Abstract
The crown leaves of pineapple possess a wealth of smooth and glossy silk medium-length fibers, primarily composed of cellulose and lignin, accompanied by constituents such as fats, waxes, pectin, uronic acid, anhydride, pentosan, color pigments, and inorganic substances. These fibers exhibit an anisotropic nature and are characterized by hydrogen bonding interactions, rendering them effective in conjunction with semiconductor oxide (TiO2) through their cellulosic fibrils. The dye extracted from Pineapple Crown Leaves (PCL) using ethanol was subjected to FTIR and UV–visible spectroscopy. The FTIR analysis revealed absorption peaks at 3268 cm−1 and 2922 cm−1, confirming the presence of –OH and –CH stretching attributed to the fibrils within the dye. UV–visible spectroscopy further demonstrated absorption within the visible region of the electromagnetic spectrum. Additionally, a photoluminescence study of the dye showcased emission within the visible range of the electromagnetic spectrum. Subsequently, a solar cell incorporating this dye underwent JV characterization, yielding an efficiency of 1.0034%, along with fill factor, open-circuit voltage, and short-circuit current density values of 0.40644, 0.7058 V, and 3.4906 mA/cm2, respectively. To gain deeper insights and facilitate optimization for large-scale installations, a simulation model utilizing PC1D was proposed to explore the influential parameters of the Dye-sensitized solar cell (DSSC).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solar cells represent vital devices for generating electrical energy to meet the demands of our daily lives. Among these, third-generation solar cells, particularly dye-sensitized solar cells (DSSCs) have garnered significant attention due to their cost-effectiveness, ease of fabrication, and eco-friendly attributes. Evaluating the performance of DSSCs can be achieved through experimental methodologies employing solar simulators equipped with electrometers. Alternatively, computational simulations using specialized software provide a means to assess solar cell performance without conducting physical experiments. Numerous experiments have been undertaken to explore the potential of DSSCs, employing a wide array of natural and synthetic dyes and delving into the pigments present within these dyes. Additionally, researchers have engaged in solar cell simulations, manipulating various parameters to comprehensively analyze and optimize their performance.
Martin Esteves et al. [1] have used Lithium Titanate nanotubes as photoanode in dye-sensitized solar cell and undergone DFT simulation to determine the properties. Results have shown that the proposed cell produced an efficiency of 7.7% which is higher than the hydrogen titanate nanotube. Subrata Sarker and Ding Min Kim [2] have conducted a study on DSSC with reduced graphene oxide in counter electrode and proposed an analytical model to evaluate the performance of the cell. It has been found that the counter electrode with small charge transfer resistance is more effective than platinum-based counter electrode. Kavitha et al. [3] have used luminescence high thermal stability to analyze the bioimaging and has shown faster to produce higher efficiency to intermolecular metal-legend. Varadarajan et al. [4] have utilized single crystal to study the lysine activates process and enhanced the optoelectronics devices for the growth of single crystal.
Weiyi Zhang et al. [5] have studied three dyes with different donors and acceptors comprising dithiafulvenyl, phenothiazine and cyanoactylc acid with different π groups by conducting molecular dye by simulation. It is observed that suitable groups with appropriate configuration led to the enhancement of the performance of the dye. Sangiorgi et al. [6] have made a thin layer of TiO2 of thickness 5 μm and used three thiazolo [5,4-d] thiazole dyes as sensitizers and named as fibre-shaped dye-sensitized solar cell. The experimental results have proved that the proposed cell with TTZ7 dye in DSSC has shown a power conversion efficiency of 0.80% and it is higher than the cell with N719 dye.
Following this, Shanmugan et al. [7] have undergone thorough antibacterial activites using Ag silver nanoparticles from citrus aurantifolia. Inferences of the studies by various researchers have been presented precisely for the benefits for medicine for antiseptic additives. Latha Devi and Shanmugan [8] have proposed a new model to simulate the performance of spatial patterrns by varying the weather conditions. Results of the study have proven the efficacy of the model to evaluate the performance of the domain of the southeastern sites. Vinu et al. [9] have conducted a simulation study for dye-sensitized solar cell with molybdenum doped TiO2 in photoanode by 10% and 5% by weight. The results of the study have proven that 5% wt of molybdenum with TiO2 has shown good results compared to bare TiO2. Latha Devi and Shanmugan [10] have undergone a simulation of weather conditions and Pearson correlation coefficient efficiency is 0.96. Dheeraj Devadiga et al. [11] have experimented a dye-sensitized solar cell incorporated with a novel organic sensitizer of azine group co-sensitized with Z907. It is found that the cell has produced efficiency of 9.70% higher than the cell without Z907. Casadio et al. [12] have used TTZ5 sensitizer in fibre-shaped DSSC which is long and flexible with 5 µm thickness of photoanode. It has been found that the cell has given efficiency of 1.23% which can be optimized. Jae Ho Kim et al. [13] have embedded metal–organic framework (mesoporous TiO2). It is used as a photo anode in DSSC. The performance analysis of the study revealed that the usage of metal–organic framework has reduced the series resistance and increased the shunt resistance by controlling the recombination of electrons. Ruba et al. [14] have varied the concentration of eosin-y dye in the photoanode of DSSC and examined the performance. It is observed that the concentration of 10 mg of eosin-y dye has given an efficiency of 0.39% compared to other concentrations. Sowmya et al. [15] have used the dyes extracted from leaves of prosopisjuliflora and used in the photoanode of DSSC using the solvents ethanol and acetone and experimented. It is observed that the cell fabricated with the dried leaves of prosopisjuliflora using acetone has given an efficiency of 0.322%.
