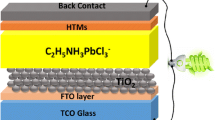
Abstract
Future revolution in photovoltaics will be hinged mainly on cost, health implication, and material stability and performance. Based on these criteria, lead-based inorganic photovoltaics, organic–inorganic hybrid, and silicon photovoltaics are screened-out. According to the literature, the lead-free inorganic perovskite solar cell is favorably disposed to cost and safe-health. However, the simultaneous solution to material stability, high defect density, and low power conversion efficiency (PCE) still remains a mystery that has not been solved. This research proposed the green-based modifiable CaZnBr4 as a potential candidate for lead-free solar cell application based on the principle of A-site cation with green-based additive incorporation. The green-based additive was obtained from Kola Nitida, Carica Papaya, Ficus Exasperata, and Musa paradisiaca. The elemental characterization of the green-based additives was performed using X-ray fluorescence spectroscopy (XRF). The optical, crystalline, and electronic properties were characterized using ultraviolet–visible (UV–Vis) spectroscopy, X-ray diffractometry, Quantum Espresso, scanning electron microscopy and SCAPS-1D. The green-base-modified CaZnBr4 showed significant PCE improvement by 3% with significant film and crystallinity formation. The stressed state of the parent compound CaZnBr4 shows that it may be better suited for thermovoltaics application. It is recommended that better results could be obtained when different synthetic routes and green-based additives are used to initiate the defect passivation protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are several kinds of perovskites based on their constituent elements and also their chemical formulas. The former includes pure inorganic as well as organic–inorganic hybrid perovskites [1]. The latter includes single, double and possibly triple perovskites [2].
Perovskites have been shown to have excellent photoelectric properties, such as high optical absorption coefficients and lower exciton binding energy [3]. In addition, they also have piezoelectric, conducting, antiferromagnetic, and superconducting properties [4]. These properties make them suitable for use in several electrical devices, particularly those that require semiconductors. The lead-based perovskite had demonstrated much prospect due to its structural stability but the long-term concerns when the material becomes waste are known to be disastrous especially considering the ejection of > 35 g of lead per solar panel into the environment.
In the search for a lead-free solar cell, it has been observed that though it has high absorption coefficients which may not translate to high power conversion efficiency (PCE) considering its fundamental challenge of structural instability [5]. Based on various result that had been obtained both experimentally or theoretically, we reviewed studies on the elemental impact on solar cell (Table 1) and how they can be harnessed to give a good inorganic solar cell. The calcium was adapted because it is usually used as an electron transportation layer in most layered solar cell [6]. Zinc is also used as charge carrier [7], buffer layer [8] or hole blocking layer [9] in solar cell application. Bromine is used for passivating defect states at grain boundaries and interfaces in solar cells [10, 11]. Based on the above, we came up with the idea of CaZnBr4 which in itself cannot be categorized as perovskite. However, it is noted that the application of certain green-based additives that would have significant influence on the structural stability of the compound. The green-based additives are expected to reduce the high defect density. The only limitation may be the creation of a new compound via bromine reduction as reported by Zhu et al. [12] presented in Eq. (1):
Methods
The procedure for the green-base additives that was used in the fabrication began with the collection of leaves from of the following tropical plants, i.e., Kola Nitida, Carica Papaya, Ficus Exasperata, and Musa paradisiaca. The green additives were used based on their perceived optical properties as presented in Emetere and Ahiara [31, 32]. The leaves were washed using distilled water and dried in the laboratory. They were washed the second time before blending.
The leaves were blended with 80 ml of methanol and sieved into beakers. The extracts were then heated at 100 °C for 30 min in order to remove the methanol content. Subsequently, 2 ml from the sieved green-based additives was collected into PET Bottles for UV–Vis analysis and labeled appropriately. After that, the remaining samples were heated in an oven at 200 °C for 5 h to evaporate the water content. The dried extracts were afterward ground with mortar and pestle, and then stored in PET bottles as powder and labeled appropriately before sending it for XRF characterization.
