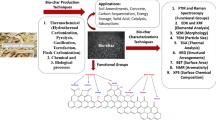
Abstract
Briquetted biomass, like sugarcane bagasse, a by-product of sugar mills, is a renewable energy source. This study aimed at the production and characterization of bagasse briquettes. The production of briquettes was carried out with different blending ratios (5, 10, and 15%) and average particle sizes (0.75, 2.775, and 4.8 mm) with various binders of cow dung, waste paper, and admixture (molasses and wastepaper). The bagasse underwent drying, size reduction, sieving, binder addition, and densification using a manual press during the briquetting process. Characterization of the physical and combustion parameters of briquettes, such as density, shatter resistance, proximate, and calorific value, followed the American Society for Testing and Materials procedures. The result shows that the maximum density of briquettes was 0.804 g/cm3, while shatter resistance varied from 83.051 to 94.975% (4.8mm, 5% cow dung and 0.75mm, 5% admixture binders respectively). ANOVA analysis showed that the factors and their interactions had a significant influence (p value < 0.05) on the physical properties. The optimum parameters of briquettes achieved were 14.953% admixture binder, 0.776 mm particle size, 0.805 g/cm3 density, and 95.811% shatter resistance. Bagasse briquettes with a 5% cow dung binder achieved a high calorific value of 39927.05 kcal/kg. The ultimate analysis revealed a composition of 47.49% carbon (C), 5.133% hydrogen (H), 1.557% nitrogen (N), 0.374% sulfur (S), and 45.446% oxygen (O). Therefore, bagasse has a high calorific value and can be used for briquetting to replace fossil fuel and firewood in different applications. In addition, due to its availability, utilizing as fuel source has economic advantage.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energy plays an important role in human life, affecting many different aspects of daily life. The world energy system is heavily dependent on fossil fuels, but there is growing interest in renewable energy sources, in which biomass plays a significant role [1]. Due to the growing global energy demand, there is a trend to use alternative resources for energy production. Fossil fuels currently dominate global energy consumption, accounting for more than 80% of the global energy consumption. However, these energy sources contribute to greenhouse gas emissions, climate change and rising temperatures [2]. The economy of a developing country is heavily influenced by fossil fuel imports, making the production of renewable energy from biomass significant [3]. Renewable energy sources such as solar, wind and biomass are considered clean and sustainable alternative energy sources that do not never run out [4]. Biomass, from trees, crops, organic waste and various industries, is a promising renewable energy source for 50% of the world’s population, after coal and oil, providing about 1250 million tons of equivalent oil [5, 6]. Biomass has the potential to be converted into energy and fuel through a various of technologies, including direct combustion, thermochemical, biochemical processes and biomass gasification [7, 8]. However, direct burning of biomass is inefficient and leads to the release of unburned carbon and smoke into the environment, while also causing respiratory health problems [9]. Sugarcane bagasse (SCB) is an abundant source of agricultural waste derived from sugarcane processing. Researchers are actively studying its various applications, particularly, in the field of energy and environmental sustainability. Globally, sugarcane production generates about 1.6 billion tons per year, approximately to about 279 million metric tons of sugarcane bagasse. India is the world’s second largest sugarcane producer after Brazil globally. SCB is a major agricultural waste product, with an annual output of 513 million tons worldwide [10,11,12].
Challenges in Ethiopia’s energy sector include modernization and dependence on traditional biomass energy to meet growing demand [13]. Ethiopia has significant unexploited potential in the biomass sector, with 75 million metric tons of biomass, 1120 million metric tons of firewood and 20 million metric tons of agricultural waste [14]. Firewood, crop residues and dungs play a major role in Ethiopia’s energy balance, indicating a significant dependence on these traditional biomass sources [15]. The Wonji Sugar Factory (WSF) in Ethiopia produces a significant amount of sugarcane bagasse as a by-product. The factory’s current production capacity is about 174,946 tons of sugar/year, and the sugarcane crushing capacity/day is 6250 tons of sugarcane [16]. The factory plans to increase sugar crushing capacity/day to 12,500 tons and produce 220,700 tons of sugar/year. Each ton of pressed sugarcane can produce about 300 kg of bagasse [17]. WSF currently produces about 1,800 tons of bagasse per day, corresponding to an annual output of about 540,000 tons in 300 working days. Currently, WSF directly burns part of the bagasse; at 50% moisture, although using this method is neither energy efficient nor environmentally friendly. Excess sugarcane bagasse in the factory causes many problems in terms of storage, management and disposal. This leads to environmental pollution and health risks [18, 19]. To meet the country’s growing energy needs and restore a healthy environment requires a combination of continuous renewable energy supplies and efficient technology [5]. Therefore, converting excess bagasse waste into briquettes through a process that increases their density offers a sustainable solution.
The economic benefits of briquettes lie in their ability to provide an inexpensive alternative to domestic cooking fuel, especially in low-income areas. Energy sources such as wood, charcoal, and LPG can be expensive and harmful to environment. Briquettes, made from agricultural residue such as sugarcane bagasse, coconut shells, and banana peels, offer a sustainable solution. They improve energy efficiency, reduce environmental impact and deliver the lowest life cycle costs compared to fuelwood, wood charcoal, and LPG. Briquettes also contribute to waste management, energy efficiency, and versatile applications, creating additional economic opportunities [20,21,22]. Mixed briquettes, which contain a higher ratios of sugarcane bagasse offer economic benefits because they are cheaper than conventional fuel. They provide a cost-effective alternative to traditional cooking fuels [20]. The use of biomass-based briquettes offers a sustainable and economically viable solution for power generation, reducing reliance on traditional diesel fuel. Several studies have demonstrated the potential of biomass as a viable alternative source for fuel in various energy systems [5, 7, 23, 24]. These studies have demonstrated significant diesel fuel savings, improved engine performance and reduced emissions through biomass gasification. In general, briquette fuel has an advantage due to its renewability properties, higher calorific value, and lower ash content than coal [25].
Various binders can be used in the briquetting process, such as inorganic binders (clay, lime, cement, sodium silicate) and organic binders (biomass, tar pitch, and petroleum bitumen) [19]. Messay et al. [26] studied the production of carbonized sugarcane bagasse briquettes using clay and molasses as binder. However, using clay as binder reduces the combustion efficiency and calorific value, and increases the ash content [27]. In addition, the higher ash content is undesirable in boiler operations involving fouling and slag [28]. Co-gasification of different feedstocks raw materials has shown promising results in dual-fuel engines, further improving the feasibility of biomass-based produced gas [5]. Optimization techniques such as response surface methodology (RSM) have been proven to be effective in improving engine characteristics and significan replacing diesel engines [5, 7]. Inuma et al. (2023) explored the use of waste paper and clay as binders in briquettes production from sugarcane bagasse and promoted sustainable biomass utilization and optimize production using the Box–Behnken Design feature of Design Expert software [29].
