Abstract
Rice husks are not readily biodegradable making their disposal challenging due to the common disposal method of open burning which has negative environmental effects. Additionally, banana, sweet potato and cassava peelings form a large percentage of organic municipal solid waste. Therefore, this study developed rice husk biochar briquettes with organic municipal peelings waste as binders. Rice husks biochar was formed via carbonization processes in a step-down kiln at temperatures ranging between 400 and 500 °C. Organic binders were mixed with the rice husk biochar at different ratios of 10% and 15% before being compacted at a pressure ≤ 7 MPa into briquettes. Thermogravimetric results showed that the developed briquettes had high ash contents ranging from 44% to 47%. Rice husk biochar briquettes with the highest particle density were observed for briquettes with 15% cassava peel binder at 427.1 kg/m3. The highest HHV and maximum attainable flame temperature of 21.75 MJ/kg and 828.7 °C were obtained for rice husk biochar briquettes with 15% matooke peeling organic binder. For all rice husk biochar briquettes, increasing the organic peeling binder had a positive impact of reducing the ash content, while at the same time increasing the peak temperatures, thus contributing to their enhanced thermal stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbonized briquettes continue to be discussed as alternatives for charcoal and firewood in developing countries because they are majorly produced from biochar from the carbonization of agricultural residues such as rice husks, coffee husks, groundnut shells, bagasse, maize cobs, etc. and are thus a potentially renewable and sustainable feedstock for domestic energy production [1,2,3,4,5,6]. Therefore, the development of briquettes from carbonized agricultural residues reduces a significant waste stream category through thermo-chemical processes that convert this waste into energy as a valorized product. The continued emphasis on research and development of carbonized briquettes from agricultural residues is further supported by the fact that currently firewood and charcoal use accounts for over 80% of domestic energy fuels in sub-Saharan Africa, whose population is expected to increase to over 3 billion by 2050 [7]. This implies that efforts to mitigate the effects of climate change and global warming will not yield much progress unless alternatives to firewood and charcoal are continuously developed and promoted, especially in sub-Saharan Africa.
One of the major problems faced in the development of carbonized briquettes from agricultural residues is the fact that starch derived from edible portions of crops such as cassava and corn (or maize) continues to be the main binder for biochar agglomeration in briquette production [5, 6, 8,9,10]. Despite the relatively small amounts of edible food-based starch used in biochar briquette production, criticism has persisted due to perception that their continued use in the production of biochar briquettes is unsustainable, hinders commercialization and promotes energy at the expense of food for growing populations. Therefore, significant research effort in the development of carbonized briquettes has been given to using alternative binders [6, 11,12,13,14,15,16,17,18,19]. One interesting alternative to cassava starch as binder is organic municipal peeling waste. Municipal Solid Waste (MSW) continues to pose a major management problem for most countries in sub-Saharan Africa due to limited financial and technical capacity to sustainable handle it disposal and disintegration [20]. Majority of MSW composition in developing countries is organic in nature, consisting mainly of organic food peelings and waste food, whose handling is problematic due to its bulky nature as a result of high moisture contents, and yet this organic waste is a potential energy recovery resource [21, 22]. Therefore, there is a need to provide alternative options for the handling and management of the organic component of municipal solid waste in view of achieving a sustainable circular economy [23].
Very few studies exist in the literature where organic municipal peelings have been used as binder material in briquette production. Recent studies have called for the briquetting of municipal solid wastes and the application of multi-functional desulfurizing bio-binders a solid waste management strategy [24]. Briquettes were formed from dried and pulverized vegetable market waste and without using any external binding agent. The calorific value for four different types of the waste was low and ranged between 10 and 13.7 MJ/kg of dry matter [25]. In another study, briquettes were developed from Pterocarpus indicus leaves with inedible rejected pineapples as binder material. Highest calorific value was obtained for a combination of 95% Pterocarpus leaves and 5% binder [26]. Other studies have used municipal solid food market waste as blending material with fecal sludge to develop non-charred briquettes in order to solve energy and sanitation problems in slums [27]. Co-briquetting of cassava peels with sawdust at different torrefaction pre-treatment conditions resulted in high quality briquettes with improved strength for torrefied cassava peels [28]. Cassava peels and plantain peels were combined with corn cobs as bio-binders to produce briquettes with optimal performance which peaked at 35% thermal efficiency and 3.5 kW for fire power for briquettes with 20% cassava peels, 20% plantain peels and 60% corn cobs [29]. Briquettes developed from citrus peels (pear orange, ponkan tangerine, and Tahiti lemon) and rice husks showed potential to replace traditional sources of heat in development of sustainable bioenergy fuels [30]. Briquettes were also developed using municipal waste compositing char and sawdust char at ratios ranging from 100:0 to 20:80. Results showed that municipal waste composting char to sawdust char at ratios of 20:80 was the most suitable for producing solid fuel briquettes [31]. Blends of cornhusk and cassava peels were used to produce briquettes for application in co-firing of coal plants [32]. The potential of starch remaining from torrefied orange peels, banana peels, dry leaves and tree branch to act as the natural binder in a hot binderless briquetting process were studied. The results showed that the starch material contributed to additional briquette strength of torrefied MSW material [33]. Rice husk briquettes were developed, characterized and their performance compared for rice husk briquettes with cassava peels and cassava starch as binders. The properties of rice husk briquettes made with cassava peels were superior to those with cassava starch binder [34].