Further, Sowmya et al. [16] have fabricated solar cell devices with bulk TiO2 coated over the FTO substrate and tested for JV characteristics. It has been found that the device did not show cell characteristics and revealed that bulk TiO2 could not be used for making the device. Gireesh Baiju et al. [17] have proposed a mathematical model for a flexible dye-sensitized solar cell made of flexible polymer comprising of TiO2 film deposited indium tin oxide coated polyethylene teripthalate. It has been found that the flexible DSSC can be optimized using various control parameters of the solar cell. Inbarajan et al. [18] have undergone an experiment with a dye-sensitized solar cell incorporated with the dye extracted from allium cepa using the solvent ethanol directly and by Soxhlet extractor. It has been found that the cell made by direct extraction of dye using ethanol has produced a higher efficiency of 1.17%. Pooja Prakash et al. [19] have extracted the dyes from poinsettia, fruits of tamarillo, seeds from annatto and pungent chillies and used them separately as sensitizer as well as the cocktail of dyes in photoanode of DSSC and studied the performance. It is found that the cell with the dye from bracts of poinsettia has give higher efficiency of 1.74% and also shows the stability of anthocyanin pigments. Akula Surya Teja et al. [20] have conducted a review of the recent processing technology and the effect of various dyes extracted from different parts of the plants and the inferences of the study have been presented precisely. Futuristic approach for the enhancement of the performance of the solar cell has been highlighted with precise research works carried out.
Shabaan A.K. Elroby et al. [21] have fabricated a solar cell with phenothiazine phenoxazine and 5,10-dimethyphenazine donors and architecture of phenyl with D-A’-(π-A)2 followed by the conduction of DFT calculation. Results have been presented precisely with geometrical, electrochemical and optical properties of the dye. In a study conducted by Meena et al. [22], experiments were conducted using dye-sensitized solar cells incorporating a layer of P25 TiO2 nanoparticles (NPs) applied onto a fluorine-doped tin oxide substrate. The research outcomes reported maximum photo-conversion efficiencies of 7.35%, 6.84%, and 7.04% for beetroot, grapes, and red cabbage, respectively. Pooja and Janarthanan [23] have prepared Kumkum dye using turmeric and CaCO3 and used it as sensitizer in photoanode of DSSC for the performance. The experimental results of the study have shown that the dye rich in flavonoid polyphenol is effective for absorbing solar radiation to a larger extent. Abdulmohsen [24] conducted a study involving the creation of a nanocomposite utilizing cobalt (II) chloride (CoCl2), thiourea (CH4N2S), and silicon dioxide (SiO2) to produce a novel hybrid nanofluid with varying concentrations of up to 60%. This hybrid nanofluid was developed with the aim of improving heat transfer within the system. Results of the various researches have been presented which will be helpful for future researchers. Gandhi et al. [25] conducted a study on an energetic SiO2/TiO2 nanolayer, and it was observed that a system with a thermal conductivity ratio of 30% resulted in a 40.241% improvement in thermal efficiency. This enhancement in thermal efficiency led to improved herbal extract production and high-quality natural purified water. Rajesh Kumar et al. [26] focused on the green synthesis of silicon dioxide nanoparticles using leaf extract of Jatropha curcas L. (JCL). They evaluated the photocatalytic treatment of the system, and SiO2 NPs/JCL showed increased energy absorption, contributing to enhanced nighttime performance through distillation. Emad et al. [27] explored the use of cobalt oxide in PV evacuated tubes to extend the heat-integrated energy system. Arulraj Simon Prabu et al. [28] and Rajasekar et al. [29] studied Cu2ZnSnS4 thin films, while Dedeepya et al. [30] developed leaf extract of siriyanangai using DSSC for energy enhancement. Asha et al. [31] synthesized TiO2/Jackfruit peel for enhanced energy storage material. Shanmugan et al. [32] discussed solar cells from an absorption viewpoint, and Shanmugan et al. [33] focused on biogenic silver nanoparticles.
Doranehgard et al. [34] produced graphite powder mixed with black paint on the absorber plate to generate more heat energy. Kavitha et al. [35] improved plant extracts with silver nanoparticles, and Palanikumar et al. [36] developed nanocomposite/PCM. Furthermore, Palanikumar et al. [37] created a film of Ta2O5 nanoparticles with doping SnO2–Ag on solar absorber material, and Thamizharasu et al. [38] developed a SiO2/TiO2 nanolayer. Furthermore, a comprehensive exploration and documentation of natural fibers sourced from various plants, renowned for their robust and durable characteristics, have been carried out extensively. Further various types of natural fibers derived from various plants and their harder and stronger nature have been studied extensively and documented by Issam et al. [39].
To gain a comprehensive understanding of the current landscape in dye-sensitized solar cells (DSSCs), an extensive review and analysis of literature encompassing research on dyes as sensitizers and simulation modeling conducted by fellow researchers were conducted. In the present study, a pivotal endeavor was undertaken to extract dye rich in fibers from the crown leaves of pineapple fruit. The extracted dye was subsequently subjected to FTIR and UV–visible spectroscopy to ascertain its properties. Additionally, a thorough examination of the dye's fibrils was carried out to understand its functional groups and their anchoring effects on the semiconductor oxide material, specifically TiO2. The surface morphology of the film comprising TiO2/dye was examined through SEM photography, and the stability of the resulting solar cell was assessed. Furthermore, to provide a more precise assessment of the factors influencing the performance of the proposed solar cell, a simulation model employing PC1D was formulated. This model allows for the determination of the impact of various parameters on the solar cell's performance, and the results are presented and interpreted in detail.