The objective of this study was to produce and characterize sugarcane bagasse briquettes by analyzing the effects of mixing ratio, particle size, and organic binder. This study contributes to strengthening the knowledge base on biomass briquette production by exploring alternative binder compositions and optimizing briquette properties. In addition, it provides valuable information for developing suitable optimized briquette formulations tailored for different applications and using experimental designs and statistical analyses (including three-level factorial designs and analysis of variance using ANOVA) to analyze and examine the physical and combustion properties of the sugarcane bagasse briquettes. Unlike previous studies that focused on conventional binders such as starch or clay, this study introduces a unique combination of binders, including cow dung, waste paper, and molasses, to improve the quality of briquettes. It explores different particle sizes and compositions, from 5 to 15% to optimize briquette properties. The study was conducted at Wonji Sugar Factory in Oromia, Ethiopia, which uses locally available resources such as sugarcane bagasse, cow dung, and waste paper, thereby contributing to sustainable energy practices and strategies waste management. This study also provides potential implications for large-scale production and implementation in Ethiopia’s energy sector.
Materials and methods
Study area location
Bagasse was collected from the WSF, located in Oromia region, near Adama town, about 110 km southeast of Addis Ababa. The specific geographic coordinates for the factory’s location are provided in Fig. 1.
Materials and instruments
The primary raw material used in briquette production was sugarcane bagasse, while potential binder sources included cow dung, waste paper, and molasses. The following equipment and instruments, along with their respective models, were used in the procedure:
Oven (BIE & BERNTSEN; A-S), Convection dryer (CE-130 Convection dryer), mortar Grinder (CE-261 study apparatus), mill grinder (HERZOG, D-49086), Weighing balance (electronic beam balance; ISO 9001, National Metrology institute calibration S, No (7) 206308), Sieve analyzer (CE–264 Analytical sieve shaker), Manual Press, Desiccators, Furnace, Crucible, and Oxygen Bomb Calorimeter (IKA-Calorimeter C4000 Adiabatic).
Briquette production process
The process of producing briquettes was carried out by adapting the process described in Bot et al. (2023) [31] and Sharma et al. (2015) [32] while by modifications using the locally available raw materials and equipment (see Scheme 1).
Raw material preparation
Samples were collected and dried using a convection dryer at 75 ℃. Subsequently, it was taken to a grinding unit to reduce it into smaller particles. The ground sugarcane bagasse then was subjected to sieve analysis using sieves with average diameter mesh sizes of 0.75, 2.77, and 4.8 mm.
Binder preparation and mixing
Cow dung, waste paper, and mixture of waste paper and molasses (50:50 w/w) were used as binders. The waste paper was soaked in water to activate the starch. Samples with different particle sizes were mixed with the appropriate binders based on mass ratio. The mixing operation was performed manually with the composition of binder corresponding to binder type.
Energy densification process
The densification was conducted using a manual press with a design pressure of 5 MPa and a cylindrical mold similar to the method used by Thabuot et al. (2015) [33]. Briquettes were prepared by mixing the biomass. The prepared mixture was pressed in a manual press in a cylindrical mold outer diameter of 6.4 cm and inner diameter 2.4 cm. After the compression step, the briquettes were sent for the drying process (Scheme 1, Fig. 2).
Experimental design
Design expert-11 software with Central composite design (CCD) one of the response surface methodologies (RSM) was used in designing the experiments. The study focused on three key factors (waste paper, M + WP, and cow dung), particle size (0.5–5.6 mm), and composition of binder (5–15%). The responses studied include density (g/cm3) and shatter resistance (%). A total of 27 experiments were conducted using non-carbonized sample briquettes, which were subsequently tested for bulk density and shatter resistance.
Characterization of produced briquettes
Physical properties characterization
The bulk density of the samples
The volume of the briquettes was calculated by the difference between the outer volume and the volume of the inner space volume to obtain the actual volume of the briquettes similar to the method used based on Ajimotokan et al. (2019) (Eq. (1)) [34]:
where ρ is the bulk density (g/cm3), m the mass of biomass and briquette (g) and v the volume of briquette (cm3). The cylindrical volume was calculated using the area A and the height of the briquette h:
where R is the radius of the exterior circle and r is the radius of an interior circle of cylindrical mold.
The volume (V) was determined using Eq. (3):
Shatter resistance
The standard method for measuring shatter resistance was used according to ASTM D440-86 [35]. The shatter resistances of the briquette were calculated using the following formula:
where \({W}_{1}\) is the weight of the briquette before shattering (g) and \({W}_{2}\) the weight of the briquette after shattering (g):
Combustion properties characterization of briquette
Proximate analysis (ash basis)
(a) Moisture content (MC) The moisture content on a dry basis was calculated based on Oriabure et al. (2017) [36]; in an oven dryer at 105 \(^\circ{\rm C}\):
where W1 is the initial weight of the sample and W2 is the final weight of the bagasse sample after drying.
(b) Volatile matter (VM) The volatile content was determined according ASTM standards. A 5 g sample was weighed and put into a crucible, which was then subjected to a furnace at a constant temperature of 950 °C. After heating for 7 min, the crucibles were taken out, allowed to cool, and reweighed. The same procedures were applied to both biomass and briquette samples. The volatile matter (%) was calculated as
where \({w}_{i}\) is the initial weight of the sample (g) and \({w}_{f}\) is the final weight of the sample (g).
(c) Ash content Ash content was determined by the gravimetric method using a furnace according to ASTM Standards. The dried biomass and briquette samples 5 g were placed into crucibles and placed in a furnace set to 600 °C for 4 h. Then crucibles containing the samples were removed and cooled in desiccators. The weight of the crucible and the sample was then recorded and the percentage of ash was determined using Eq. (8):
where W1 is the weight of the crucible, W2 is the combined weight of the dry crucible and the dry sample of biomass and W3 is the weight of the dry crucible along with the ash.
(d) Fixed carbon (%FC): The %FC was calculated using the volatile matter, moisture content, and ash amount by difference (9):
where FC is the fixed carbon and VM is volatile matter content.