In this study, rice husks carbonized briquettes (RHB) were developed with three different organic municipal peeling waste as binders, namely: cassava peels (CS), banana (for cooking, locally called “matooke”) peels (M), and sweet potato peels (SP). The developed rich husk biochar briquettes were characterized for their proximate analysis, mechanical, and thermal properties.
Materials and methods
Briquette development
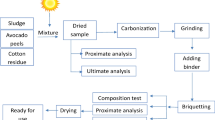
Rice husks were obtained from Zhong’s Industries Limited company that specializes in commercial growing K98 rice varieties. K98 rice husks were loaded into 80 L capacity air-tight ceramic saggars. The ceramic saggars were then place inside a step-down kiln for 4–5 h for carbonization to take place with temperatures maintained between 400 °C and 500 °C. The carbonization process was enhanced due to limited oxygen supply into the ceramic saggars which allowed for slow pyrolysis to take place [4, 5]. The resulting rice husk biochar was sieved using a 600 μm mesh (See Fig. 1).
Sweet potato peels, matooke peels and cassava peels were collected from markets in Kampala City, Uganda, where these foods are regularly consumed. The peels were washed in clean water to remove all dirt from them. Sun drying was done for 12 days until the moisture content in the peels was less than 10%. The peel sizes were then mechanically reduced to powder form with particle sizes less than 500 μm. Municipal solid waste peeling powder/flour were then weighed into 100 g (10%) and 150 g (15%) portions using a digital balance. The proportions of peeling powder/flour were mixed with 500 ml of water and brought to a boil at approximately 100 °C to form a homogeneous uniformly mixed starchy paste for each peeling powder/flour. This starchy paste was mixed with rice husk biochar to form a total 1000 g weight. After being mixed thoroughly, the rice husk biochar and binder mixture was placed into hollow cylindrical molds (with outer diameter 4.7 cm, center inner protrusion diameter of 1.65 cm and height of 5.7 cm). This composite mixture was then compressed under low pressure (≤ 7 MPa). The developed briquettes were then carefully pushed out using a piston revealing the formation of the hollow rice husk bio-char briquette developed using rice husk biochar and organic municipal peeling powder/flour of cassava, sweet potato and matooke peels. The developed briquettes were dried in a natural convection oven at 75 °C over a 24-hour period until their moisture content was < 5%. The abbreviations of the different briquettes are provided in Table 1.
Briquette characterization
Thermogravimetric analysis (TGA) was done using an Eltra Thermostep non-isothermal TGA at 16 °C/min from 25 °C to 900 °C. Prior to TGA analysis high purity compressed air was used to clean the reaction chamber and nitrogen gas was used a purge gas during pyrolysis reactions taking place on the samples at a maintained flow rate of 1 L/min [5]. TGA was used to determine moisture content, ash content, fixed carbon and volatile matter for the rice husks, rice husk biochar, organic municipal peeling waste (cassava peels, matooke peels, and sweet potato peels) and for the developed rice husk biochar briquettes with different amounts of peeling powder used (10% and 15%) [4, 5]. TGA results were further used to determine the weight loss vs. temperature and derivative thermogravimetry (DTG) curves for the developed rice husk biochar briquettes. Burning rates for the developed rice husk bio-char briquettes was determined as the ratio of the fuel weight difference (calculated between the fuel weight at a particular time and its initial weight) to time (this is the time taken for the fuel to attain its new weight in the weight loss vs. temperature curve. The average values of ash content (AC), fixed carbon (FC) and moisture content (MC) for the developed rice husk biochar briquettes obtained from TGA analysis were used to determine the higher heating value for each briquette with different organic peeling powder/flour amounts. The commonly used equation from Garcia et al. (2014) was used in this study (See Eq. 1) [35].
Standard water boiling tests were done to determine the maximum attainable flame temperature, ignition time and boiling time for 200 g of developed rice husk biochar briquettes to boil 0.5 L of water in a flat-bottomed cylindrical cross-section aluminium pan using an improved domestic cookstove, typically used in sub-Saharan Africa [5]. Temperature measurements were done every minute using a DT-8865 non-contact infrared thermometer gun. Drop strength for the developed rice husk biochar briquettes were determined as the ratio of the weight of briquette after being dropped on a thick steel plate to the weight of the developed briquettes before being dropped. The drop test was carried out by allowing each rice husk briquette to be dropped from a height of 2 m onto a thick steel metal plate. Drop strength gives a measure of the impact resistance of the briquette when subjected to impact loads that are typically present during packaging, storage, and transportation [36, 37]. Particle density was determined as the ratio of the mass to the volume of each briquette sample.
