Abstract
This work presents the synthesis and evaluation of Pd-Ni aerogels toward the urea oxidation reaction (UOR). The incorporation of Ni led to a 0.13 V reduction in the energy required for the oxidation and reduction of PdO compared to monometallic Pd, both in alkaline medium with and without urea. Varying the Ni ratios in Pd (Pd-Ni 4:1, Pd-Ni 1:1, and Pd-Ni 1:4) led to significant changes in the electrochemical behaviour. In alkaline medium without urea, PdNi 4:1 showed the formation of NiOOH at 1.35 V, which promoted oxygen diffusion on the electrode surface and increased the current density, confirming the increase in the active sites of NiOOH and NiPdOOH and enabling urea-based electrolysis at these sites. While palladium aerogels alone are ineffective for UOR, the presence of nickel plays a key role in enhancing the UOR efficiency. On the other hand, physicochemical characterisation revealed that PdNi 4:1 has a crystal size of 4.37 nm and a larger shift in the 2θ positions of the (111) and (200) planes, which favours electronic changes that were investigated by XPS. These changes affected the electrocatalytic activity, which is primarily related to electronic effects. The results of SEM and TEM studies and nitrogen adsorption-desorption isotherm confirmed that the aerogels are highly porous and have an effective surface area and abundant active sites for reactions that allow efficient mass transfer and low diffusion resistance. TEM observations revealed interconnected nanochains indicating optimal electrocatalytic activity for both ORR and UOR due to high mass transfer. These interconnected networks are crucial for improving electrocatalytic activity in the urea oxidation reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are a plethora of different ways of generating energy that can replace the fossil fuels that currently exist in the world. The growing demand for energy has led researchers to improve electrochemical devices for energy production. Electrochemical water splitting leads to an oxygen evolution reaction (OER) which is the most environmentally sustainable approach to bypassing the use of conventional fossil fuels. But OER is a very slow process, so it has low kinetics and requires a higher overvoltage due to the four-electron transfer. This discourages industrialists from utilising the OER process. To solve this problem and increase the activity of OER, we need to use precious metal-based electrocatalysts, such as palladium (Pd), which is chosen for this type of application due to its high electrocatalytic activity and stability [1]. The palladium atoms can be arranged in such a way that they can be manipulated to obtain the shape and size of the particles for the generation of different active sites, thus increasing the exposed surface area. Various nanostructured palladium materials such as nanoparticles [2], nanowire arrays [3], core-shell materials [4] have been widely explored, to name a few. Palladium aerogels are self-supporting mesoporous 3-dimensional nanostructured materials characterised by low density with a high number of active sites, reducing the required mass of the electrocatalyst while still achieving high catalytic activity [5,6,7].
However, palladium itself is still scarce and very expensive, so it is not profitable to use it in large quantities. Transition metals such as nickel can be used together with Pd, resulting in a bimetallic electrocatalyst that exhibits improved electrocatalytic activity, higher steady-state anodic current densities and greater tolerance to CO poisoning [8,9,10]. This nickel-containing palladium aerogel can improve the electrocatalytic activity of the complex.
Considering that the rate of the cathodic hydrogen evolution reaction (HER) in water electrolysis is conditioned by the corresponding anodic oxygen evolution reaction (OER) [11]. Since the kinetics of the 4-electron transfer process in the OER is slow and requires a higher overpotential, the overall efficiency of the water electrolysis process is low and the energy consumption increases. However, by using the urea oxidation reaction (UOR CO(NH2)2 + 6OH- →N2 + CO2 + 5H2O + 6e-) as the anode to assist in water splitting and H2 production, the theoretical voltage (0.37 V vs. RHE) is significantly lower than the voltage of conventional water electrolysis (1.23 V vs. RHE). This enables the production of H2 with lower energy consumption [11]. The slow six-electron transfer and the complicated gas evolution steps in UOR lead to slow kinetics. Therefore, nickel-containing palladium aerogels can be efficiently utilized for the urea oxidation process in such a way that the Ni species adopts a high valence state and thus increases the catalytic activity of the UOR. The palladium-nickel aerogel on the electrode is oxidised by applying a higher potential, which leads to the formation of a higher valence state of the nickel species. This supports the degradation of urea to nitrogen and CO2. To enable the oxidation of the urea, the nickel site on the electrode surface must be specifically activated. The nickel species in the Pd/Ni catalyst can capture protons in an alkaline medium and thus improve the electron transfer and therefore the UOR kinetics. The ratio of Pd and Ni is very crucial for enhancing the electrochemical performance and electrical conductivity. In this paper, a series of self-supporting three-dimensional mesoporous PdNi aerogels are synthesised by sol-gel and microwave heating to improve the electrocatalytic performance of urea oxidation reaction in alkaline media. This aerogel led to the formation of the mesoporous catalyst, which results in faster electron transfer during the electrocatalytic reaction.