Calorific value
The calorific value was determined using a bomb calorimeter (IKA-Calorimeter C4000 Adiabatic) at the Messebo cement factory, Ethiopia. The temperature in the jacket was kept constant as the temperature inside the vessel. It was done by taking 0.5 g mass and the cover was then closed and filled with oxygen at 30 bars. When the sample was fully burned the temperature rise was measured and the heat released was determined.
Ultimate (elemental) analysis
The elemental composition of sample (carbon, nitrogen, hydrogen, and sulfur) components was determined using an EA 1112 Flash CHNS/O-analyzer at Addis Ababa University, Department of Chemistry, under carrier gas flow rate of 120 ml/min, reference flow rate 100 ml/min, oxygen flow rate 250 ml/min, furnace temperature of 900 °C, and oven temperature of 75 °C. The ultimate analysis was done for the bagasse and briquette with the highest calorific value 5% cow dung binder. The oxygen fraction was determined by the difference using Eq. (10):
Statistical data analysis
Analysis of variance (ANOVA) was carried out to determine whether there were statistically significant differences in responses due to different treatments. The distribution plot of the bulk density and shatter resistance were analyzed to check for normal distribution by looking for low p-values to identify important terms in the model.
Results and discussion
The effects of particle size, binder type, and composition of binders on the physical and combustion properties of briquettes were tabulated, graphically represented, and discussed.
Physical characterization of briquettes
The physical properties of the briquettes were analyzed and the factors that affect the briquette production, density, and shatter resistance are summarized in Table 1.
The effect of particle size and binder type on the density of briquettes
As shown in Table 1 and Fig. 3, the density of the briquettes varied between 0.395 and 0.804 g/cm3. The highest density achieved was 0.804 g/cm3 when using a binder additive (M + WP). Increasing the binder composition from 5 to 15% and decreasing the average particle size from 4.8 to 0.75 mm resulted in a significant increase in density. Compared to cow dung and waste paper, the admixture binder type consistently provided higher densities across all particle size ranges as shown in Table 1, while waste paper gave the lowest density. The higher density observed with the M + WP binder type can be attributed to the sticky starch quality of molasses, which facilitates closer particle packing. This characteristic allows for a denser arrangement of particles within the briquettes. Factor 3 (binder type) appeared to have a notable effect on the density of the briquettes. Anggraeni et al. reported that the density of the briquettes also depends on the moisture content, and compressed and expanded state of briquettes [37]. Higher results are achieved in this study than the density reported in the literature density of briquette from sugarcane bagasse of 0.470 g/cm3 [31] and the results were in line with those obtained from [38].
As shown in Table 1, briquettes produced using M + WP as the binder (Runs 4, 5, 9, 13, 24, and 25) gave higher densities than briquettes produced using cow dung or waste paper. This means that the selection of binder type can influence the density of the briquettes. The results of the physical characterization of the briquettes show that the use of M + WP can positively affect the density of briquettes. These findings underline that optimizing the binder type in the production process can help improve the quality and durability of briquette.
The density of briquettes varied depending on the binder type. In the waste paper binder, density initially increased as the binder composition increased from 5 to 10% then decreased in 15% binder. However, for other binder types, such as cow dung and admixture (M + WP), the density gradually increases as the binder’s composition varied from 5 to 15%. In fact, waste paper is lighter than bagasse. These gave rise to particles of bagasse compacting and filling the porosities by the binders. Similar trends were obtained and reported by Zepeda in the sawdust briquetting process [39]. The best composition for production of briquettes was determined at a 15% of binder other than the lowest density waste paper binder. Hence, to improve the density, the composition of the binder should be maintained at its highest value, except for waste paper. All binder types had a significant interaction effect on density, as shown in Figs. 4, 5.
However, the most significant effect of binder type and binder composition on the density of the briquettes observed was due to admixture (molasses and waste paper) binder. In a report on waste management and energy recovery on binders press mud, bagasse, and molasses, Olugbade discussed that molasses is the best binder having excellent calorific value similar to these results [40]. Bagasse Briquetting with 15% binder is preferable over other lower binder ratios because it exhibits a more stable and durable briquette during mass storage and long-distance transportation. A similar trend was observed in other studies increasing the molasses content from 0 to 10% resulted in a significant increment in relaxed density. This was attributed to the higher specific gravity of molasses compared to the wood char particle density [39, 41].
Like the density results, the binder type (Factor 3) has an impact on the shatter resistance of the briquettes. Runs 13, 24, and 25, which used M + WP as the binder, achieved higher shatter resistance values compared to the other runs. This indicates that the use of M + WP as a binder can enhance the resistance to shattering and structural integrity of the briquettes.
The effect of particle size, binder type, and binder level on shatter resistance
It can be observed that as the average particle size increased from 0.75 to 4.8 mm, the shatter resistance decreased for all binder types. For the cow dung binder type, the shatter resistance decreased from 88.492 to 84.62%. Similarly, for the admixture (M + WP) binder type, the shatter resistance decreased from 93.944 to 92.78%. The waste paper binder type also exhibited a decrease in shatter resistance, from 89.738 to 88.757%, as the average particle size increased. The smaller the particles, the higher the surface area, which enhances the degree of crystallinity and ability to withstand the forces of shattering. Higher resistance to shattering was achieved with the best binder type of molasses and waste paper admixture. Figure 6 shows the overall interaction of particle size and binder type on the shatter resistance of briquettes.
This show the produced briquettes are highly durable and can withstand resistance during transportation. Zepeda obtained similar with small particle sizes, larger surface area, and improved bonding [39]. In addition, shatter resistance of briquettes should be higher than 90% for handling and transportation on paper pulp and Mesua ferrea briquette mixture [37]. Runs 3, 4, 5, 9, 10, 13, 23, 24, 25, and 27 are shatter resistance higher than 90% so they meet transportation and handling requirements and can be used for various household and industrial applications.
The effect of mixing ratio and particle sizes with molasses and binder type of waste paper is shown in Fig. 6. The study observed the highest shatter resistance of 94.975% in briquettes made from molasses and waste paper binders, while the lowest shatter resistance of 89.62% was observed in briquettes made with an admixture binder type. This finding shows that these briquettes experience minimal weight loss due to shattering or physical forces, indicating their durability and the ability to withstand transportation and delivery without disintegration or weight loss. Similarly, briquettes produced with waste paper binder were analyzed, revealing a range of shatter resistance between 85.984 and 92.792% as the particle size decreased from 4.8 to 0.75 mm, and the binder composition increased from 5 to 15%. This finding aligns with the earlier discussion where the inclusion of a binder resulted in a slight increase in the shatter resistance [41].