Results and discussions
Proximate analysis results for raw materials
Proximate analysis results for the different organic MSW peeling binders (cassava peel, matooke peel and sweet potato peel), rice husks and rice husk biochar are shown in Fig. 2. For the MSW peeling binders, matoke peeling binder had the highest moisture content, ash content and fixed carbon at 11.8%, 5.1% and 20.6%, respectively. Cassava peeling binders had the highest volatile matter at 74.5%. The results for cassava peels are similar to results in other studies [38]. For all the MSW peeling binders ash content were ≤5%, which implies that their utilization as binders in the rice husk biochar briquettes will not affect the thermal performance of the developed briquettes. It further indicates that acidic or alkaline pre-treatment of the MSW peeling binders to de-ash them further is not necessary [39,40,41]. Rice husk biochar had higher fixed carbon compared to the rice husks. Volatile matter was much lower for the rice husk biochar at approximately 10% compared to 57% for the rice husks. This is expected as expended volatile compounds are released during the slow pyrolysis process involved in the carbonization process. This further indicates that the ignition ability is much higher at higher flame temperature for rice husk biochar briquettes [37]. The higher moisture content in rice husk biochar (22.5%) is explained by the expression on more pores for adsorption processes developed during the carbonization process as devolatilization takes place. Whereas higher moisture content is typically associated with low energy output, difficulty in ignition, lower combustion energy as most energy is used to expend heat of vaporization, this moisture has a positive role it plays in providing adhesive properties when the briquettes are formed using simple low-compression approaches [42]. The high ash content of rice husk biochar is due to the presence of silica oxides that have significant thermal stability and resistance to oxidation during the pyrolysis process. This implies that the flame for combustion will last longer at lower flame speeds and combustion rates for rice husk biochar briquettes. This will also contribute to increased ignition time due to the high ash content and silica presence in the rice husk biochar [5, 36, 43,44,45,46,47].
Proximate analysis results for the developed rice husk biochar briquettes
The results for ash content, fixed carbon, volatile matter and moisture content for the rice husk biochar briquettes developed with different percentages of municipal solid waste cassava, sweet potato and matooke peeling binders (10% and 15%) are shown in Fig. 3. Moisture content for all the developed briquettes ranged from 7 − 9%, which is in line with values for carbonized briquettes reported in other studies because higher moisture content values are reported to result in lower overall heat and energy output, lower combustion temperatures, and reduced shelf life of the developed briquettes [37, 44,45,46]. A low moisture content is because hydroxyl groups are destroyed during the carbonization process [36]. Some studies have reported that moisture contents less than 5% are detrimental to the overall thermal efficiency and stability of rice husk pellets because the excessive dryness causes a very high combustion efficiency, which results in the fuel burning out very quickly [48]. Low moisture content is desirable because it implies that less energy is expended for drying and evaporation of inherent moisture in the briquette allowing for the availability of more effective calorific energy [44,45,46, 49]. The inherent low moisture content in the municipal solid waste peeling binders also contributed to the overall low moisture content in the developed rice husk biochar briquettes (See Fig. 2). Ash content of the developed rice husk biochar briquettes was high ranging from 44 − 47%. A very slight decrease in ash content was observed when the cassava, matooke and sweet potato peeling binders were added. High ash content was expected because of rice husks being carbonized in a slow pyrolysis process to form rice husk biochar resulting in the formation of mainly amorphous silica and some amorphous carbon with insoluble cristobalite (SiO2) and tridymite (SiO2) and other alkaline minerals [50]. The formation of SiO2 during oxidation of silica in the rice husks during slow pyrolysis imparts thermal stability, but further retards the burning rates and ignitability of the developed rice husk biochar briquettes [46, 49,50,51]. Increase in the municipal solid waste peeling binders for 10–15% resulted in an increase in volatile matter of the developed rice husk biochar briquettes. For all the developed rice husk biochar briquettes, volatile matter ranged from 15 − 18.5%. This result is a compound effect of the volatile matter of the individual municipal solid waste peeling binders (62.4 − 74.5%) and rice husk biochar (9.6%). The relatively high volatile matter for the municipal solid waste peeling binders is an indicator of their positive contribution in the energy content of any resulting fuel into which they are included. The relatively low volatile matter of the developed rice husk briquettes contributes to their reduced ignitability and reduced smoke production during combustion [5]. Marginally higher fixed carbon results were observed for rice husk biochar briquettes developed with 10% matooke peeling binder. Fixed carbon percentages were higher for the rice husk biochar briquettes than for the rice husk biochar and that for the municipal solid waste peeling binders considered separately. Increase in fixed carbon is a direct result of reduced volatile matter and moisture content of the resulting rice husk biochar briquettes [36, 52].