Materials and methods
Synthesis of aerogels
Aerogels are synthesised by changing the stoichiometry of noble and transition metals. The procedure is as follows: 20 ml of 2 mg/L of PdCl2 (99% Sigma-aldrich) and NiCl2.6H2O (99% Sigma-aldrich) with the volume ratios as shown in Table 1 were taken and then reduced with a solution of sodium carbonate (99.5%, JT Baker) and glyoxylic acid monohydrate (98% Sigma Aldrich) using a microwave method.
[25]. During the synthesis, a microwave heater (MW) was used as a heat source connected to a PID controller. A thermocouple was introduced into the precursor mixture to maintain a temperature of 67 °C at atmospheric pressure in a closed system. The duration of the synthesis was set to 7 h, during which the nanoparticles were formed and the gelation process reached its peak.
The removal of by-products and unreacted components from the PdXNiX aerogels was possible by repeated washing with deionised water, ethanol and acetone. Finally, the aerogels were freeze-dried wiht liquid nitrogen and the solvent (deionised water) was completely removed in a lyophilizer maintained at -110 0C for 24 h.
Physico-chemical characterisation
The structural characterisation of the aerogels to understand the morphology of the gels was possible with a scanning electron microscope (SEM, manufactured by Quanta FEG 650 microscopes from FEI). The crystal planes and lattice structure were analysed by X-ray diffraction (XRD; D8-Advance diffractometer Bruker) at a step size of 0.02º 2θ. Nitrogen adsorption-desorption isotherms at -196ºC (Micromeritics ASAP 2020) were used to determinate the specific surface area of the aerogels and the pore size was also analysed when the gels were degassed at 120 0C for one night. The surface analysis of the aerogels was performed using X-ray photoelectron spectroscopy (XPS; K-Alpha + spectrometer equipped with the Avantage Data System from Thermo Scientific™).
Electrochemical measurements
Electrocatalytic activity in half-cell configuration
For the electrochemical analysis of the aerogels, the Biologic VMP3 potentiostat/galvanostat was used, which works with a cell with three electrodes and 20 mV s− 1 scan rate. The three electrodes used in the cell were a glass-carbon electrode (3 mm) (working electrode), an Ag/AgCl electrode (reference electrode) and a Pt wire (counter electrode). The Pd-Ni aerogel inks were prepared from each type of aerogels mixed in 200 µL deionised water and 50 µL Nafion® (5%) per milligramme of aerogels. After sonication of each of the four types of ink, only 10 µL were used for electrode preparation. There were two sets of electrolytes, one with 1 M KOH solution and the other with 1 M KOH solution mixed with urea, and finally the concentration of urea solution was increased to 0.3 M. For both solutions, 50 mL were used in the cell and purged with nitrogen for thirty minutes to remove dissolved oxygen. Subsequently, electrochemical analysis was performed on all four different types of gels using the 1 M KOH solution in the presence and absence of urea at room temperature.
Results and discussions
The different volume ratios of Pd-Ni aerogels were prepared as follows: (a) Pd, (b) Pd-Ni (4:1), (c) Pd-Ni (1:1) and (d) Pd-Ni (1:4). The aerogels were synthesised using the microwave method and then freeze-dried. The physicochemical properties of the aerogels were investigated by N2 adsorption/desorption isotherms, XRD, SEM, TEM, HR-TEM and X-ray photoemission spectroscopy (XPS) as well as electrochemical analysis.
