolytes for stable operation of PEM fuel cells at high temperature. Electrochim. Acta. 54, 4111–4117 (2009). https://doi.org/10.1016/j.electacta.2009.02.040
Baglio, V., Aricò, S., Blasi, Di, Antonucci, V., Antonucci, P.L., Licoccia, S., Traversa, E., Fiory, F.S.: Nafion–TiO2 composite DMFC membranes: Physico-chemical properties of the filler versus electrochemical performance. Electrochim. Acta. 50, 1241–1246 (2005). https://doi.org/10.1016/j.electacta.2004.07.049
Barbora, L., Acharya, S., Verma, A.: Synthesis and ex-situ characterization of Nafion TiO2 Composite membranes for.pdf. Macromol. Symp. 277, 177–189 (2009)
Daiko, Y., Klein, L.C., Kasuga, T., Nogami, M.: Hygroscopic-oxides/Nafion® hybrid electrolyte for direct methanol fuel cells. J. Memb. Sci. 281, 619–625 (2006). https://doi.org/10.1016/j.memsci.2006.04.033
Chen, S.Y., Han, C.C., Tsai, C.H., Huang, J., Chen-Yang, Y.W.: Effect of morphological properties of ionic liquid-templated mesoporous anatase TiO2 on performance of PEMFC with Nafion/TiO2 composite membrane at elevated temperature and low relative humidity. J. Power Sources. 171, 363–372 (2007). https://doi.org/10.1016/j.jpowsour.2007.06.047
Nicotera, I., Khalfan, A., Goenaga, G., Zhang, T., Bocarsly, A., Greenbaum, S.: NMR investigation of water and methanol mobility in nanocomposite fuel cell membranes. Ionics (Kiel). 14, 243–253 (2008). https://doi.org/10.1007/s11581-007-0178-8
Dorothy, A.A., Panigrahi, P.: First principles study of optical properties of Ni- and Pd-doped TiO2 as visible light catalyst. Mater. Renew. Sustain. Energy. 12, 47–52 (2023). https://doi.org/10.1007/s40243-023-00228-5
González-Verjan, V.A., Trujillo-Navarrete, B., Félix-Navarro, R.M., de León, J.N.D., Romo-Herrera, J.M., Calva-Yáñez, J.C., Hernández-Lizalde, J.M., Reynoso-Soto, E.A.: Effect of TiO2 particle and pore size on DSSC efficiency. Mater. Renew. Sustain. Energy. 9, 1–8 (2020). https://doi.org/10.1007/s40243-020-00173-7
Poiana, R., Lufrano, E., Tsurumaki, A., Simari, C., Nicotera, I., Navarra, M.A.: Safe gel polymer Electrolytes for High Voltage Li-Batteries. Electrochim. Acta. 401, 139470 (2021). https://doi.org/10.1016/j.electacta.2021.139470
Simari, C., Lufrano, E., Godbert, N., Gournis, D., Coppola, L., Nicotera, I.: Titanium dioxide grafted on graphene oxide: Hybrid nanofiller for effective and low-cost proton exchange membranes. Nanomaterials. 10, 1–18 (2020). https://doi.org/10.3390/nano10081572
Tursi, A., Beneduci, A., Nicotera, I., Simari, C.: MWCNTs decorated with TiO2 as highly performing Filler in the Preparation of Nanocomposite membranes for Scalable Photocatalytic Degradation of Bisphenol A in Water. Nanomaterials. 13 (2023). https://doi.org/10.3390/nano13162325
Calva-Yáñez, J.C., de la Solís, M., Ramírez-Vargas, M., Rincón, M.E.: Photoelectrochemical performance and carrier lifetime of electrodes based on MWCNT-templated TiO2 nanoribbons. Mater. Renew. Sustain. Energy. 7, 1–6 (2018). https://doi.org/10.1007/s40243-018-0126-8
Enotiadis, A., Boutsika, L.G., Spyrou, K., Simari, C., Nicotera, I.: A facile approach to fabricating organosilica layered material with sulfonic groups as an efficient filler for polymer electrolyte nanocomposites. New. J. Chem. 41 (2017). https://doi.org/10.1039/c7nj01416c
Tanner, J.E.: Use of the stimulated echo in NMR diffusion studies. J. Chem. Phys. 52, 2523–2526 (1970)
Nicotera, I., Kosma, V., Simari, C., Ranieri, G.A., Sgambetterra, M., Panero, S., Navarra, M.A.: An NMR study on the molecular dynamic and exchange effects in composite Nafion/sulfated titania membranes for PEMFCs. Int. J. Hydrogen Energy. 40, 14651–14660 (2015). https://doi.org/10.1016/j.ijhydene.2015.06.137