Thermal properties
The thermogravimetric behavior of the developed rice husk biochar briquettes with cassava, matooke and sweet potato peeling binders is shown in Fig. 4. Rice husk biochar briquettes with lower municipal solid waste peeling binders had less weight loss as temperature increased. This further implies that thermal stability of the developed rice husk biochar briquettes reduced with an increase in the binder material (See Fig. 4). Briquette samples with 15% matooke peeling binder had the most weight loss followed by rice husk biochar briquettes with 15% sweet potato peeling binders. Therefore, rice husk biochar briquettes with the lowest weight loss had cassava peeling binder. The starch generated from cassava is known for imparting strong particle to particle bonding between the biochar particles, which would imply that a higher threshold is necessary to initiate thermal degradation of rich husk biochar briquettes developed with cassava peeling binders [53,54,55]. The weight loss behavior with temperature is typical for the thermal degradation of lignocellulosic materials where evaporation (105°C is followed by the devolatilization (200°C − 600°C) during which hemicellulose (150°C-350°C), cellulose 275°C-350°C), and lignin (300°C− 900°C) are decomposed until ash is formed (≤900°C) [5]. After 350°C, weight loss of the rice husk biochar briquettes reduced more as municipal solid waste peeling binders were increased in the overall briquette matrix. This result correlates very well with the high volatile matter results for the municipal solid waste peeling binders (See Fig. 2). This weight loss is predicated by oxidation of biochar carbon during the pyrolysis process [50]. This implies that enhanced chemical reactions take place as aliphatic side chains split off from aromatic rings in the structures of the municipal solid waste peeling wastes [48,49,50, 56].
Derivative Thermogravimetric (DTG) analysis profiles for the rice husk biochar briquettes with different municipal solid waste peeling binders are shown in Fig. 5. The weak peaks in the DTG curves in the first stage corresponds to removal of moisture content at approximately 100 °C. Further degradation rates for the developed rice husk biochar briquettes are characterized by two distinct peaks representing hemicellulose, cellulose, and lignin decomposition [5, 57]. Maximum decomposition took place at 483 °C and 489 °C for rice husk biochar briquettes with 10% and 15% cassava peelings binder, respectively, corresponding to a devolatilization rate of 0.007%/min and 0.01%/min respectively. Maximum decomposition of rice husk biochar briquettes took place at 497 °C and 504 °C for 10% and 15% matooke peeling binder, respectively, at a rate of 0.018%/min and 0.021%/min, respectively. The maximum decomposition of rice husk biochar briquette with sweet potato peeling binder ratios of 10% and 15% were 511°C and 496 °C, respectively, at rates of 0.013%/min and 0.019%/min. Therefore, this implies the rice husk biochar briquettes with matooke peelings biochar had the most weight loss and those with cassava peeling binders had the least weight loss.
Results for burning rates for the developed rice husk biochar briquettes are shown in Fig. 6. After the 8th minute, rice husk biochar briquettes with a higher cassava peeling binders (15%) had higher burning rates than rice husk biochar briquettes with lower cassava peeling binders (10%). All the developed rice husk biochar briquettes displayed a similar burning rate between 10 and 90 min. Rice husk biochar briquettes with 10% matooke peeling binder had the higher burning rates than rice husk briquettes with 15% matooke peeling binder. Rice husk briquettes with sweet potato peeling binder showed similar results with rice husk briquettes with higher binder percentages had higher binder rates than those developed with briquettes with lower binder percentages. An increase in burning rates with an increase in municipal solid waste peeling binders is due to the reduction of rice husk biochar in the total briquette volume which means that these briquettes have reduced silica and SiO2 in the briquette matrix while increasing the municipal solid waste peeling binders which have high volatiles [50].
Heating values
Higher heating values (HHV) for the developed rice husk biochar briquettes for different percentages (10% and 15%) of municipal solid waste peeling binders are shown in Fig. 7. For all the developed rice husk biochar briquettes a marginal increase in higher heating value was observed for briquettes with 15% municipal solid waste peeling binder added. Rice husk biochar briquettes with 15% matooke peeling waste binder had higher heating values of 21.8 MJ/kg, which were the highest for all the developed briquettes. The lowest higher heating value result was recorded for rice husk biochar briquettes with 10% cassava peeling binder at 16.8 MJ/kg. These results were expected as rice husk biochar briquettes with matooke peeling binders had the highest fixed carbon and lowest volatile matter of all the other municipal solid waste peeling wastes (See Fig. 2) [58].
Maximum flame temperature, ignition time and boiling time
The maximum attainable flame temperature, ignition, and boiling time for the developed rice husk briquettes with different proportions of municipal solid waste peeling binders are shown in Table 2. For all the developed rice husk biochar briquettes, the maximum attainable flame temperature increased with an increase in the amount of municipal solid waste peeling binder from 10 to 15%. From the results it was noted that rice husk biochar briquettes with 15% matooke peeling binder had the highest flame temperature of 828.7 °C. This is because matooke peeling binders had the highest fixed carbon and lowest volatile matter percentages of all the municipal solid waste peeling binders. However, once the matooke peeling binders were reduced from 15 to 10%, then the ignition time for the developed rice husk biochar briquette increased significantly from 7 min to 10 min. The maximum flame temperatures were directly correlated to the results for ignition time and time taken to boil 0.5 L of water. Rice husk biochar briquettes with sweet potato peeling binders required the highest time to boil 0.5 L of water at 17 min. Maximum flame temperature, ignition time and boiling time are very important parameters in determining the viability of the developed rice husk biochar briquettes because they provide an indication of the briquette performance during actual domestic cooking application [59].