The crystalline nature of the aerogels was analysed using X-ray diffraction patterns. The diffraction patterns of the aerogels are shown in Fig. 1. The diffraction peaks appear at the 2θ positions 40.15°, 46.80°, 68.29° and 82.28°, which correspond to the planes (111), (200), (220) and (311) and are characteristic of palladium with a face-centred cubic crystal system (JCPDS No. 01-087-0641) [12, 13]. Pd, which has (111) as a sharp peak, is the most important facet involved in the active oxidation of organic molecules. When nickel is incorporated into palladium, a decrease intensity and a broadening of the Pd peaks corresponding to (111), (200), and (220) is observed, along with shifts in the 2θ positions, especially in the (111) and (200) planes, with increasing Ni content in the aerogels. Nickel atoms displace several Pd atoms, leading to in an expansion of the lattice due to the proximity between the two metals. These changes induced by the addition of Ni alter the electronic properties of Pd (which will be studied by XPS), possibly affecting the adsorption energies of urea [9]. The absence of signals from nickel oxides and hydroxides in the PdNi 4:1 ratio indicates that the Ni atoms are in amorphous form or the concentration is very low, as signals from nickel are observed in the PdNi 1:1 and PdNi 1:4 ratios [14]. The XRD of Pd-Ni (1:1) and Pd-Ni (1:4) exhibits the presence of Ni in the patterns with (100) and (103) facets, which agree well with JCPDS No. 04-0850 for Ni. In addition, using the Scherrer equation [15], the crystallite sizes for Pd, Pd-Ni (4:1), Pd-Ni (1:1) and Pd-Ni (1:4) were determined to be 9.88, 4.37, 3.43 and 2.78 nm,, respectively. The size of the crystallites decreased in the following order: Pd > Pd-Ni (4:1) > Pd-Ni (1:1) > Pd-Ni (1:4), indicating that the addition of Ni decreases the size of Pd.
Figure 2 shows the isotherms for the nitrogen adsorption, the desorption of the aerogels and the determination of the pore size of Pd, Pd-Ni (4:1), Pd-Ni (1:1) and Pd-Ni (1:4). The hysteresis curve of all four combinations shows a type IV adsorption/desorption hysteresis, which indicates that the aerogels have an ordered mesoporous structure of the Pd and Pd-Ni combinations. The adsorption isotherms shown in Fig. 2 displays multi-layered adsorption of nitrogen and even at higher relative pressures no visual plateau can be recognised, which indicates that the materials predominantly have a meso- or macroporous structure. From the graphs in Fig. 2 it can be seen that the bimetallic Pd-Ni aerogels have a larger surface area, which can be confirmed by SEM and TEM images. The pore size distributions of the aerogels confirm that there are micro-, meso- and macropores with pores ranging from 2 to 100 nm in size. Therefore, the diffusion barriers in such hierarchical porous systems are minimised and play an important role in catalytic applications.
The morphological structure of the mesoporous Pd and Pd-Ni network was determined using a scanning electron microscope. According to the SEM images in Fig. 3, the aerogels have an open-pored 3D network structure. In contrast to Pd-Ni (1:1) and Pd-Ni (1:4), the Pd and Pd-Ni (4:1) aerogels exhibit remarkable mesopores, which have channels and thus favour mass diffusion processes. This is consistent with the results of the surface analysis discussed above.
In the case of the Pd-Ni (1:1) and Pd-Ni (1:4) samples, these aerogels have a spider web-like appearance with fine fibres; the particle size is smaller than that of the aerogels with a higher nickel content. Another notable change due to the addition of Ni is the distribution. In aerogels with more Ni, the particles have a less homogeneous distribution and isolated chains. This architecture allows for increased interaction with the electrolyte, exposing extensive active sites for the urea oxidation reaction. These active sites are responsible for improved electron transfer kinetics and maintain the stability of the mesoporous structures during the inimic electrochemical cycling process.
In addition to SEM, transmission electron microscopy (TEM) was also used to understand the morphological properties of the aerogels. Figure 4 shows TEM images of both monometallic (palladium aerogel) and bimetallic (palladium and nickel aerogel) three-dimensional structures with an extensive network of nanochains. These nanochains form an interconnected network with an abundance of open pores and channels. Figure 4a, c, e and g show the palladium, Pd-Ni (4:1), Pd-Ni (1:1) and Pd-Ni (1:4) aerogels respectively, which clearly present the extended network of nanochains.