These results demonstrate that lower particle sizes and higher binder ratio lead to greater shatter resistance. This indicates that the waste paper binder exhibits considerable resistance against disintegration and weight loss during storage and transport. However, it should be noted that the study mentioned a decrease in shatter resistance at high molasses ratios when binder composition increased, as reported in a previous study by Zepeda-Cepeda et al. (2021) [19]. Accordingly, it is crucial to apply an optimal binder composition for a given particle size during the briquetting process to achieve high-quality briquettes, regardless of the type of binder used. The shatter resistance of the briquettes was influenced by the combined effects of particle size, binder type and binder level. Smaller particle sizes, binder types, and higher binder levels generally resulted in improved shatter resistance. These results emphasize the importance of optimizing these factors during the briquette production process to enhance the durability and resistance to breakage of the briquettes.
Density analysis of variance ANOVA result
The Model F value of 63.01 implies the model is significant; this is a test for comparing the source’s mean square to the residual mean square. There is only a 0.01% chance that an F value this large could occur due to noise. P values below 0.05 indicate model terms are significant. In this case, A, B, C, BC, and B2 are significant model terms. The values above 0.1 indicate the model terms are not significant. Df (Degree of Freedom) indicates the number of independent elements in the sum of squares. In addition, mean square is the ratio of the sum of squares to degrees of freedom. The ANOVA of the density of briquettes produced Table 2 indicates that at a 5% level of significance, binder type, the composition of binder, particle size, and their interactions had significant effects on the density of the briquettes produced (p value < 0.05). Cor Total: shows the amount of variation around the mean of the observations.
Shatter resistance ANOVA result
The model F value of 49.71 shows the model is significant. There is only a 0.01% chance that an F value this large could occur due to noise and P values less than 0.05 shows that model terms are significant. In this case, A, B, C, and Ac are significant model terms. Values greater than 0.1 indicate the model terms are not significant. Besides, Df (Degree of Freedom) designates the number of independent elements in the sum of squares. The Mean Square, the ratio of the sum of squares to degrees of freedom and the ANOVA of the shatter resistance of briquettes produced. Table 3 indicates that at a 5% level of significance, binder type, the composition of binder, particle size, and their interactions had significant effects on the shatter resistance of the briquettes produced (p value < 0.05). Cor Total illustrates the quantity of variation about the mean of the observations. The particle size, the composition of the binder, binder type, and the interaction of particle size and binder type significantly affects the shatter resistance of briquettes. All the factors (particle size, the composition of the binder, binder type) and the interactions have a significant effect on shatter resistance of briquettes with a p value lower than 0.05.
Generally, the statistical analysis of the model’s significance, reflected by the Model F values of 63.01 and 49.71, suggests a low probability of random noise. P values below 0.05 confirm the importance of model terms (A, B, C, BC, B2, AC), while values above 0.1 indicate insignificance. The ANOVA results for briquette density indicate significant effects of binder type, composition, particle size, and their interactions, impacting the density (Table 2). Similarly, for shatter resistance, binder type, composition, particle size, and their interactions significantly influence durability and structural integrity (see Table 3). These findings provide valuable insights for optimizing the production and quality of briquettes. This result is consistent with the statistical analysis reported in the previous studies discussed that the models for responses were statistically significant, with interactive terms (AB, AC, and BC) and quadratic were deemed significant as their values were below 0.05 [9, 29].
The high R2 and adjusted R2 values indicate good predictive ability of the density and shatter resistance models. The density model has a higher R2 value of 0.9253 compared to the shatter resistance model’s R2 of 0.8847, suggesting a slightly better fit (see Table 4). Adeq Precision values for both models indicate an adequate signal-to-noise ratio (density: 30.668, shatter resistance: 27.088), making them effective for process design. The density model has a low coefficient of variation (C.V.) of 0.0202, indicating high precision in the measurements. Similarly, the shatter resistance model has a C.V. of 0.8819, suggesting precision in the experimental treatments. The density model has a small standard deviation of 0.0202, while the shatter resistance model has a larger standard deviation of 0.7911, indicating wider data spread. In conclusion, both models have good predictive abilities, with the density model performing slightly better. These findings provide valuable insights for decision-making processes. Similar results on fit statistics have been reported in the previous studies [29, 42].
Numerical optimization
Numerical optimization was used to find a good set of conditions that will meet all the goals as shown in Fig. 7. The target of optimization was to maximize the density and shatter resistance because they determine the physical properties of the produced briquettes like durability and strength. The process examines for a combination of factors and levels that concurrently satisfy the criteria placed on each of the responses and factors. Factors were automatically included “in range” and the responses were maximized.
Numerical optimization was used in the models to search the factor space for the best trade-offs to achieve multiple goals. The factors particle size, binder level and binder type were optimized in range but the responses density and shatter resistances were set to be maximized. Similarly numerical optimization was used for sugarcane briquettes using binders waste paper and clay [29].
The desirability denotes the objective function which ranges from 0 to 1. Desirability ‘0’ defines outside of the design, while desirability ‘1’ indicates the design is at a goal. Based on this, a particle size of 0.776 mm, the composition of binder 14.953%, with a binder-type molasses and waste paper admixture, a density of 0.805 g/cm3, and shatter resistance of 95.811% are the optimum values obtained.
Validation
The model validation was performed by experimentation again after optimizing the process parameters, to achieve this for the optimized factors experiments were conducted three times at the constant optimized parameters then checked for the responses. The percentage error for each experiment was calculated and the average density was obtained 0.783 \(\pm\) 2.77 g/cm3. The percentage error was relative to an optimized density of 0.806 g/cm3. The average shatter resistance was found 94.84% \(\pm\) 1.41%. In most engineering process design, an error up to 5% is compromised. Therefore, the design model used was valid and it can be applied in related optimization engineering applications. The same validation process has been reported by [43].