Mechanical properties
Particle density is a very important measure for determining the compactness of a developed briquette. It provides a metric that determines the ability of a briquette to be transported without disintegration [5, 37]. The highest particle density of 427.11 kg/m3 was recorded for rice husk biochar briquettes with 15% cassava peeling binder. Rice husk biochar briquettes with 15% municipal solid waste peeling binders had higher particle densities than rice husk biochar briquettes developed with 10% municipal solid waste peeling binders (See Fig. 8). This implies that an increase in municipal solid waste binders enhanced the mechanical property of the developed briquettes. The carbonization process enhances compaction of the rice husk biochar with the binder due to the availability of free radicals available for bonding [5].
Drop strength results for the developed rice husk biochar briquettes are shown in Table 3. The drop strength results are clearly positively correlated with the particle density results (See Fig. 8). An increase in the municipal solid waste binder from 10 to 15% in the developed rice husk biochar briquette resulted in an increase in the briquette drop strength. Rice husk biochar briquettes with 15% matooke peeling binder had the highest value for drop strength at 87.1%, while rice husk biochar briquettes with 10% sweet potato peeling binder had the lowest value for drop strength at 68%. Higher drop strengths for the rice husk biochar briquettes as municipal solid waste peeling binders are increased is due to increased intermolecular hydrogen bonds between starch constituents and the possible formation of solid bridges [53, 60]. Higher drop strength values are desirable because it implies that the developed briquettes can be transported without disintegration [5, 37].
Conclusions
Rice husks biochar was formed via carbonization processes in a step-down kiln at temperatures ranging between 400 and 500 °C. Municipal solid waste peeling binders from cassava, matooke and sweet potato peelings, were mixed with the rice husk biochar at different ratios of 10% and 15% before being compacted at a pressure ≤ 7 MPa into briquettes. Thermogravimetric results showed that the developed briquettes had high ash contents ranging from 44% to 47%. Rice husk biochar briquettes with the highest particle density were observed for briquettes with 15% cassava peeling binder at 427.1 kg/m3. The highest HHV and maximum attainable flame temperature of 21.75 MJ/kg and 828.7 °C were obtained for rice husk biochar briquettes with 15% matooke peeling organic binder. For all rice husk biochar briquettes developed it was noted that an increase in the municipal solid waste binders led to an increase in the peak temperatures, reduction in ignition time and boiling time. The results from the study indicate that rice husk biochar briquettes are suitable sustainable domestic cooking fuels.
Data availability
Data will be made available upon reasonable request to the corresponding author.
References
Bot, B.V., Tamba, J.G., Sosso, O.T.: Assessment of biomass briquette energy potential from agricultural residues in Cameroon. Biomass Convers. Biorefinery. 14, 1905–1917 (2024). https://doi.org/10.1007/s13399-022-02388-2
Sunnu, A.K., Adu-Poku, K.A., Ayetor, G.K.: Production and characterization of charred briquettes from various agricultural waste. Combust. Sci. Technol. 195(5), 1000–1021 (2023). https://doi.org/10.1080/00102202.2021.1977803
Nagarajan, J., Prakash, L.: Preparation and characterization of biomass briquettes using sugarcane bagasse, corncob and rice husk. Materials Today: Proceedings 47: 4194–4198 (2021). https://doi.org/10.1016/j/matpr.2021.04.457
Yiga, V.A., Nuwamanya, A., Birungi, A., Lubwama, M., Lubwama, H.N.: Development of carbonized rice husks briquettes: Synergy between emissions, combustion, kinetics and thermodynamic characteristics. Energy Rep. 9, 5977–5991 (2023). https://doi.org/10.1016/j.egyr.2023.05.066
Lubwama, M., Yiga, V.A., Muhairwe, F., Kihedu, J.: Physical and combustion properties of agricultural residue bio-char bio-composite briquettes as sustainable domestic energy sources. Renew. Energy. 148, 1002–1016 (2020). https://doi.org/10.1016/j.renene.2019.10.085
Olugbade, T., Ojo, O., Mohammed, T.: Influence of binders on combustion properties of biomass briquettes: A recent review. Bioenergy Res. 12, 241–259 (2019). https://doi.org/10.1007/s12155-019-09973-w