HR-TEM images of the four different types of aerogels were analysed in Fig. 4 (b, d, f and h) to determine the crystalline structure and interplanar spacing. All four images of palladium, Pd-Ni (4:1), Pd-Ni (1:1), Pd-Ni (1:4) aerogels show a face-centred cubic (FCC) structure and (111) and (200) lattice planes, which are mainly visible along with the stacking faults and lattice boundaries in the aerogels. The most important parameter for any bimetallic aerogel is its chemical reactivity and catalytic activity. Such aerogels, which have lattice boundaries and stacking faults due to the interdiffusion of the metal atoms, thus also become active sites for many types of reactions such as the urea oxidation reaction. Palladium and nickel have different lattice parameters and therefore interdiffusion of the metal atoms of palladium with nickel atoms occurs, creating a three-dimensional network of the bimetallic aerogel. The distances between the lattice planes (111) and (200) for the four mono- and bimetallic aerogels are listed in Table 2. By applying Bragg’s law [16], the interplanar distances with minimal changes during synthesis were identified. These small variations lead to electronic changes that are further investigated by XPS. In previous studies [17, 18], changes in interplanar spacing were observed at maximum compositions of PdNi 1:1 where only Pd peaks were detected. This could be due to the synthesis method (polyol method), where Ni peaks may not be visible due to their low concentration or because they are in amorphous form. In addition, changes in composition were detected, with certain alloying fractions obtained by synthesis methods without exceeding the 1:1 ratio with PdNi. Therefore, it is possible that the material appears as a mixture and the slight variations in lattice parameters, crystallite size and peak shifts in the (111) and (200) planes can be attributed to the proximity between Ni and Pd. This is because Ni penetrates the Pd lattice due to its proximity and thus alters the electronic properties observed by XPS of Pd.
High-resolution XPS was performed to analyse the components and the electronic state of the surface of these aerogels in more detail (Fig. 5). Before analysing, it is important to mention that all spectra were calibrated using the C 1s peak at 284.5 eV, while the Shirley baseline was used to remove the background and the Gaussian and Lorentz functions was used to deconvolve the peaks. The overall spectrum of the samples is shown in Fig. 5a and confirms the presence of C, Pd and Ni. Figure 5b-d displays the Pd 3d spectra and compares the oxidation states in the PdNi samples with different compositions. Each spectrum was deconvoluted into the Pd 3d5/2 and Pd 3d3/2 orbitals and shows peaks at 337 eV and 342.35 eV, indicating Pd2+. In contrast, the peak at 335.41 eV and 340.7 eV show a more pronounced presence of Pd0 and, to a lesser extent, PdO, possibly due to environmental exposure [19,20,21]. However, the Pd0 3d5/2 showed a slight shift to a higher binding energy, + 0.3 eV for PdNi 4:1; these chemical changes indicate a modification of the electronic structure of the Pd atoms induced by electron transfer between Ni and Pd atoms, these changes may modify the adsorption energies of urea [22]. The Ni 2p spectrum can be seen in Fig. 5e-g, deconvoluted into Ni 2p3/2 and Ni 2p1/2, at 855 eV and 872.48 eV, corresponding to Ni2+, while the peaks at 856.5 eV and 874.0 eV refer to Ni3+, along with their satellite structure (labelled as Sat.) for both materials. The XPS peaks at bond energies 855 eV and 856.5 eV correspond to Ni(OH)2 and NiOOH, respectively [23,24,25,26]. This indicates that the Ni species in all samples consist mainly of Ni(OH)2 and NiOOH. The atomic percentage calculated for the PdNi 1:1 material is 22.44% for Ni and 16.08% for Pd, while for PdNi 1:4 it indicates 22.5% Ni and 5.7% Pd. Finally, PdNi 4:1 exhibits 8.7% Ni and 34.6% Pd, which is consistent with their different compositions.
Electrochemical measurements
Pd-Ni aerogel samples were analysed by cyclic voltammetry with a three-electrode cell in alkaline medium (1 M KOH) with and without urea solution. The electrochemical profiles are shown in Fig. 6. In previous reports, the peaks of hydrogen desorption and adsorption are in the potential ranges of 0.30 to 0.54 V vs. RHE and 0.11 to 0.18 V vs. RHE, respectively [27, 28]. Similarly, the formation of Pd (II) oxides takes place in the profiles between 1.3 and 1.4 V vs. RHE, while the reduction of these oxides is indicated by a reduction peak between 0.27 and 0.50 V vs. RHE [29, 30]. Figure 6 shows a change in the current for the different aerogels. The current at 0.5 V decreases (this is the characteristic peak of Pd) when the sample has a larger amount of nickel in the structure.
In the KOH profiles, a small current peak in the range of 1.4–1.52 V vs. RHE can be seen, which is associated with the characteristic of the Ni (II)/Ni(III) species as a function of reaction (1). When urea is present in the solution, the current increases noticeably.
When Ni is incorporated into the aerogels, the anodic Pd combines with the anodic NiOOH, resulting in NiPdOOH as the active site. Reactions (2) and (3) are fairly obvious as a consequence and then regenerate NiPd(OH)2, which in turn can oxidise another molecule of urea.