Experiment
Materials and methods
The production process of dye-sensitized solar cells encompasses several distinct stages, including dye extraction, photoanode fabrication, and electrolyte preparation, counter-electrode construction, and the final assembly of the solar cell. Each of these fabrication steps is elaborated upon in the subsequent sections.
Extraction of dye
The crown leaves from the queen variety of pineapple are collected in a tray and completely washed using deionized water to remove the dust impurities. Then the leaves are dried in a shadow region for more than 2 days, such that to stimulate retted fibres which will be strong enough without deterioration of their properties. The Soxhlet extractor is provided with the 50gms of crushed powder of the crown leaves and 500 mL of ethanol has been introduced into the round-bottomed flask. The apparatus is set into the position using the clamps and tap water is allowed to circulate around the condenser to increase the condensation process to get the extract. Figure 1 shows the photograph of the extraction process of the dye (PCL) using the Soxhlet extractor. The structure of the lignin, cellulose and hemicellulose structures [39, 40] have been derived from the researchers and is shown in Fig. 2. It is clear from the structure that, the edges of the compounds has –OH group and the bonding of the dye with TiO2 is through the group and clearly seen in the structure.
Preparation of the electrolyte
The iodine/triiodide redox couple was prepared by combining key precursors, namely acetic acid, lithium iodide, and iodine solution, which play a critical role in regenerating the dye as photoelectric current flows through the system. Specifically, 0.67 g of lithium iodide, 10 ml of acetonitrile, and 0.13 g of iodine were thoroughly mixed in a beaker for a duration of 15 min. This resulting mixture served as the electrolyte within the designed solar cell.
Counter electrode
The counter electrode was fashioned using graphite, which was uniformly applied to the FTO substrate, as an alternative to employing a platinum-based counter electrode. Subsequently, the photoanode and counter electrode were securely sandwiched together using a binder clip, with the liquid electrolyte introduced in between to form a thin layer. Electrical connections were established by applying silver paste to both the photoanode and counter electrode. A visual representation of the assembled solar cell can be found in Fig. 3.
The photoanode of the constructed solar cell boasts an effective surface area measuring 1.5 cm × 1.5 cm. It features a uniform coating of TiO2 infused with the dye extracted from the crown leaves of the pineapple. The TiO2 nanopowder, sourced from Sigma Aldrich, exhibits an average particle size of 20 nm, with a molecular weight of 79.87 g/mol. This nanopowder boasts a density of 4.26 g/cm3 and is insoluble in water, dilute acids, and organic solvents. Notably, the nanopowder comprises both anatase and rutile phases, with proportions of 85% and 15%, respectively.
The photoanode of the fabricated solar has an effective area of 1.5 cm × 1.5 cm which has the homogeneous coating of TiO2 soaked with the dye extracted from the crown leaves of the pineapple. The TiO2 nanopowder is bought from Sigma Aldric which has an average particle size of 20 nm with a molecular weight of 79.87 g/mol. The density is 4.26 g/cm3 which is insoluble in water, dilute acids and organic solvents. The nanopowder has both anatase and rutile phase of 85% and 15%, respectively.
Results and discussions
FTIR spectroscopy of the dye
FTIR spectroscopy of the dye has been recorded using IRAffinity with MIRacle 10 in ATR mode and presented in Fig. 4. From the FTIR spectroscopy, it is observed that the significant hydroxyl group with hydrogen bonded accompanied with –OH stretching is obtained at 3268 cm−1 and it is broad. This is due to the fact that the presence of lignocellulosic fibre present in the dye has hydrogen bonding with the hydroxyl group. The second prominent peak obtained at 2922 cm−1 corresponding to the C–H asymmetric/symmetric stretching with methylene group (> CH2). The C–H stretching extends extensively in the dye to respond to the photonic energy for absorption and transfer of the energy to LUMO level electron which excites into the energy level equal to that of semiconductor oxide material. Further, the peak at 1609.99 cm−1 corresponds to the C=C stretching which is a double bond representing the stability of the dye as the cleavage of the bond is quite difficult. Aromatic primary amine with CN stretching is present in the dye and represented in the peak at 1335.95 cm−1. In addition to stretching, there exist C–C vibrations represented by the peaks at 1226.30 cm−1, 1148.66 cm−1 and 1012.69 cm−1 is corresponding to the methyne group. The peak corresponding to 1012.69 cm−1 resembles and approaches the threshold wave number of 1033.33 cm−1 to undergo absorption in a significant manner. Methylene group is also present and peak obtained at 763.48 cm−1. The functional groups have a strong impact in making the perfect anchor leading to a strong bond with the TiO2 material. It is confirmed that, the major compounds in the dye comprising of cellulose, hemicellulose and lignin [39, 41,42,43] with the functional groups as obtained from FTIR. The structure of the lignin, cellulose and hemicellulose [44] has been derived from the researchers. It is clear from the structure that, the edges of the compounds has –OH group and the bonding of the dye with TiO2 is through the group and clearly seen in the structure as shown in Fig. 2.