Combustion properties of briquettes
Calorific value
Moisture content first vaporizes and condenses into water. Likewise, the vapor formed during combustion is condensed into liquid water. Latent heat from the condensation of steam is recovered. Thus, the calorific value determined using a bomb calorimeter was a gross calorific value (GCV), and the results are given in Fig. 8. The proximate and calorific analyses were conducted for both raw material bagasse biomass and selected briquettes having the best physical properties (i.e., density and shatter resistance), as stated in Fig. 3. The calorific value of raw bagasse was 17.29 MJ/kg (4116.736 kcal/kg), higher than the study on briquette production from sugarcane bagasse investigated by [26], which obtained 3813.83 Cal/g. There are divergences or differences in the results between the two studies, which can be attributed to factors such as variations in sampling, seasonality, and soil fertility. These factors can influence the composition and properties of the bagasse, thereby affecting its calorific value. The calorific value of the briquettes was decreased by adding binders to sugarcane bagasse in all cases. This result is consistent with the studies Awwal et al. and Thabuot et al. who reported that the calorific value of briquettes decreases with adding molasses binders [33, 34]. This result come to agreement with the investigation on the calorific values decrease with increasing binders reported by [7]. Besides, relatively better results are achieved than the calorific value of carbonized briquettes result using clay and molasses report [26]. However, the calorific value is influenced by the type of binder, moisture content, and type of biomass used. It depends on the binder type applied as the binder calorific value of the binder can sometimes be higher than the calorific value of the main biomass. Therefore, the overall calorific value is influenced by the characteristics of the binder and its interaction with the main biomass components [24].
Proximate properties
Moisture content
The sugarcane bagasse was dried to moisture content 6.2% by mass. The briquettes had different moisture contents of 7.19% for waste paper, 5.86% for cow dung, and 5.29% for molasses and waste paper admixture, as shown in Fig. 8. The moisture content has a significant effect on the combustion properties of the briquette as well as its calorific value. When moisture content is high, the briquettes during the combustion process will take some time to ignite, and the amount of heat generated will remain small due to heat loss during evaporation and condensation of the moisture during combustion. This statement is supported by Motta et al., which explains how higher moisture content lowers heating value despite slowing NOX emissions [45]. Better quality briquettes have a moisture content of 5 to 10% as suggested by [46]. Similarly, in the study by Szymajda and Łaska, on biomass cow dung, the calorific value has decreased with increasing moisture content. Hence, briquettes should be dried before application to energy sources to lower their moisture content [47]. This result is consistent with the study by on the torrefaction technique of biomass briquettes [48].
Ash content
Sugarcane bagasse has an ash content of 3.25% by mass; the higher ash content of 13.8% for 5% M + WP represent a higher amount relative to the amount of incombustible, inorganic materials, and this significantly affects the calorific value. Low ash content briquettes will have a high heating value, while high ash contents briquettes have lower calorific values because they contain inorganic compounds. Sugarcane bagasse has low ash content (3.25%) naturally due to of course to the presence of small inorganic materials which is considered a positive circumstance for the use of such renewable resources as raw materials for briquette production.
The results showed that the ash content has increased with the addition of binder in all cases, because the binder used contained highly inorganic incombustible matters. Ash content should be below 20% w/w for industrial boiler applications [49]. This shows all the briquettes produced can be used for industrial boiler operations since they have an ash content of within acceptable limits. Cow dung binder has an ash content of 13% and the binders should be less than 12.5% for fertilizer applications [47]. Hence, the use of cow dung binder increases the ash content and reduces the calorific value from 17.29 to 16.49 MJ/kg. Cow dung binder has higher ash content than other binders. However, it must be noted that ash content alone cannot determine the calorific values to be higher or lower because it is the integrated dependence on moisture, volatile and fixed carbon contents.
Volatile matter content
As shown in Fig. 8, proximate analysis of sugarcane bagasse in dry basis of 74.79 wt. % shows that the raw material contains high volatile matter which comprises combustible gases such as oxides of sulfur, oxides of nitrogen, hydrogen, carbon monoxide, methane, and gases that do not burn like carbon dioxide and water. Cane bagasse briquettes reduced their volatile matter from originally 74.79% of raw feedstock bagasse to 52.89% of cow dung binder-type briquettes. It is observed that, to have a high calorific value, the volatile matter needed to be balanced such that if the volatile matter is too high, the ignition time and burning time will be too short and the calorific value will be minimized. It was reported by Bot et al., that high volatile matter content is easy to ignition and the briquette would freely burn with a high flame during combustion [31, 50].
Fixed carbon
Based on proximate analysis, 15.76 wt. % fixed carbon was obtained. The amount of fixed carbon has increased from 15.76% raw bagasse to 27.85% for 5% Cd non-carbonize briquette for all briquettes added. After the volatile matters are emitted from the potential bagasse fuel, the remaining part is the fixed carbon [51]. This is responsible for the burning of the char and is used as a main heat generation stream in the fuel. Carbon is a measure of the solid combustible material in solid fuel after the removal of volatile matter content. Fixed carbon is responsible for heat generation, whereas high volatile matter content encourages the ignition of fuel easily [52]. With this result, 5% cow dung briquette binder contributes 27.85% fixed carbon which shows that it can generate higher heat during combustion than other briquettes.
Ultimate analysis
The ultimate (elemental) composition of sugarcane bagasse and 5% cow dung the sample with the highest calorific value, briquettes was analyzed. Bagasse showed 47.785% carbon, 5.495% hydrogen, 0.2157% nitrogen, 0.02% sulfur, and 46.4823% oxygen are given in Table 5. A study [48] supports these findings, stating that carbon is the predominant component (30–60% of dry matter), followed by oxygen (30–40%) and hydrogen (5–6%). Nitrogen, sulfur, and chlorine are typically present in small quantities (< 1%) [50, 53, 54]. However, there is a notable difference in nitrogen content between the samples. The 5% cow dung briquette has higher nitrogen content (1.557%) compared to the sugarcane biomass (0.2157%). The literature suggests that nitrogen content in dry biomass is typically less than 1%. Therefore, the higher nitrogen content in the 5% cow dung briquette is attributed to the presence of cow dung as a component. Regarding sulfur content, both samples exhibit relatively low values (0.02% and 0.325%). These findings are consistent with the literature reports, which state that sulfur is typically present in small quantities in dry biomass [53].
The high oxygen content in bagasse, from its main constituents (cellulose, hemicellulose, and lignin), is due to alcohol (OH) and carboxylic acid (COOH) groups [55]. The result revealed that there is a reduction in O–C ratio due to the briquetting effect this is due to the consumption of elemental oxygen. Hydrocarbon fuels with higher hydrogen-to-carbon ratio tend to have a higher energy content [43]. The H/C ratio is an important factor in determining the fuel’s energy potential. Hydrogen has a higher calorific value per unit weight compared to carbon. When hydrogen combines with oxygen during combustion, it releases a significant amount of energy. Therefore, fuels with higher hydrogen content can release more energy during combustion. The quantity of carbon and hydrogen are mainly responsible for the combustibility of the bagasse briquettes. The same trends are achieved with Akowuah et al. (2012) who reported that as the hydrogen-to-carbon ratio and oxygen-to-carbon ratio increase, the overall mass of the briquette fuel decreases during a volatile combustion phase [54].