Ferronato, N., Mendoza, I.J.C., Portillo, M.A.G., Conti, F., Torretta, V.: Are waste-based briquettes alternative fuels in developing countries? A critical review. Energy. Sustain. Dev. 68, 220–241 (2022). https://doi.org/10.1016/j.esd.2022.03.013
Kumar, T.A., Ramesh, S.: Sustainable production of cashew nutshell briquettes: Experimental assessment and optimization of factors affecting the physical and fuel characteristics. Biomass Convers. Biorefinery. 13, 16969–16990 (2023). https://doi.org/10.1007/s13399-021-02234-x
Oladeji, J.T., Enweremadu, C.C.: The effects of some processing parameters on physical and densification characteristics of corncob briquettes. Int. J. Energy Eng. 2(1), 22–27 (2021). https://doi.org/10.5923/j.ijee.20120201.04
Khlifi, S., Lajili, M., Belghith, S., Mezlini, S., Tabet, F., Jeguirim, M.: Briquettes production from olive mill waste under optimal temperature and pressure conditions: Physico-Chemical and mechanical characterizations. Energies. 13(5), 1214 (2020). https://doi.org/10.3390/en13051214
Nazari, M.M., Idroas, M.Y., Miskam, M.A.: Effects of organic and inorganic binders on the carbonized empty fruit bunch (EFB) briquette properties. AIP Conference Proceedings 2541, 030003 (2022). https://doi.org/10.1063/5.0117528
Narzary, A., Das, A.K.: Study of effects of addition of charcoal and binder derived from taro on physiochemical properties of briquettes made from tree leaves. Sustainable Energy Technol. Assessments Part. B. 52, 102119 (2022). https://doi.org/10.1016/j.seta.2022.102119
Akbar, A., Aslam, U., Asghar, A., Aslam, Z.: Effect of binding materials on physical and fuel characteristics of bagasse based pellets. Biomass Bioenerg. 150, 106118 (2021). https://doi.org/10.1016/j.biombioe.2021.106118
Kivumbi, B., Jande, Y.A.C., Kirabira, J.B., Kivevele, T.T.: Production of carbonized briquettes from charcoal fines using African Elemi (Canarium Schweinfurthii) resin as organic binder. Energy Sour. Part a Recover. Utilization Environ. Eff. (2021). https://doi.org/10.1080/15567036.2021.1977870
Yank, A., Ngadi, M., Kok, R.: Physical properties of rice husk and bran briquettes under low pressure densification for rural applications. Biomass Bioenerg. 84, 22–30 (2016). https://doi.org/10.1016/j.biombioe.2015.09.015
Muazu, R.I., Stegemann, J.A.: Biosolids and microalgae as alternative binders for biomass fuel briquetting. Fuel. 194, 339–349 (2017). https://doi.org/10.1016/j.fuel.2017.01.019
Cahyono, R., Santoso, J.: Miliati. Biomass briquettes using Indonesia Durian seeds as binder agent: The effect of binder concentration on the briquette properties. Chem. Eng. Trans. 56, 1663–1558 (2017). https://doi.org/10.3303/CET1756278
Wang, T., Wang, Z., Zhai, Y., Li, S., Liu, X., Wang, B., Li, C., Zhu, Y.: Effect of molasses binder on the pelletization of food waste bydrochar for enhanced biofuel pellets production. Sustainable Chem. Pharm. 14, 100183 (2019). https://doi.org/10.1016/j.scp.2019.100183
Zubairu, A., Gana, S.A.: Production and characterization of briquette charcoal by carbonization of agro-waste. Energy Power. 4(2), 41–47 (2014). https://doi.org/10.5923/j.ep.20140402.03
Amulen, J., Kasedde, H., Serugunda, J., Lwanyaga, J.D.: The potential of energy recovery from municipal solid waste in Kampala City, Uganda, by incineration. Energy Convers. Management: X. 14, 100204 (2022). https://doi.org/10.1016/j.ecmx.2022.100204
Ngusale, G.K., Oloko, M., Awuor, F.O.: Briquetting as a means of recovering energy from organic market waste. Energy Sources part. A: Recovery Utilization Environ. Eff. (2021). https://doi.org/10.1080/15567036.2021.1925784
Nsubuga, D., Banadda, N., Kabenge, I., Wydra, K.D.: Potential of jackfruit waste for biogas briquettes and as a carbondioxide sink – A review. J. Sustainable Dev. 13, 60–75 (2020). https://doi.org/10.5539/jsd.v13n4p60
Jamaludin, H., Elmaky, H.S.E., Sulaiman, S.: The future of foodwaste: Application of circular economy. Energy Nexus. 7, 100098 (2022). https://doi.org/10.1016/j.nexus.2022.100098
Rawat, S., Kumar: Critical review on processing technologies and economic aspect of bio-coal briquette production. Prep. Biochem. Biotechnol. 52(8), 855–871 (2022). https://doi.org/10.1080/10826068.2021.2001754
Srivastava, N.S.L., Narnaware, S.L., Makwana, J.P., Singh, S.N., Vahora, S.: Investigating the energy use of vegetable market waste by briquetting. Renew. Energy. 69, 270–275 (2014). https://doi.org/10.1016/j.renene.2014.01.047
Gotama, G.J., Anggono, W., Sutrisno, Suprianto, F.D., Jonoadji, N., Arifin, F.X.Y.: Investigation of briquette derived from Pterocarpus indicus leaves and rejected pineapples as inedible sources of renewable energy. IOP Conf. Ser.: Mater. Sci. Eng. 1034, 012074 (2021). https://doi.org/10.1088/1757-899X/1034/1/012074