UV–visible spectroscopy
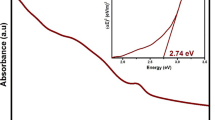
The UV–visible spectroscopic analysis of the dye was conducted using a Shimadzu UV–visible spectrometer from the UV-1700 series. Figure 5 illustrates the UV–visible spectroscopy results obtained for the dye extracted from pineapple crown leaves. The analysis revealed that the dye exhibits responsiveness across a wide range of wavelengths, encompassing both ultraviolet and visible regions within the electromagnetic spectrum. In the ultraviolet region, a prominent peak was observed at 330 nm, indicating the dye's capacity to efficiently absorb energy from the ultraviolet spectrum without any degradation of the pigments present in the dye. Moving into the visible region, the dye exhibited absorption from violet to red, with varying absorption intensities ranging from 0.9 to 0.2 absorbance units (A.U.). Notably, an absorption peak at 680 nm demonstrated the dye's ability to absorb even low-energy photons, which subsequently excite electrons, a phenomenon referred to as surface plasmons. Additionally, It is also confirmed the presence of chlorophyll pigments in the dye, responsible for the photogeneration of electron–hole pairs, was confirmed. Furthermore, a minor bump in the absorption spectrum at 680 nm, with absorption of 0.45 A.U., provided evidence for the efficient photogeneration of electrons by the dye molecules.
FTIR and UV–Visible Spectroscopy of the Photoanode
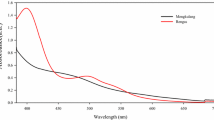
As a confirmation of the good anchoring of the dye with the TiO2 is confirmed by the FTIR and UV–visible spectroscopic study of the soaked TiO2-coated FTO substrate with the extracted dye. Figure 5a, b represent the FTIR and UV–visible spectroscopy of the FTO/TiO2/dyeto confirms the anchorage and sensitization of the dye for the intercepting radiation over the photoanode. From Fig. 5a it is seen that the peak at 3268 cm−1 and 2922 cm−1 for the dye solution indicating the OH and CH stretching has disappeared for the photoanode confirming the bonding of the stretching group with the TiO2 semiconductor oxide material. Further, the absence of a peak greater than 1000 and lesser than 1500 cm−1 reflects the presence of solvent and its interaction with the TiO2 material. From Fig. 5b, it is clear that TiO2 with the dye has shown broad absorption between 285 to 315 nm confirms the presence of chlorophyll as it shows absorption stronger which is similar to the results obtained. It is observed that there is a redshift with a decrease in intensity of absorption for the TiO2/dye sample indicating the chlorophyll absorption which is sensitizing the photons without fail.
Photoluminescence study of the dye
The dye has been subjected to photoluminescence spectroscopy and it is represented in Fig. 6. The PL emission spectra revealed that the most prominent emission occurred at 430 nm which clearly inferred that the dye has significant absorption of violet color wavelength. It has been concluded that the dye has the ability to absorb energy in the visible region effectively. Further, the emission is absorbed at 570 nm nearing the yellow region of the visible electromagnetic spectrum. From the observation, it is clear that the excitation of the photoelectrons occurred at the visible region of the electromagnetic spectrum which is intact with the inferences obtained from the UV–visible spectroscopy. Further, it is clear that the dye is an effective sensitizer and sensitizes the photon with variable energy corresponding to the wavelength varying from violet to red colour of the visible wavelength. The solar cell utilized in this study possesses a strategic design, enabling it to efficiently extract protons from solar energy using crown leaves from the queen variety of pineapple. Unlike conventional silicon-based solar cells, this particular solar cell does not require the expensive TiO2-coated FTO substrate, resulting in significantly reduced manufacturing costs. However, despite the cost advantage, the commercial adoption of these solar cells has been hindered by their relatively poor chemical stability and low photo conversion efficiency (PCE). To address these limitations, the utilization of natural dyes as alternatives to ruthenium in DSSCs has been explored. Notable examples include chlorophyll, beta-carotene, and anthocyanin, which have demonstrated the potential to enhance energy conversion efficiency. Figure 7 shows the SEM photograph of the photoanode with the incorporation of dye on the TiO2-coated FTO substrate.
JV characteristics of the dye
Keithley 2400 Graphical series SMU model provided with a solar simulator with an Xenon lamp of 100 mW/cm2 is used to take the JV characteristics of the fabricated solar cell. Experiment has been conducted with the fabricated dye-sensitized solar cell such that the ambient temperature is about 32 °C and inside the physics laboratory. The fabricated solar cell with the sensitizers derived from the crown leaves of pineapple shows solar cell characteristics. Graph has been plotted between the voltage and current density and represented in Fig. 8. From the figure, the solar cell parameters of short circuit current density, open circuit voltage, maximum voltage, maximum current density, fill factor and efficiency is 3.4906 mA/cm2, 0.7058 V, 0.3998, 2.5026, 0.40644 and 1.00134%. The solar cell has shown efficiency greater than the cell supported with the sensitizers containing pigments of flavonoids and chlorophyll derived from the various plants rather than the crown leaves of pineapple. The crown leaves of pineapple are rich in lignocellulosic fibres comprising cellulose and lignin which are stronger and harder to withstand without degradation.