Conclusion
This study examined the physical and combustion properties of sugarcane bagasse briquettes as an energy source. The findings contribute to the ongoing development of renewable and sustainable energy sources, specifically in the field of biomass application through briquetting technology. By combining molasses and waste paper binders at a composition of 15% binder and a particle size of 0.75 mm, the briquettes achieved a maximum density of 0.804 g/cm3 and a shattering resistance of 94.975%. This suggests that the briquettes are sufficiently robust to withstand disintegration during storage and transportation. The density of the waste paper binder initially increased as the binder composition increased from 5 to 10%, but decreased at 15% binder composition. However, for the cow dung and admixture (M + WP) binders, the density gradually increased as the binder composition changes from 5 to 15%. This indicates that a higher binder composition should be maintained to improve density, except for waste paper binders. An increase in particle size results to decrease in density and shatter resistance for all binders, leading to lower-quality briquette fuels. In contrast, smaller particles with a larger surface area exhibited enhanced crystallinity and better resistance against shattering. Proximate analysis revealed that the raw material had a higher volatile matter, resulting in a high combustion rate, low ash content, and low fixed carbon at given moisture content compared to the produced briquettes. The briquettes with a 5% cow dung binder recorded the highest calorific value of 16.49 MJ/kg, which was lower than the raw material calorific value of 4116.736 kcal/kg. Based on the ANOVA analysis, the optimal values obtained were a particle size of 0.776 mm, a binder composition of 14.953% (molasses and waste paper), a density of 0.805 g/cm3, and a shatter resistance of 95.811%. The ultimate analysis revealed a composition of 47.49% carbon (C), 5.133% hydrogen (H), 1.557% nitrogen (N), 0.374% sulfur (S), and 45.446% oxygen (O) This indicates that briquettes with high carbon and nitrogen contents tend to have a significant amount of energy. This suggests that the addition of binders decreases the calorific values, although the effect depends on the nature of the binders. Generally, the briquettes produced can be utilized for domestic and industrial applications. Further research should focus on carbonized sugarcane bagasse briquettes. The impact of briquetting pressure and temperature on the physical and combustion properties of the briquettes should be investigated in future studies.
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- ASTM:
-
American Society for Testing and Materials
- CCD:
-
Central composite design
- CHNS/O:
-
Carbon, hydrogen, nitrogen, sulfur and oxygen
- CD:
-
Cow dung
- C.V.:
-
Coefficient of variation
- Df:
-
Degree of freedom
- FC:
-
Fixed carbon
- GCV:
-
Gross calorific value
- H:C:
-
Hydrogen to carbon ratio
- Kcal:
-
Kilocalories
- kg:
-
Kilogram
- LPG:
-
Liquefied petroleum gas
- MC:
-
Moisture content
- mm:
-
Millimeter
- M + WP:
-
Molasses + wastepaper
- O:C:
-
Oxygen to carbon ratio
- RSM:
-
Response surface methodologies
- SCB:
-
Sugarcane bagasse
- VM:
-
Volatile matter
- WP:
-
Waste paper
- WSF:
-
Wonji Sugar Factory
References
Bórawski, P., Bełdycka-Bórawska, A., Holden, L.: Changes in the polish coal sector economic situation with the background of the European Union energy security and eco-efficiency policy. Energies (2023). https://doi.org/10.3390/en16020726
Daniyanto, S.D., Budiman, A.: Torrefaction of Indonesian sugar-cane bagasse to improve bio-syngas quality for gasification process. Energy Proc. 68, 157–166 (2015). https://doi.org/10.1016/j.egypro.2015.03.244
Ujjinappa, S., Sreepathi, L.K.: Evaluation of physico-mechanical-combustion characteristics of fuel briquettes made from blends of Areca Nut Husk, Simarouba Seed Shell and Black Liquor. Int. J. Renew. Energy Dev. 7(2), 131–137 (2018). https://doi.org/10.14710/ijred.7.2.131-137
Raj, R., Tirkey, J.V., Jena, P.: Gasification of briquette, mahua wood, and coconut shell and application to CI engines: comparative performance and optimisation analysis. Ind. Crops Prod. 199(December), 116758 (2023). https://doi.org/10.1016/j.indcrop.2023.116758
Raj, R., Tirkey, J.V.: Techno-economic assessment of sugarcane bagasse pith-based briquette production and performance analysis of briquette feed gasifier-engine system. J. Environ. Manage. 345(August), 118828 (2023). https://doi.org/10.1016/j.jenvman.2023.118828
Cantero-Tubilla, B., Sabolsky, K., Sabolsky, E.M., Zondlo, J.W.: Investigation of pretreated switchgrass, corn stover, and hardwood fuels in direct carbon fuel cells. Int. J. Electrochem. Sci. 11(1), 303–321 (2016). https://doi.org/10.1016/s1452-3981(23)15845-7
Raj, R., Tirkey, J.V., Jena, P.: Gasification of Briquette, Mahua wood, and Coconut shell and application to CI engines: comparative performance and optimisation analysis. Ind. Crops Prod. 199(4), 116758 (2023). https://doi.org/10.1016/j.indcrop.2023.116758
Masud, M.H., Ananno, A.A., Arefin, A.M.E., Ahamed, R., Das, P., Joardder, M.U.H.: Perspective of biomass energy conversion in Bangladesh. Clean Technol. Environ. Policy 21(4), 719–731 (2019). https://doi.org/10.1007/s10098-019-01668-2
Gibson, F., Thulu, D., Kachaje, O.: A study of combustion characteristics of fuel briquettes from a blend of banana peelings and Saw Dust in Malawi. Int. J. Thesis Proj. Diss. 4(March), 135–158 (2019). https://doi.org/10.13140/RG.2.2.16237.64485