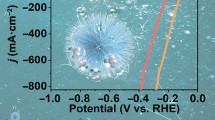
The polarisation curve in Fig. 6c shows that 1.72, 1.69, 1.61 and 1.73 V vs. RHE are enough for Pd, Pd-Ni (4: 1), Pd-Ni (1:1) and Pd-Ni (1:4) towards the OER to reach 10 mAcm− 2 of current density.
This difference is due to the presence of nickel in the aerogel, which improves the transfer of OH− ions to the surface of the electrode. For the OER-urea (Fig. 6d), the current density is achieved at 1.48, 1.35, 1.49 and 1.44 V vs. RHE for Pd, Pd-Ni (4:1), Pd-Ni (1:1), and Pd-Ni (1:4), respectively. This catalyst behavior with and without urea at 10 mA cm− 2 is illustrated in Fig. 6e.
The preliminary stability tests are shown in Fig. 7. The current density decreases rapidly for all four aerogels. However, the Pd-Ni (1:1) and (1:4) samples take less time to reach a stable state. The noise is due to the formation of N2 and CO2 bubbles on the surface of the electrode [31]. The Pd and Pd-Ni (1:1) aerogels exhibit a current density of ~ 21 mA cm− 2, and the Pd-Ni (4:1) and Pd-Ni (1:1) aerogels present a current density of ~ 29 mA cm− 2. NiOOH is the main active site for the OER –urea electrolysis as there are a large number of active sites that can increase the current.
All the above characterisations including the electrochemical studies clearly show that the Pd-Ni aerogels are a classical candidate for the urea oxidation reaction and can effectively catalyse the reaction. Based on the results of SEM, TEM and nitrogen adsorption-desorption isotherm, it was confirmed that the aerogels are highly porous structures with and effective surface area, possessing open pores and channels to allow good mass transport, no resistance for the diffusion process and highly active sites for the reactions. The defects in the bimetallic aerogels are clearly responsible for the catalytic activity. The TEM examination revealed that the nanochains are linked together like a chain. This is an indicator of optimal electrocatalytic activity for both ORR and UOR due to the high mass transfer. These interconnected, extended networks are important for durability and efficient mass and energy transfer, which improves the electrocatalytic activity for the urea oxidation reaction.
By achieving a current density of 10 mA cm−² at 1.35 V vs. RHE, Pd-Ni (4:1) outperforms several of the previously developed UOR catalysts [32,33,34,35,36,37]. Meanwhile, Pd-Ni aerogels are not as affective for OER as other reported electrocatalysts Table 3.
Conclusions
Physicochemical characterisation revealed that PdNi 4:1 exhibits a reduction in crystallite size of 4.37 nm, together with a larger shift in the 2θ positions of the (111) and (200) planes, which favours electronic changes that were investigated by XPS. These changes affected the electrocatalytic activity, which is primarily related to electronic effects. The results of SEM and TEM studies and nitrogen adsorption-desorption isotherms confirmed that the aerogels are highly porous and have an effective surface area and abundant active sites for reactions that allow efficient mass transfer and low diffusion resistance. TEM observations revealed interconnected nanochains indicating optimal electrocatalytic activity for both ORR and UOR due to high mass transfer. These interconnected networks are crucial for improving the electrocatalytic activity for the urea oxidation reaction. In addition, the addition of Ni led to a decrease in the potentials required for the oxidation and reduction of PdO by 0.13 V and 0.02 V, respectively, compared to monometallic Pd, both in alkaline medium in the presence or absence urea. The variation of the Ni content in Pd led to significant changes in the electrochemical behaviour. In the alkaline medium without urea, PdNi 4:1 showed the formation of NiOOH at 1.35 V, which facilitated oxygen diffusion on the electrode surface and increased the current density, confirming the increase in the active sites of NiOOH and NiPdOOH and enabling urea-based electrolysis at these sites. While palladium aerogels alone are not effective for the urea oxidation reaction (UOR), the presence of nickel plays a crucial role in increasing the efficiency of UOR.
Leiru2016@.