Simulation modelling using PC1D software
Solar spectrum
The solar spectrum has been thoroughly analyzed using the PC1D software on the particular day of testing the fabricated solar cell and the output values are represented which include the climatic parameters in the local climatic conditions of Coimbatore, Tamilnadu, India and represented in Table 1. Followed, the output values of power density perpendicular, power density module, and photocurrent perpendicular and photocurrent module for direct, diffuse and global solar radiation have been found with respect to the input values of climatic parameters and represented in Table 2. Figure 9 represents the spectral distribution with respect to the wavelength for direct, diffuse and global solar radiation from 280 to 4000 nm obtained from the simulation software.
Equations governing carrier dynamics
The fabricated solar cell has produced current due to the excited photoelectrons generated due to the absorption of energy of the photon by the dye extracted from the crown leaves of pineapple and the transfer of electrons to the semiconductor oxide of the photoanode. When the cell works, the total current density can be written as
Here \({J}_{\mathrm{L}}\) and \({J}_{0}\) are the light-generated current density and saturation current density. V represents the voltage and m is the ideality factor that influences the curvature of the JV characteristics curve. The open circuit voltage which is meant as the voltage across the cell when the current withdrawn from the cell is zero is determined using the expression given by
Also, \({J}_{\mathrm{L}}\) also represents the number of excited photocarriers when light illuminates the solar cell whereas \({J}_{0}\) is the dark or saturation current due to leakage of charge carriers. The fabricated solar cell is modelled using an equivalent circuit model using PC1D software. The equivalent circuit is drawn such that a current source is considered equal to the intensity of incident light and the source is parallel to a non-resistive diode. A series resistance (Rs) and shunt resistance (Rsh) are included such that the former determines the series resistive losses at the contacts while the latter determines the total leakage through the entire solar cell device. Figure 10 represents the equivalent circuit model which is used for simulation using PC1D.
With the equivalent circuit with the shunt and series resistance, the total current density can be written as
The simulation results using PC1D have been found and the graphs including light JV, Log JV and Log mV curve have been drawn which are represented in Figs. 10, 11 and 12 for the fabricated solar cell. The results of the simulation with the inputs and light JV outputs have been tabulated in Table 3.
To eradicate the resistive losses at the contacts, the series resistance should be very small and negligible. It is found from the simulation that; the series resistance is 1.2 Ωcm2. The shunt resistance should be very high to overcome the leakage current throughout the cell and it is found to be 10 kΩcm2. Following the light collected current density is determined as 38 mA/cm2 with saturation current density \({J}_{0}\) of 1pA/cm2. Figure 13 represents the light JV curve obtained from the simulation using PC1D and from the figure it is found that the fill factor is 0.7669 which influences the efficiency of the solar cell.
Further, the solar cell is reproducible for the surface area of 2.25 cm2 which is confirmed by the fabrication of solar cells of five. Among the five solar cells, the output of the solar cell in terms of short circuit current density and open circuit voltage of the four cells are up to the benchmark characteristics with least error. The study has successfully confirmed a high level of reproducibility, achieving an 80% consistency rate through the precise fabrication and synthesis of the necessary materials. These results are in favorable comparison to the findings reported in previous studies [46], highlighting the improved performance of the Pineapple Crown Leaves (PCL) in this new research context.
Conclusions
The study has led to the following conclusions:
-
i.
The dyes extracted from the crown leaves have exhibited strong anchoring and bonding with TiO2, as evidenced by the presence of –OH and –CH stretching signals at 3268 cm−1 and 2922 cm−1 in the FTIR spectra. These fibrils have demonstrated superior durability compared to dyes obtained from ordinary leaves.
-
ii.
UV–visible absorption analysis of the dye has revealed absorption in the visible region of the electromagnetic spectrum, indicating its efficacy in harnessing solar energy.
-
iii.
The solar cell incorporating this dye achieved an efficiency of approximately 1.0034%, surpassing dyes rich in chlorophyll.
-
iv.
The simulation results for the fabricated solar cell have provided valuable insights, particularly in terms of the light JV characteristics under varying input voltages.
-
v.
Equivalent circuit simulation modeling using PC1D software has yielded solar cell parameters consistent with experimental values.
-
vi.
The ideality factor, which reflects the recombination mechanism concerning varying voltage, indicated a shift in light Jsc from the dark or saturation current density.
-
vii.
The high shunt resistance value of approximately 12 kΩcm2 suggests that higher shunt resistance leads to enhanced output while minimizing current leakage throughout the solar cell device. Additionally, the low series resistance value obtained from simulation modeling implies minimal resistance at the cell’s contact points.
-
viii.
The study found remarkable agreement between simulation results using PC1D and experimental data, with minimal and reasonable error.
Availability of data and material
The designed solar cell and data of results of characterization are available.