Pan, S., Zabed, H.M., Wei, Y., Qi, X.: Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: current status, challenges and future outlook. Ind. Crops Prod. (2022). https://doi.org/10.1016/j.indcrop.2022.115684
Yogitha, B., Karthikeyan, M., Muni Reddy, M.G.: Progress of sugarcane bagasse ash applications in production of eco-friendly concrete—review. Mater. Today Proc. 33, 695–699 (2020). https://doi.org/10.1016/j.matpr.2020.05.814
Ajala, E.O., Ighalo, J.O., Ajala, M.A., Adeniyi, A.G., Ayanshola, A.M.: Sugarcane bagasse: a biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour. Bioprocess. (2021). https://doi.org/10.1186/s40643-021-00440-z
Hailu, A.D., Kumsa, D.K.: Ethiopia renewable energy potentials and current state. AIMS Energy 9(1), 1–14 (2020). https://doi.org/10.3934/ENERGY.2021001
Tiruye, G.A., Besha, A.T., Mekonnen, Y.S., Benti, N.E., Gebreslase, G.A., Tufa, R.A.: Opportunities and challenges of renewable energy production in ethiopia. Sustain 13(18), 1–25 (2021). https://doi.org/10.3390/su131810381
Mondal, M.A.H., Bryan, E., Ringler, C., Mekonnen, D., Rosegrant, M.: Ethiopian energy status and demand scenarios: prospects to improve energy efficiency and mitigate GHG emissions. Energy 149, 161–172 (2018). https://doi.org/10.1016/j.energy.2018.02.067
Bati Fedi, G., Dereje Asefa, F., Tafa Waktole, A.: Farm households’ perception about sugarcane outgrowers’ scheme: empirical evidence around Wonji/Shoa Sugar Factory. Cogent Econ. Financ (2022). https://doi.org/10.1080/23322039.2021.2009664
Sajad Hashemi, S., Karimi, K., Majid Karimi, A.: Ethanolic ammonia pretreatment for efficient biogas production from sugarcane bagasse. Fuel 248(February), 196–204 (2019). https://doi.org/10.1016/j.fuel.2019.03.080
Kabeyi, M.J.B., Olanrewaju, O.A.: Bagasse electricity potential of conventional sugarcane factories. J. Energy 2023, 1–25 (2023). https://doi.org/10.1155/2023/5749122
Zhang, G., Sun, Y., Xu, Y.: Review of briquette binders and briquetting mechanism. Renew. Sustain. Energy Rev. 82(January 2017), 477–487 (2018). https://doi.org/10.1016/j.rser.2017.09.072
Bot, B.V., Axaopoulos, P.J., Sakellariou, E.I., Sosso, O.T., Tamba, J.G.: Economic viability investigation of mixed-biomass briquettes made from agricultural residues for household cooking use. Energies 16(18), 6469 (2023). https://doi.org/10.3390/en16186469
Bot, B.V., Axaopoulos, P.J., Sosso, O.T., Sakellariou, E.I., Tamba, J.G.: Economic analysis of biomass briquettes made from coconut shells, rattan waste, banana peels and sugarcane bagasse in households cooking. Int. J. Energy Environ. Eng. 14(2), 179–187 (2023). https://doi.org/10.1007/s40095-022-00508-2
Raj, R., Kumar Singh, D., Vachan Tirkey, J.: Performance simulation and optimization of SI engine fueled with peach biomass-based producer gas and propane blend. Therm. Sci. Eng. Prog. 41(May), 101816 (2023). https://doi.org/10.1016/j.tsep.2023.101816
Raj, R., Kumar Singh, D., Vachan Tirkey, J.: Performance simulation and optimization of SI engine fueled with peach biomass-based producer gas and propane blend. Therm. Sci. Eng. Prog. 41, 101816 (2023). https://doi.org/10.1016/j.tsep.2023.101816
Raj, R., Kumar Singh, D., Tirkey, J.V.: Co-gasification of Low-grade coal with Madhuca longifolia (Mahua) biomass and dual-fuelled mode engine performance: Effect of biomass blend and engine operating condition. Energy Convers. Manag. 269(March), 116150 (2022). https://doi.org/10.1016/j.enconman.2022.116150
Elsisi, S., Omar, M., Samak, A., Gomaa, E., Elsaeidy, E.: Production the briquettes from mixture of agricultural residues and evaluation its physical, mechanical and combustion properties. Agric. Eng Misr J (2023). https://doi.org/10.21608/mjae.2023.219214.1107
Messay, E.G., Birhanu, A.A., Mulissa, J.M., Genet, T.A., Endale, W.A., Gutema, B.F.: Briquette production from sugar cane bagasse and its potential as clean source of energy. Afr. J. Environ. Sci. Technol. 15(8), 339–348 (2021). https://doi.org/10.5897/AJEST2021.3006
Obi, O.F., Pecenka, R., Clifford, M.J.: A Review of biomass briquette binders and quality parameters. Energies 15(7), 1–22 (2022). https://doi.org/10.3390/en15072426
Hariana, A., Prismantoko, G.A., Ahmadi, A., Darmawan, A.: Ash evaluation of indonesian coal blending for pulverized coal-fired boilers. J. Combust. (2021). https://doi.org/10.1155/2021/8478739
Inuma, F.M., Mohammed, J., Bawa, M.A.: Production and optimization of briquettes from sugarcane bagasse using blends of waste paper and clay as binders. J. Appl. Sci. Environ. Manag. 27(3), 571–577 (2023). https://doi.org/10.4314/jasem.v27i3.22
Dengia, A., Dechassa, N., Wogi, L., Amsalu, B.: Analysis of declining trends in sugarcane yield at Wonji-Shoa Sugar Estate, Central Ethiopia. Exp. Results (2023). https://doi.org/10.1017/exp.2023.13
Bot, B.V., Sosso, O.T., Tamba, J.G., Lekane, E., Bikai, J., Ndame, M.K.: Preparation and characterization of biomass briquettes made from banana peels, sugarcane bagasse, coconut shells and rattan waste. Biomass Convers. Biorefinery 13(9), 7937–7946 (2023). https://doi.org/10.1007/s13399-021-01762-w
Sharma, M.K., Priyank, G., Sharma, N.: Biomass briquette production: a propagation of non-convention technology and future of pollution free thermal energy sources Manoj Kumar Sharma, Gohil Priyank. Nikita Sharma. Am. J. Eng. Res. (AJER) 2, 44–50 (2015)
Thabuot, M., Pagketanang, T., Panyacharoen, K., Mongkut, P., Wongwicha, P.: Effect of applied pressure and binder proportion on the fuel properties of holey bio-briquettes, vol. 79. Elsevier B.V (2015). https://doi.org/10.1016/j.egypro.2015.11.583
Ajimotokan, H.A., Ibitoye, S.E., Odusote, J.K., Adesoye, O.A., Omoniyi, P.O.: Physico-mechanical properties of composite briquettes from corncob and rice husk. J. Bioresour. Bioprod. 4(3), 159–165 (2019). https://doi.org/10.12162/jbb.v4i3.004