Kizito, S., Jjagwe, J., Ssewaya, B., Nekesa, L., Tumutegyereize, P., Zziwa, A., Komakech, A.J.: Biofuel characteristics of non-charred briquettes from dried fecal sludge blended with food market waste: Suggesting a waste-to-biofuel enterprose as a win-win strategy to solve energy and sanitation problems in slums settlements. Waste Manage. 140, 173–182 (2022). https://doi.org/10.1016/j.wasman.2021.11.029
Akogun, O.A., Waheed, M.A., Ismaila, S.O., Dairo, O.U.: Co-briquetting characteristics of cassava peel with sawdust at different torrefaction pretreatment conditions. Energy sources, part A: Recovery, utilization and Environmental effects (2020). https://doi.org/10.1080/15567036.2020.1752333
Samomssa, I., Nono, Y.J., Cârâc, G., et al.: Optimization of fuel briquette production from cassava peels, plantain peels and corn cobs. J. Mater. Cycles Waste Manag. 23, 1905–1917 (2021). https://doi.org/10.1007/s10163-021-01260-1
Magnago, R.F., Costa, S.C., Ezirio, M.J., Saciloto, V., Parma, G.O.C., Gasparotto, E.S., et al.: Briquettes of citrus peel and rice husk. J. Clean. Prod. 276, 10, 123820 (2020). https://doi.org/10.1016/j.clepro.2020.123820
Prasityousil, J., Muenjina, A.: Properties of solid fuel briquettes produced from rejected material of municipal waste compositing. Procedia Environ. Sci. 17, 603–610 (2013). https://doi.org/10.1016/j.proenv.2013.02.076
Waheed, M.A., Akogun, O.A.: Quality enhancement of fuel briquette from cornhusk and cassava peel blends in coal thermal plant. Int. J. Energy Res. 45(2), 1867–1878 (2020). https://doi.org/10.1002/er.5865
Irhamna, A.R., Utama, A.I., Hardianto, T.: Preliminary investigation on the starch potential as natural binder in the hot binderless briquetting process of torrefied municipal solid waste. AIP Conf. Proc. 1984(030001) (2018). https://doi.org/10.1063/1.5046622
Arewa, M.E., Daniel, I.C., Kuye, A.: Characterization and comparison of rice husk briquettes with cassava peels and cassava starch as binders. Biofuels. 7(6), 671–675 (2016). https://doi.org/10.1080/17597269.2016.1187541
Garcia, R., Pizarro, C., Lavin, A.G., Bueno, J.L.: Spanish biofuels heating value estimation. Part II: Proximate analysis date. Fuel. 117, 1139–1147 (2014). https://doi.org/10.1016/j.fuel.2013.08.049
Lubwama, M., Yiga, V.A.: Characteristics of briquettes developed from rice and coffee husks for domestic cooking applications in Uganda. Renew. Energy. 118, 43–55 (2018). https://doi.org/10.1016/j.renene.2017.11.003
Lubwama, M., Yiga, V.A.: Development of groundnut shells and bagasse briquettes as sustainable fuel sources for domestic cooking applications in Uganda. Renew. Energy. 111, 532–542 (2017). https://doi.org/10.1016/j.renene.2017.04.041
Kayiwa, R., Kasedde, H., Lubwama, M., Kirabira, J.B.: Characterization and pre-leaching effect on the peels of predominant cassava varieties in Uganda for production of activated carbon. Curr. Res. Green. Sustainable Chem. 4, 100083 (2021). https://doi.org/10.1016/j.crgsc.2021.100083
Menya, E., Olupot, P.W., Storz, H., Lubwama, M., Kiros, Y.: Characterization and alkaline pretreatment of rice husk varieties in Uganda for potential utilization as precursors in the production of activated carbon and other value-added products. Waste Manage. 81, 104–116 (2018). https://doi.org/10.1016/j.wasman.2018.09.050
Nanssou, P.A.K., Nono, Y.J., Kapseu, C.: Pretreatment of cassava stems and peelings by thermohydrolysis to enhance hydrolysis yield of cellulose in bioethanol production process. Renew. Energy. 97, 252–265 (2016). https://doi.org/10.1016/j.renene.2016.05.050
Adekunle, A., Orsat, V., Raghavan, V.: Lignocellulosic bioethanol: A review and design conceptualization study of production from cassava peels. Renew. Sustain. Energy Rev. 64, 518–530 (2016). https://doi.org/10.1016/j.rser.2016.06.064
Antwi-Baosiako, C., Acheampong, B.B.: Strength properties and calorific values of sawdust briquettes as wood-residue energy generation source from tropical hardwoods of different densities. Biomass Bioenerg. 85, 144–152 (2016). https://doi.org/10.1016/j.biombioe.2015.12.006
Karam, D.S., Nagabovanalli, P., Rajoo, K.S., Ishak, C.F., Abdu, A., Rosli, Z., Muharam, F.M., Zulperi, D.: An overview on the preparation of rice husk biochar, factors affecting its properties, and its agriculture application. J. Saudi Soc. Agricultural Sci. 21, 149–159 (2022). https://doi.org/10.1016/j.ssas.2021.07.005