References
Xue, Q., Wang, Z., Ding, Y., Li, F., Chen, Y.: Chemical functionalized noble metal nanocrystals for electrocatalysis. Chin. J. Catal. Dalian Inst. Chem. Phys. Chin. Acad. Sci. (2023). https://doi.org/10.1016/S1872-2067(22)64186-X
Schreyer, O.A.H., Quinson, J., Escudero-Escribano, M.: Toward overcoming the challenges in the comparison of different pd nanocatalysts: Case study of the ethanol oxidation reaction. Inorganics. (2020). https://doi.org/10.3390/inorganics8110059
Liu, C., Adams, E., Li, Z., Yu, P., Wong, H.W., Gu, Z.: Effect of metal substrate on Electrocatalytic Property of Palladium Nanowire Array for high performance ethanol electro-oxidation. Langmuir Am. Chem. Soc. (2019). https://doi.org/10.1021/acs.langmuir.9b02060
Zeng, Q., Liu, D., Liu, H., Cui, P., Hu, C., Chen, D., Xu, L., Wu, X., Yang, J.: Electronic and lattice strain dual tailoring for boosting pd electrocatalysis in oxygen reduction reaction. IScience Elsevier Inc. (2021). https://doi.org/10.1016/j.isci.2021.103332
Douk, A.S., Saravani, H., Farsadrooh, M.: Three-dimensional inorganic polymer of pd aerogel as a highly active support-less anode catalyst toward formic acid oxidation. Int. J. Hydrogen Energy Elsevier Ltd. (2019). https://doi.org/10.1016/j.ijhydene.2019.05.084
Martínez-lázaro, A., Ramírez-montoya, L.A., Ledesma-garcía, J., Montes-morán, M.A., Gurrola, M.P., Menéndez, J.A., Arenillas, A., Arriaga, L.G.: Facile synthesis of unsupported pd aerogel for high performance formic acid microfluidic fuel cell. Mater. (Basel). (2022). https://doi.org/10.3390/ma15041422
Douk, A.S., Saravani, H., Abad, Y., Noroozifar, M.Z.: Controlled organization of building blocks to prepare three-dimensional architecture of Pd–Ag aerogel as a high active electrocatalyst toward formic acid oxidation. Compos. Part. B Eng. Elsevier Ltd. (2019). https://doi.org/10.1016/j.compositesb.2019.05.021
Sui, L., An, W., Feng, Y., Wang, Z., Zhou, J., Hur, S.H.: Bimetallic Pd-Based surface alloys promote electrochemical oxidation of formic acid: Mechanism, kinetics and descriptor. J. Power Sources Elsevier B V. (2020). https://doi.org/10.1016/j.jpowsour.2020.227830
Eshghi, A., Sadati Behbahani, E., Kheirmand, M., Ghaedi, M.: Pd, Pd–Ni and Pd–Ni–Fe nanoparticles anchored on MnO2/Vulcan as efficient ethanol electro-oxidation anode catalysts. Int. J. Hydrogen Energy Elsevier Ltd. (2019). https://doi.org/10.1016/j.ijhydene.2019.08.236
Juárez-Marmolejo, L., Pérez-Rodríguez, S., Montes de Oca-Yemha, M.G., Palomar-Pardavé, M., Romero-Romo, M., Ezeta-Mejía, A., Morales-Gil, P., Martínez-Huerta, M.V., Lázaro, M.J.: Carbon supported PdM (M = Fe, Co) electrocatalysts for formic acid oxidation. Influence of the Fe and Co precursors. Int. J. Hydrogen Energy. (2019). https://doi.org/10.1016/j.ijhydene.2018.11.112
Gong, Y., Zhao, H., Ye, D., Duan, H., Tang, Y., He, T., Zhang, J.: High efficiency UOR electrocatalyst based on crossed nanosheet structured FeCo-LDH for hydrogen production. Appl. Catal. A. (2022). https://doi.org/10.1016/j.apcata.2022.118745
Goswami, C., Saikia, H., Tada, K., Tanaka, S., Sudarsanam, P., Bhargava, S.K., Bharali, P.: Bimetallic palladium-nickel nanoparticles anchored on Carbon as High-Performance electrocatalysts for Oxygen reduction and formic acid oxidation reactions. ACS Appl. Energy Mater. (2020). https://doi.org/10.1021/acsaem.0c01622
Houache, M.S.E., Hughes, K., Ahmed, A., Safari, R., Liu, H., Botton, G.A., Baranova, E.A.: Electrochemical valorization of glycerol on Ni-Rich Bimetallic NiPd nanoparticles: Insight into product selectivity using in situ polarization Modulation Infrared-Reflection absorption spectroscopy. ACS Sustain. Chem. Eng. (2019). https://doi.org/10.1021/acssuschemeng.9b01070