Dohm, E., Luttrell, G., Bratton, R., Ripepi, N., Taulbee, D.: Evaluation of methods used to quantify the durability of coal-biomass briquettes. In: 29th Annu. Int. Pittsburgh Coal Conf. 2012, PCC 2012, vol. 1, no. September 2016, pp. 148–157 (2012)
Oriabure, E.D., Terzungwue, T.E., Emoh, O.F.: Density, shatter index and heating value of briquettes produced from the leaves of terminalia mentalis and sawdust of daniela oliveri. Int. J. Pure Agric. Adv. 1(1), 38–44 (2017). https://doi.org/10.20448/813.11.38.44
Anggraeni, S., Girsang, G.C.S., Nandiyanto, A.B.D., Bilad, M.R.: Effects of particle size and composition of sawdust/carbon from rice husk on the briquette performance. J. Eng. Sci. Technol. 16(3), 2298–2311 (2021)
Onukak, I.E., Mohammed-Dabo, I.A., Ameh, A.O., Okoduwa, S.I.R., Fasanya, O.O.: Production and characterization of biomass briquettes from tannery solid waste. Recycling (2017). https://doi.org/10.3390/recycling2040017
Zepeda-Cepeda, C.O., et al.: Effect of sawdust particle size on physical, mechanical, and energetic properties of pinus durangensis briquettes. Appl. Sci. (2021). https://doi.org/10.3390/app11093805
Olugbade, T., Ojo, O., Mohammed, T.: Influence of binders on combustion properties of biomass briquettes: a recent review. Bioenergy Res. (2019). https://doi.org/10.1007/s12155-019-09973-w
Tanui, J.K., Kioni, P.N., Kariuki, P.N., Ngugi, J.M.: Influence of processing conditions on the quality of briquettes produced by recycling charcoal dust. Int. J. Energy Environ. Eng. 9(3), 341–350 (2018). https://doi.org/10.1007/s40095-018-0275-7
Ossei-bremang, R. N., Kemausour, F.: Industrial symbiosis and circularization: optimal shelf life of waste-based briquettes,” pp. 0–16 2023.
Yue, L., Li, G., He, G., Guo, Y., Xu, L., Fang, W.: Impacts of hydrogen to carbon ratio (H/C) on fundamental properties and supercritical cracking performance of hydrocarbon fuels. Chem. Eng. J. 283(September 2018), 1216–1223 (2016). https://doi.org/10.1016/j.cej.2015.08.081
Awwal, O.A., Israel, O.K., Yashim, Z.: Physico-chemical, calorific, and emission performance of briquettes produced from maize cob, sugarcane bagasse, and polythene composites. Avicenna J. Environ. Heal. Eng. 6(1), 49–54 (2019). https://doi.org/10.34172/ajehe.2019.07
Motta, I.L., Miranda, N.T., Maciel Filho, R., Wolf Maciel, M.R.: Biomass gasification in fluidized beds: a review of biomass moisture content and operating pressure effects. Renew. Sustain. Energy Rev. 94(2017), 998–1023 (2018). https://doi.org/10.1016/j.rser.2018.06.042
Aransiola, E.F., Oyewusi, T.F., Osunbitan, J.A., Ogunjimi, L.A.O.: Effect of binder type, binder concentration and compacting pressure on some physical properties of carbonized corncob briquette. Energy Rep. 5, 909–918 (2019). https://doi.org/10.1016/j.egyr.2019.07.011
Szymajda, A., Łaska, G.: The effect of moisture and ash on the calorific value of cow dung biomass, p. 4 (2019). https://doi.org/10.3390/proceedings2019016004
Saeed, M.A., Ahmad, S.W., Kazmi, M., Mohsin, M., Feroze, N.: Impact of torrefaction technique on the moisture contents, bulk density and calorific value of briquetted biomass. Polish J. Chem. Technol. 17(2), 23–28 (2015). https://doi.org/10.1515/pjct-2015-0024
Navalta, C.J.L.G., Banaag, K.G.C., Raboy, V.A.O., Go, A.W., Cabatingan, L.K., Ju, Y.H.: Solid fuel from Co-briquetting of sugarcane bagasse and rice bran. Renew. Energy 147, 1941–1958 (2020). https://doi.org/10.1016/j.renene.2019.09.129
Ajimotokan, H.A., Ehindero, A.O., Ajao, K.S., Adeleke, A.A., Ikubanni, P.P., Shuaib-Babata, Y.L.: Combustion characteristics of fuel briquettes made from charcoal particles and sawdust agglomerates. Afr Sci (2019). https://doi.org/10.1016/j.sciaf.2019.e00202
Iryani, D.A., Kumagai, S., Nonaka, M., Sasaki, K., Hirajima, T.: Characterization and production of solid biofuel from sugarcane bagasse by hydrothermal carbonization. Waste Biomass. Valoriz. 8(6), 1941–1951 (2017). https://doi.org/10.1007/s12649-017-9898-9
Patil, R.A., Deshannavar, U.B., Ramasamy, M., Emani, S., Issakhov, A., Khalilpoor, N.: Briquetting of dry sugarcane leaves by using press mud, cow dung, and buffalo dung as binders. J. Chem. Eng. Int. (2021). https://doi.org/10.1155/2021/8608215
Chaney, J. O.: Combustion characteristics of biomass briquettes by Joel Chaney, MSci Physics with French,” p. 229 (2010)
Akowuah, J.O., Kemausuor, F., Mitchual, S.J.: Physico-chemical characteristics and market.pdf. Int. J. Energy Environ. Eng. 3(20), 1–6 (2012)
Raj, R., Kumar Singh, D., Vachan Tirkey, J.: Performance simulation and optimization of SI engine fueled with peach biomass-based producer gas and propane blend. Therm. Sci. Eng. Prog. 41(6), 101816 (2023). https://doi.org/10.1016/j.tsep.2023.101816
Funding
This study was supported by Mekelle University, PO Box 231, Mekelle, Ethiopia through Grant No.:PG/MSc/EiTM/MU-NMBU/40/2019.
Author information
Authors and Affiliations
Contributions
AGM and GGB conceived the problem of the study. All the authors prepared the research proposals and developed the design of the experiments. AGM and GGB prepared the first draft of the manuscript, and all the authors reviewed it to produce the final draft. All the authors reviewed and approved the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mekonen, A.G., Berhe, G.G., Desta, M.B. et al. Production and characterization of briquettes from sugarcane bagasse of Wonji Sugar Factory, Oromia, Ethiopia. Mater Renew Sustain Energy 13, 27–43 (2024). https://doi.org/10.1007/s40243-023-00248-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-023-00248-1