Kipngetich, P., Kiplimo, R., Tanui, J.K., Chisale, P.: Effects of carbonization on the combustion of rice husks briquettes in a fixed bed. Clean. Eng. Technol. 13, 100608 (2023). https://doi.org/10.1016/j.clet.2023.100608
Saeed, A.A.H., Harun, N.Y., Bilad, M.R., Afzal, M.T., Parvez, A.M., Roslan, F.A.S., Rahim, S.A., Vinayagam, V.D., Afolabi, H.K.: Moisture content impact on properties of briquettes produced from rice husk waste. Sustainability. 13(6), 3069 (2021). https://doi.org/10.3390/su13063069
Kipngetich, P., Kiplimo, R., Tanui, J.K., Chisale, P.C.: Optimization of combustion parameters of carbonized rice husk briquettes in a fixed bed using RSM technique. Renew. Energy. 198, 61–74 (2022). https://doi.org/10.1016/j.renene.2022.07.130
Akolgo, G.A., Awafo, E.A., Essandoh, E.O., Owusu, P.A., Uba, F., Adu-Poku, K.A.: Assessment of the potential of charred briquettes of sawdust, rice and coconut husks: Using water boiling and user acceptability tests. Sci. Afr. 12, e00789 (2021). https://doi.org/10.1016/j.sciaf.2021.e00789
Missagia, B., Guerrero, C., Narra, S., Sun, Y., Ay, P., Krautz, H.J.: Physiochemical properties of rice husk pellets for energy generation. Energy Fuels. 25(12), 5786–5790 (2011). https://doi.org/10.1021/ef201271b
Ndindeng, S.A., Mbassi, J.E.G., Mbacham, W.F., Manful, J., Graham-Acquaah, S., Moreira, J., Dossou, J., Futakuchi, K.: Quality optimization in briquettes made from rice milling by-products. Energy. Sustain. Dev. 29, 24–31 (2015). https://doi.org/10.1016/j.esd.2015.09.003
Prakongkep, N., Gilkes, R.J., Wiriyakitnateekul, W., Duangchan, A., Darunsontaya, T.: The effects of pyrolysis conditions on the chemical and physical properties of rice husk biochar. Int. J. Mater. Sci. 3, 97–103 (2013)
Adan, S.N.F.S., Aiman, J.H.M., Zainuddin, F., Hamdan, Y.: Processing and characterization of charcoal briquettes made from waste straw as a renewable energy alternative. J. Physics: Conf. Ser. 2080. 012014 (2021). https://doi.org/10.1088/1742-6596/2080/1/012014
Kabenge, I., Omulo, G., Banadda, N., Seay, J., Zziwa, A., Kiggundu, N.: Characterization of banana peels waste as potential slow pyrolysis feedstock. J. Sustainable Dev. 11(2), 14–24 (2018). https://doi.org/10.5539/jsd.v11n2p14
Muazu, R.I., Stegemann, J.A.: Effects of operating variables on durability of fuel briquettes from rice husks and corn cobs. Fuel Process. Technol. 133, 137–145 (2015). https://doi.org/10.1016/j.fuproc.2015.01.022
Ajimotokan, H.A., Ibitoye, S.E., Odusote, J.K., Adesoye, O.A., Omoniyi, P.O.: Physico-mechanical properties of composite briquettes from corncob and rice husk. J. Bioresources Bioprod. 4(3), 159–165 (2019). https://doi.org/10.12162/jbb.v4i3.004
Obi, O.F., Pecenka, R., Clifford, M.J.: A review of biomass briquette binders and quality parameters. Energies. 15(7), 2426 (2022). https://doi.org/10.3390/en15072426
Nouar, Y., Nekkaa, S., Fernandez-Garcia, M., Lopez, D.: The thermal and thermomechanical behaviors of Spartium junceum flour reinforced polypropylene composites: Effects of treatment and flour content. Compos. Interfaces. 25(12), 1067–1089 (2018). https://doi.org/10.1080/09276440.2018.1459126
Lui, J., Jiang, X., Cai, H., Gao, F.: Study of combustion characteristics and kinetics of agriculture briquettes using thermogravimetric analysis. ACS Omega. 6, 15827–15833 (2021). https://doi.org/10.1021/acsomega.1c01249
Yusuf, A.A., Inambao, F.L.: Characterization of Ugandan biomass wastes as the potential candidates towards bioenergy production. Renew. Sustain. Energy Rev. 117, 109477 (2020). https://doi.org/10.1016/j.rser.2019.109477
Atan, N.A., Nazari, M.M., Azizan, F.A.: Effect of torrefaction pre-treatment on physical and combustion characteristics of biomass composite briquette from rice husk and banana residue. MATEC Web of Conferences 150, 06011 (2018). https://doi.org/10.1051/matecconf/201815006011
Tako, M., Hizukuri, S.: Gelatinization mechanism of potato starch. Carbohydr. Polym. 48(4), 397–401 (2002). https://doi.org/10.1016/S0144-8617(01)00287-9
Acknowledgements
This work was financially supported by Yosevi Engineering Services Limited, Uganda, www.yosevi.com. Technical support from Mr. Andrew Wabwire from the Department of Mechanical Engineering, Makerere University, Uganda is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lubwama, M., Birungi, A., Nuwamanya, A. et al. Characteristics of rice husk biochar briquettes with municipal solid waste cassava, sweet potato and matooke peelings as binders. Mater Renew Sustain Energy 13, 243–254 (2024). https://doi.org/10.1007/s40243-024-00262-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-024-00262-x