References
Esteves, M., Fernández-Werner, L., Pignanelli, F., Montenegro, B., Belluzzi, M., Pistón, M., Rodríguez Chialanza, M., Faccio, R., Mombrú, Á.W.: Synthesis, characterization and simulation of lithium titanate nanotubes for dye sensitized solar cells. Ceram. Int. 45(11), 708–717 (2019). https://doi.org/10.1016/j.ceramint.2018.09.233
Sarker, S., Kim, D.M.: Measurement and simulation of current-voltage relation in dye-sensitized solar cells with reduced graphene oxide at the counter electrodes. Sol. Energy 176, 656–662 (2018). https://doi.org/10.1016/j.solener.2018.10.075
Kavitha, A., Ram Kumar, M., Ravichandran, S., Shanmugan, S., Selvaraju, P., Srinivas Prasad, M.V.V.K.: Luminescence, high thermal stability of Erbium-Ytterbium Schiff base metal complexes for bioimaging application. Mater Today Proc 51(Part 1), 1087–1095 (2022). https://doi.org/10.1016/j.matpr.2021.07.102
Varadarajan, S., Senthil Kumar, M., Shanmugan, S., Arputhalatha, A., Chithambaram, V., Geetha, P.: A new class single crystal L-lysine hydrogen chloride (LLHC) for optoelectronic applications. J. Mater. Sci. Mater. Electron. 32, 26351–26358 (2021). https://doi.org/10.1007/s10854-021-06987-z
Zhang, W., Zhang, Y., Su, H., Zhu, X., Wang, L., Zhang, J.: The effects of dye aggregation on the performance of organic dyes in dye-sensitized solar cells: From static model to molecular dynamics simulation. J. Lumin. 205, 7–13 (2019). https://doi.org/10.1016/j.jlumin.2018.08.084
Sangiorgi, N., Sangiorgi, A., Dessì, A., Zani, L., Calamante, M., Reginato, G., Mordini, A., Sanson, A.: Improving the efficiency of thin-film fiber-shaped dye-sensitized solar cells by using organic sensitizers. Sol. Energy Mater. Sol. Cells 204, 110209 (2020). https://doi.org/10.1016/j.solmat.2019.110209
Shanmugan, S., Ram Kumar, M., Selvaraju, P., Ravichandran, S.: Systematic growth on antibacterial activities use of silver nanoparticles with citrus aurantifolia. Mater Today Proc 51(1), 998–1005 (2022). https://doi.org/10.1016/j.matpr.2021.07.055
Latha Devi, N.S.M.P., Shanmugan, S.: An approach of renewable energy based on spatial patterns of radiation flux for solar thermal applications. Mater. Today Proc. 51(1), 1151–1156 (2022). https://doi.org/10.1016/j.matpr.2021.07.115
Vinu, V., Anandu, S., Ancy, A., Sreekala, C.O.: Molybdenum doped titanium dioxide as photoanode in dye sensitized solar cell—a simulation study. Mater. Today Proc. 46(Part 8), 3005–3010 (2021). https://doi.org/10.1016/j.matpr.2020.12.841
Latha Devi, N.S.M.P., Shanmugan, S.: Analysis of weather condition on thermal behavior utilization in solar device. Mater. Today Proc. 51(1), 1079–1086 (2022). https://doi.org/10.1016/j.matpr.2021.07.099
Dheeraj Devadiga, M., Selvakumar, D.D., Selvaraj Paramasivam, A., Prakasha Shetty, T.N., Senthil Kumar, S.: Organic sensitizer with azine π-conjugated architecture as co-sensitizer and polymer-based electrolyte for efficient dye-sensitized solar cell. Surf. Interfaces 33, 102236 (2022). https://doi.org/10.1016/j.surfin.2022.102236
Casadio, S., Sangiorgi, N., Sangiorgi, A., Dessì, A., Zani, L., Calamante, M., Reginato, G., Mordini, A., Sanson, A.: Highly efficient long thin-film fiber-shaped dye sensitized solar cells based on a fully organic sensitizer. Sol. Energy Mater. Sol. Cells 224, 110986 (2021). https://doi.org/10.1016/j.solmat.2021.110986
Kim, J.H., Park, H.W., Koo, S.-J., Lee, D., Cho, E., Kim, Y.-K., Shin, M., Choi, J.W., Lee, H.J., Song, M.: High efficiency and stable solid-state fiber dye-sensitized solar cells obtained using TiO2 photoanodes enhanced with metal organic frameworks. J. Energy Chem. 67, 458–466 (2022). https://doi.org/10.1016/j.jechem.2021.10.034
Ruba, N., Pooja Prakash, R.N., Sowmya, S., Janarthanan, B., Nagamani Prabu, A., Chandrasekaran, J.: Dye-sensitized solar cell using Eosin Y dye in various concentrations. Mater Today Proc 45(Part 2), 2371–2374 (2021). https://doi.org/10.1016/j.matpr.2020.10.729
Sowmya, S., Inbarajan, K., Ruba, N., Pooja Prakash, R.N., Janarthanan, B.: A novel idea of using dyes extracted from the leaves of Prosopis juliflora in dye—sensitized solar cells. Opt. Mater. 120, 111429 (2021). https://doi.org/10.1016/j.optmat.2021.111429
Sowmya, S., Pooja Prakash, R.N., Nagamani Prabu, A., Janarthanan, B., Reddy Minnam Reddy, V., Hegazy, H.H.: Fabrication of natural dye-sensitized solar cells with bulk TiO2 instead of nano-sized. Optik 242, 166205 (2021). https://doi.org/10.1016/j.ijleo.2020.166205
Gireesh Baiju, K., Nandanwar, M.N., Jayanarayanan, K., Kumaresan, D.: Numerical modelling and simulation of heat sink assisted thermal sintering of titania film on polymer substrates for the fabrication of high-performance flexible dye sensitized solar cells. Chem. Eng. Res. Design 181, 209–219 (2022). https://doi.org/10.1016/j.cherd.2022.03.013