Jiang, Y., Li, Q., Li, X., Wang, X., Dong, S., Li, J., Hou, L., Jiao, T., Wang, Y., Gao, F.: Three-Dimensional Network Pd-Ni γ-Al2O3 catalysts for highly active Catalytic Hydrogenation of Nitrobenzene to Aniline under mild conditions. ACS Omega. (2021). https://doi.org/10.1021/acsomega.1c00441
Obradović, M.D., Stančić, Z.M., Lačnjevac, U., Radmilović, V.V., Gavrilović-Wohlmuther, A., Radmilović, V.R., Gojković, S.L.: Electrochemical oxidation of ethanol on palladium-nickel nanocatalyst in alkaline media. Appl. Catal. B. (2016). https://doi.org/10.1016/j.apcatb.2016.02.039
Vicente, R.A., Neckel, I.T., Sankaranarayanan, S.K., Solla-Gullon, J., Fernández, P.S.: Bragg coherent diffraction imaging for in situ studies in electrocatalysis. ACS nano. (2021). https://doi.org/10.1021/acsnano.1c01080
Khan, M.S., Khattak, R., Khan, A., Chen, Q., Nisar, J., Iqbal, Z., Batiha, G.E.S.: Synthesis and characterizations of PdNi carbon supported nanomaterials: Studies of electrocatalytic activity for oxygen reduction in alkaline medium. Molecules. (2021). https://doi.org/10.3390/molecules26113440
Yang, H., Wang, H., Li, H., Ji, S., Davids, M.W., Wang, R.: Effect of stabilizers on the synthesis of palladium–nickel nanoparticles supported on carbon for ethanol oxidation in alkaline medium. J. Power Sources. (2014). https://doi.org/10.1016/j.jpowsour.2014.02.110
Martínez-Lázaro, A., Rodriguez-Barajas, M.H., Rey-Raap, N., Espinosa, F.I., Álvarez-Contreras, L., Ledesma-García, J., Arenillas, A., Arriaga, L.G.: Novel and high electrocatalytic activity aerogel Pd-TM (TM = co, Ni, Fe). Mater. Today Nano. (2023). https://doi.org/10.1016/j.mtnano.2023.100308
Rodríguez-Barajas, M.H., Gutiérrez, A., Martínez-Lázaro, A., Espinosa-Lagunes, F.I., Rey-Raap, N., Arenillas, A., Ledesma-García, J., Arriaga, L.G.: Palladium – cobalt aerogels for ethanol oxidation: Electrochemical study of chemical ratio effects. J. Alloys Compd. (2023). https://doi.org/10.1016/j.jallcom.2023.172390
Velázquez-Hernández, I., Torres-Pacheco, L., Álvarez-López, A., Álvarez-Contreras, L., Guerra-Balcázar, M.: Arjona, N.:Sustainable energy conversion of crude glycerol as biofuel employing PdBi nanomaterials. Appl. Surf. Sci. (2022). https://doi.org/10.1016/j.apsusc.2022.154385
Song, Y., Chen, Y., Chen, X., He, L., Wu, Y., Su, X.: High performance self-supporting 3D nanoporous PdNi alloy foam for methanol oxidation electrocatalysis. J. Porous Mater. Springer US. (2022). https://doi.org/10.1007/s10934-022-01245-x
Zhu, C., Wen, D., Oschatz, M., Holzschuh, M., Liu, W., Herrmann, A.K., Simon, F., Kaskel, S., Eychmüller: A.:Kinetically controlled synthesis of PdNi bimetallic porous nanostructures with enhanced electrocatalytic activity. Small. (2015). https://doi.org/10.1002/smll.201401432
Hosseini, M.G., Hosseinzadeh, F., Zardari, P., Mermer, O.: Pd-Ni Nanoparticle Supported on Reduced Graphene Oxide and multi-walled Carbon Nanotubes as Electrocatalyst for Oxygen Reduction Reaction. Fullerenes Nanotub Carbon Nanostructures Taylor & Francis (2018). https://doi.org/10.1080/1536383X.2018.1465049
Park, K.W., Choi, J.H., Kwon, B.K., Lee, S.A., Sung, Y.E., Ha, H.Y., Hong, S.A., Kim, H., Wieckowski, A.: Chemical and electronic effects of Ni in Pt/Ni and Pt/Ru/Ni alloy nanoparticles in methanol electrooxidation. J. Phys. Chem. B. (2002). https://doi.org/10.1021/jp013168v