Simari, C., Prejanò, M., Lufrano, E., Sicilia, E., Nicotera, I.: Exploring the structure–performance relationship of Sulfonated Polysulfone Proton Exchange membrane by a combined computational and experimental Approach. Polym. (Basel). 13, 959 (2021). https://doi.org/10.3390/polym13060959
Simari, C., Lufrano, E., Brunetti, A., Barbieri, G., Nicotera, I.: Highly-performing and low-cost nanostructured membranes based on Polysulfone and layered doubled hydroxide for high-temperature proton exchange membrane fuel cells. J. Power Sources. 471, 228440 (2020). https://doi.org/10.1016/j.jpowsour.2020.228440
Tohidian, M., Ghaffarian, S.R.: Surface modified multi-walled carbon nanotubes and nafion nanocomposite. Polym. Adv. Technol. 29, (2018)
Zelovich, T., Vogt-Maranto, L., Simari, C., Nicotera, I., Hickner, M.A., Paddison, S.J., Bae, C., Dekel, D.R., Tuckerman, M.E.: Non-monotonic temperature dependence of Hydroxide Ion Diffusion in Anion Exchange membranes. Chem. Mater. 34, 2133–2145 (2022). https://doi.org/10.1021/acs.chemmater.1c03594
Munoz, S., Greenbaum, S.: Review of recent nuclear magnetic resonance studies of ion transport in polymer electrolytes. Membr. (Basel). 8, 1–23 (2018). https://doi.org/10.3390/membranes8040120
Hallberg, F., Vernersson, T., Pettersson, E.T., Dvinskikh, S.V., Lindbergh, G., Furó, I.: Electrokinetic transport of water and methanol in Nafion membranes as observed by NMR spectroscopy. Electrochim. Acta. 55, 3542–3549 (2010). https://doi.org/10.1016/j.electacta.2010.01.064
Nicotera, I., Coppola, L., Rossi, C.O., Youssry, M., Ranieri, G.A.: NMR investigation of the dynamics of confined water in nafion-based electrolyte membranes at subfreezing temperatures. J. Phys. Chem. B. 113, 13935–13941 (2009). https://doi.org/10.1021/jp904691g
D’Epifanio, A., Navarra, M.A., Christoph Weise, F., Mecheri, B., Farrington, J., Licoccia, S., Greenbaum, S.: Composite nafion/sulfated zirconia membranes: Effect of the filler surface properties on proton transport characteristics. Chem. Mater. 22, 813–821 (2010). https://doi.org/10.1021/cm901486t
Slichter, C.: Principles of Magnetic Resonance., New York (1990)
Kim, Y., Ketpangr, K., Jaritphun, S., Park, S., Shanmugam, S.: A polyoxometalate coupled graphene oxide–nafion composite membrane for fuel cells operating at low relative humidity. J. Mater. Chem. A. 3, 8148–8155 (2015)
Vinothkannan, M., Kim, A.R., Gnana Kumar, G., Yoo, D.J.: Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 8, 7494–7508 (2018). https://doi.org/10.1039/c7ra12768e
Patel, H.A., Mansor, N., Gadipelli, S., Brett, D.J.L., Guo, Z.: Superacidity in Nafion/MOF hybrid membranes retains Water at Low Humidity to enhance Proton Conduction for fuel cells. ACS Appl. Mater. Interfaces. 8, 30687–30691 (2016). https://doi.org/10.1021/acsami.6b12240
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Isabella Nicotera: validation, resources, writing—review and editing, project administration, funding acquisition; Luigi Coppola: formal analysis, data curation, writing—original draft preparation, visualization; Cataldo Simari: Conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nicotera, I., Coppola, L. & Simari, C. Novel Nafion nanocomposite membranes embedded with TiO2-decorated MWCNTs for high-temperature/low relative humidity fuel cell systems. Mater Renew Sustain Energy (2024). https://doi.org/10.1007/s40243-024-00266-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-024-00266-7