Inbarajan, K., Sowmya, S., Janarthanan, B.: Direct and soxhlet extraction of dyes from the peels of Allium cepa and its effective application in dye—sensitized solar cells as sensitizer. Opt. Mater. 129, 112487 (2022). https://doi.org/10.1016/j.optmat.2022.112487
Pooja Prakash, R.N., Janarthanan, B., Ubaidullah, M., Al-Enizi, A.M., Shaikh, S.F., Alanazi, N.B., Alkhalifah, R.I., Ilyas, M.: Optimization, fabrication, and characterization of anthocyanin and carotenoid derivatives based dye-sensitized solar cells. J King Saud Univ Sci 35(4), 102625 (2023). https://doi.org/10.1016/j.jksus.2023.102625
Teja, A.S., Srivastava, A., Satrughna, J.A.K., Tiwari, M.K., Kanwade, A., Yadav, S.C., Shirage, P.M.: Optimal processing methodology for futuristic natural dye-sensitized solar cells and novel applications. Dyes Pigments 210, 110997 (2023). https://doi.org/10.1016/j.dyepig.2022.110997
Elroby, S.A.K., Bouzzine, S.M., Al-Ghamdi, H.A., Alotaibi, M.M., El-Shishtawy, R.M.: Molecular understanding of electron donor influences in dye-sensitized solar cells of novel series-based D-A’-(π-A)2. Mater. Sci. Semiconductor Proc. 165, 107675 (2023). https://doi.org/10.1016/j.mssp.2023.107675
Meena, M., Kavitha, A., Karthick, S., Pavithra, S., Shanmugan, S.: Effect of decorated photoanode of TiO2 nanorods/nanoparticles in dye-sensitized solar cell. Bull. Mater. Sci. 45(4), 231 (2022). https://doi.org/10.1007/s12034-022-02828-9
Pooja Prakash, R.N., Janarthanan, B.: Enhancement of sensitization and electron transfer by kumkum dye in dye-sensitized solar cell applications. Optik 287, 171093 (2023). https://doi.org/10.1016/j.ijleo.2023.171093
Alsaiari, A.O., Shanmugan, S., Abulkhair, H., Bamasag, A., Moustafa, E.B., Alsulami, A.R., Ahmad, I., Elsheikh, A.: Applications of TiO2/Jackfruit peel nanocomposite in solar still: experimental analysis and performance evaluation. Case Stud. Thermal Eng. 38, 102292 (2022). https://doi.org/10.1016/j.csite.2022.102292
Mohandass Gandhi, A., Shanmugan, S., Ravinder, K., Elsheikh, A.H., Sharifpur, M., Bewoor, A.K., Bamisile, O., Hoang, A.T., Onga, B.: SiO2/TiO2 nanolayer synergistically trigger thermal absorption inflammatory responses materials for performance improvement of stepped basin solar still natural distiller. Sustain Energy Technol Assess 52(Part A), 101974 (2022). https://doi.org/10.1016/j.seta.2022.101974
Rajesh Kumar, T., Shanmugan, S., Sunita Sundari, G., Latha Devi, N.S.M.P., Abhiram, N., Palanikumar, G.: Experimental Investigation on the Performance of a Solar Still Using SiO2 Nanoparticles/Jatropha curcas L. SILICON 14, 3501–3514 (2022). https://doi.org/10.1007/s12633-021-01119-y
Ghandourah, E.I., Sangeetha, A., Shanmugan, S., Zayed, M.E., Moustafa, E.B., Tounsi, A., Elsheikh, A.H.: Performance assessment of a novel solar distiller with a double slope basin covered by coated wick with lanthanum cobalt oxide nanoparticles. Case Stud. Thermal Eng. 32, 101859 (2022). https://doi.org/10.1016/j.csite.2022.101859
Prabu, A.S., Chithambaram, V., Bennet, M.A., Shanmugan, S., Pruncu, C.I., Lamberti, L., Elsheikh, A.H., Panchal, H., Janarthanan, B.: Microcontroller PIC 16F877A standard based on solar cooker using PV—evacuated tubes with an extension of heat integrated energy system. Environ. Sci. Pollut. Res. 29, 15863–15875 (2022). https://doi.org/10.1007/s11356-021-16863-2
Rajasekar, R., SenthilKumar, M., Shanmugan, S., Nagarajan, M.: The influence of Cu2ZnSnS4 thin films with characteristics of treatment conditions on spray pyrolysis technique for solar cells application. Indian J. Phys. 96, 707–716 (2022). https://doi.org/10.1007/s12648-020-01999-7
Dedeepya, G., Shanmugan, S., Sunita Sundari, G., Latha Devi, N.S.M.P., Meenachi, M., Gnana Kiran, M., Selvaraju, P.: Dyes prepared from leaf extract of Siriyanangai (Andrographis Paniculata) with the effect of TiO2 based DSSCs. Materials Today: Proceedings 66. Part 8, 3644–3650 (2022). https://doi.org/10.1016/j.matpr.2022.07.188
Asha, S., Shanmugan, S., Venkateswarlu, M., Meenachi, M., Sangeetha, A., Rao, M.C.: Thermal potential porous materials and challenges of improving solar still using TiO2/Jackfruit peel-enhanced energy storage material. Mater. Today Proc. 66(Part 8), 3616–3625 (2022). https://doi.org/10.1016/j.matpr.2022.07.142
Shanmugan, S., Selvaraju, P., Sivakumar, S., Nagaraj, J., Srinivas Prasad, M.V.V.K., Abhiram, N.: Solar cells absorption viewpoint of Mie theory: Experimental analysis of TiO2 doping V/Ce. Mater. Today Proc. 51(Part 1), 1124–1128 (2022). https://doi.org/10.1016/j.matpr.2021.07.110
Shanmugan, S., Selvaraju, P., Nagaraj, J., Sivakumar, S., Ravichandran, S.: Biogenic silver nanoparticles of antibacterial activities for poly-herbal extracts in novel medicine. Mater. Today Proc. 51(Part 1), 1107–1114 (2022). https://doi.org/10.1016/j.matpr.2021.07.107