Lu, X., Ahmadi, M., Disalvo, F.J., Abruña, H.D.: Enhancing the Electrocatalytic activity of Pd/M (M = ni, mn) nanoparticles for the Oxygen reduction reaction in Alkaline Media through Electrochemical Dealloying. ACS Catal. (2020). https://doi.org/10.1021/acscatal.9b05499
Pagliaro, M.V., Wen, C., Sa, B., Liu, B., Bellini, M., Bartoli, F., Sahoo, S., Singh, R.K., Alpay, S.P., Miller, H.A., Dekel, D.R.: Improving Alkaline Hydrogen Oxidation Activity of Palladium through interactions with transition-metal oxides. ACS Catal. (2022). https://doi.org/10.1021/acscatal.2c02417
Zalineeva, A., Serov, A., Padilla, M., Martinez, U., Artyushkova, K., Baranton, S., Coutanceau, C., Atanassov, P.B.: Self-supported PdxBi catalysts for the Electrooxidation of glycerol in Alkaline Media. J. Am. Chem. Soc. (2014). https://doi.org/10.1021/ja412429f
Akbari, N., Kondov, I., Vandichel, M., Aleshkevych, P., Najafpour, M.M.: Oxygen-evolution reaction by a Palladium Foil in the Presence of Iron. Inorg. Chem. (2021). https://doi.org/10.1021/acs.inorgchem.0c03746
Mohamed, I.M.A., Yasin, A.S., Barakat, N.A.M., Song, S.A., Lee, H.E., Kim, S.S.: Electrocatalytic behavior of a nanocomposite of Ni/Pd supported by carbonized PVA nanofibers towards formic acid, ethanol and urea oxidation: A physicochemical and electro-analysis study. Appl. Surf. Sci. Elsevier B V. (2018). https://doi.org/10.1016/j.apsusc.2017.11.076
Singh, R.K., Schechter, A.: Electroactivity of NiCr Catalysts for Urea Oxidation in Alkaline Electrolyte. ChemCatChem. (2017). https://doi.org/10.1002/cctc.201700451
Wang, Y., Du, Y., Fu, Z., Wang, M., Fu, Y., Li, B., Wang, L.: External and internal dual-controls: Tunable cavity and Ru–O–Co bond bridge synergistically accelerate the RuCoCu-MOF/CF nanorods for urea-assisted energy-saving hydrogen production. Int. J. Hydrog. Energy. (2023). https://doi.org/10.1016/j.ijhydene.2023.03.127
Abdelrahim, A.M., El-Moghny, A., El-Shakre, M.G., El-Deab, M.E.: Double surface modification of graphite felt using a single facile step for electrolytic hydrogen production assisted by urea. Electrochim. Acta. (2023). https://doi.org/10.1016/j.electacta.2022.141726
Bandal, H.A., Kim, H.: In situ formed Cu3P@ CuOx as an efficient electrocatalyst for urea electrooxidation. Appl. Surf. Sci. (2023). https://doi.org/10.1016/j.apsusc.2023.156925
Zequine, C., Wang, F., Li, X., Guragain, D., Mishra, S.R., Siam, K., Gupta, R.K.: Nanosheets of CuCo2O4 as a high-performance electrocatalyst in urea oxidation. Appl. Sci. (2019). https://doi.org/10.3390/app9040793
Yang, J.H., Chen, M., Xu, X., Jiang, S., Zhang, Y., Wang, Y., Yang, D.: CuO-Ni (OH)2 nanosheets as effective electro-catalysts for urea oxidation. Appl. Surf. Sci. (2021). https://doi.org/10.1016/j.apsusc.2021.150009
Chen, N., Du, Y.X., Zhang, G., Lu, W.T., Cao, F.F.: Amorphous nickel sulfoselenide for efficient electrochemical urea-assisted hydrogen production in alkaline media. Nano Energy. (2021). https://doi.org/10.1016/j.nanoen.2020.105605
Acknowledgements
The authors thanks to the Mexican Council of Humanistics, Sciences and Technologies (CONAHCYT) for financial support. Convocatoria 2023 Laboratorios Nacionales-CONAHCYT-Grant 15, Ciencia de Frontera 2023-Grants 416 and 567.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author declares that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodríguez-Buenrostro, A., Martínez-Lázaro, A., Contreras-Martínez, M.V. et al. Mesoporous Pdx-Nix aerogels for electrocatalytic evaluation of urea-assisted electrolysis. Mater Renew Sustain Energy 13, 255–264 (2024). https://doi.org/10.1007/s40243-024-00265-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-024-00265-8