Doranehgard, M.H., Essa, F.A., Shanmugan, S., Khalid, M.: Graphite powder mixed with black paint on the absorber plate of the solar still to enhance yield: An experimental investigation. Desalination 520, 115349 (2021). https://doi.org/10.1016/j.desal.2021.115349
Kavitha, A., Shanmugan, S., Awuchi, C.G., Kanagaraj, C., Ravichandran, S.: Synthesis and enhanced antibacterial using plant extracts with silver nanoparticles: therapeutic application. Inorg. Chem. Commun. 134, 109045 (2021). https://doi.org/10.1016/j.inoche.2021.109045
Palanikumar, G., Shanmugan, S., Chithambaram, V., Shiva, G., Pruncu, C.I., Essa, F.A., Kabeel, A.E., Panchal, H., Janarthanan, B., Ebadi, H., Elsheikh, A.H., Selvaraju, P.: Thermal investigation of a solar box-type cooker with nanocomposite phase change materials using flexible thermography. Renew. Energy 178, 260–282 (2021). https://doi.org/10.1016/j.renene.2021.06.022
Palanikumar, G., Shanmugan, S., Chithambaram, V., Selvaraju, P.: Synthesis, characterization of Ta2O5 nanoparticles with doping SnO2/Ag on solar absorber material and designs analysis of energy production for solar cooker. Mater. Today Proc. 30(Part 1), 190–196 (2020). https://doi.org/10.1016/j.matpr.2020.05.740
Thamizharasu, P., Shanmugan, S., Shiva, G., Pruncu, C.I., Essa, F.A., Panchal, H., Harish, M.: Improvement of thermal performance of a solar box type cooker using SiO2/TiO2 nanolayer. SILICON 14, 557–565 (2022). https://doi.org/10.1007/s12633-020-00835-1
Elfaleh, I., Abbassi, F., Habibi, M., Ahmad, F., Guedri, M., Nasri, M., Garnier, C.: A comprehensive review of natural fibers and their composites: an eco-friendly alternative to conventional materials. Results Eng. 19, 101271 (2023). https://doi.org/10.1016/j.rineng.2023.101271
Sarangi, P.K., Singh, A.K., Srivastava, R.K., Gupta, V.K.: Recent progress and future perspectives for zero agriculture waste technologies: pineapple waste as a case study. Sustainability 15(4), 1–26 (2023). https://doi.org/10.3390/su15043575
Mahmood, Z., Yameen, M., Jahangeer, M., Riaz, M., Ghaffar, A., Javid, I.: Lignin as natural antioxidant capacity, chapter 8. In: Poletto, M. (ed.) Lignin—trends and applications. InTech, New York (2018). https://doi.org/10.5772/intechopen.68464
Astuti, W., Sulistyaningsih, T., Kusumastuti, E., Thomas, G.Y.R.S., YogaswaraKusnadi, R.: Thermal conversion of pineapple crown leaf waste to magnetized activated carbon for dye removal. Biores. Technol. 287, 121426 (2019). https://doi.org/10.1016/j.biortech.2019.121426
Nadzirah, K.Z., Zainal, S., Noriham, A., Normah, I., Siti Roha, A.M.: Physico-Chemical Properties of Pineapple Crown Extract Variety N36 and Bromelain Activity in Different Forms. APCBEE Proc. 4, 130–134 (2012). https://doi.org/10.1016/j.apcbee.2012.11.022
Reichert, A.A., de Sá, M.R., da Silva, G.E.H., Beatrice, C.A.G., Fajardo, A.R., de Oliveira, A.D.: Utilization of pineapple crown fiber and recycled polypropylene for production of sustainable composites. J. Renew. Mater. 8(10), 1327–1341 (2012). https://doi.org/10.32604/jrm.2020.010291
Richards, H.L., Baker, P.G.L., Iwuoha, E.: Metal nanoparticle modified polysulfone membranes for use in wastewater treatment: a critical review. J. Surf. Eng. Mater. Adv. Technol. 2, 183–193 (2012). https://doi.org/10.4236/jsemat.2012.223029
Funding
The Department of Physics received the DST-FIRST Level-1 (SR/FST/PS-1/2018/35) initiative, and the authors express their sincere gratitude to the Department of Science and Technology (DST, Delhi), Government of India. Additionally, thanks are extended to KLEF for furnishing the current research project with the necessary infrastructure, facilities, basic equipment, and support.
Author information
Authors and Affiliations
Contributions
NP: synthesis work design and characterization of a solar cell. MR: analysis of results. KK: data validation, and editing of the manuscript. SS: analysis of results, writing the manuscript, reviewing, and editing the paper.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among the authors.
Ethical approval
The research work is ethically complied.
Consent to participate
All the authors give their consent to having participated in the current work.
Consent for publication
All the authors give their consent for publication of this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Premkumar, N., Madhavi, M.R., Kitmo, K. et al. Utilizing the lignocellulosic fibers from Pineapple Crown Leaves extract for enhancing TiO2 interfacial bonding in dye-sensitized solar cell photoanodes. Mater Renew Sustain Energy 13, 13–25 (2024). https://doi.org/10.1007/s40243-023-00245-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-023-00245-4