The chemical constituents of CaZnBr4, which is CaBr2 and ZnBr2, were mixed in the ratio 1:1 at 0.04 mol in a beaker. The mixture was stirred to obtain a clear homogenous solution. The first batch of characterization was performed at this stage using the UV–Vis spectrometry. Aside the pure samples that were dried into a powder form and deposited in PET bottle, the green-based additives, most of which were either dark brown or black, were added to the different batches of prepared CaZnBr4 samples. 0.2 g and 0.5 g of the green-based additives were added to the pure solution of CaZnBr4 and thoroughly mixed until no deposits are visibly seen below the beaker. Subsequently, 2 ml from the mixture was taken for UV–Vis analysis, and then the rest was dried at 250 °C for 12 h, after which they were put in labeled PET bottles as powdered samples.
The optoelectronic properties of the compound were investigated by SCAPS-1D using the UV–Vis results obtained from all the samples of CaZnBr4 compound. SCAPS-1D over the years had proven to be a reliable tool for solar cell application [33, 34]. The structure of the perovskite is TiO2/ZnO/Perovskite/P3HT. The parameters for the simulation used in this research are given in Table 3. The bandgaps for the perovskites are given in Table 2. The microstructural and morphology of the novel green-base-modified CaZnBr4 was characterized using the X-ray diffraction (XRD) whose specifications had been already defined in Emetere et al. (2022).
The stability of the material was carried out using the Quantum Espresso software. The Self-Consistent Field (SCF) calculations were used to calculate the internal energy, total energy, Fermi energy, total force, etc. The calculation was done using the following parameters, i.e., Bravais-lattice index of 3, lattice parameter of 15.4287 a.u., unit-cell volume of 1836.3497 (a.u.)3, number of atoms/cell of 6, number of atomic types of 3, number of electrons of 50, number of Kohn–Sham states of 32, cutoff kinetic energy of 45.0517 Ry, charge density cutoff of 405.4650 Ry, convergence threshold of 1.0E− 06, mixing beta of 0.7000, and an average number of iterations of 8. The cutoff kinetic energy was varied along 45.0517 Ry, 47.0517 Ry, 49.0517 Ry, 51.0517 Ry, and 53.0517 Ry. The analysis was carried out under 43 Marzari–Vanderbilt smearing, with width of 0.0050 Ry at starting charge of 49.99132 C that was renormalized to 50 C.
Scanning electron microscopy analysis (SEM) and X-ray diffractometry (XRD) were used to the determine the microstructural and crystallography properties of the compounds.
Results and discussion
The width of the peak of the pure CaZnBr4 in the UV analysis is between 250 and 350 nm (Fig. 1), which is in tandem with the individual components, where calcium has peak absorbance between 550 and 650 nm [36], zinc has peak absorbance between 200 and 400 nm [37], and bromine has peak absorbance between 350 and 600 nm [38]. Generally, the doped compound, i.e., CaZnBr4 has broad peak that depicts σ–σ* transition. The broadening is due to the high percentage of SiO2, CaO, and K2O in the green-based additives (as displayed by the XRF elemental analysis in Table 3), and whose wavelength peaks are at 200 nm to 320 nm [39], 250 nm and 300 nm (n–n*) and 320 nm to 400 nm (σ–σ*) [40] for SiO2 and CaO, respectively.
The component of the green-based additives was expected to either deplete the fractions of the bromine, reduce the high defect density, create structural stability or fill the pores of the compound. The bandgap, conversion efficiency, and current density are salient parameters used to show the electronics stability of the material. The broadening of the peak may be due to the following:
-
i.
The adequate filling of structural pores of the CaZnBr4 [41].
-
ii.
The enhancement of the structural stability in CaZnBr4.
-
iii.
The higher σ–σ* transitions in the compound.
Figure 1a shows the UV for the green additives. Figure 1b affirms that pore filling was dominant in the doped CaZnBr4. The peaks for the CaZnBr4 doped with Musa paradisiaca stem extract are identical, between 200 and 400 nm; however, their bandgaps differ by a relatively larger margin to the other green-based additives.
The percentage of SiO2 in Kola Nitida (P4) is marginally lower compared to other extract. The peak for 0.5 g of P4 (3.163 eV) is higher than same green-based additives with 0.2 g (3.062 eV), although both are still lower than that of pure CaZnBr4 (3.658 eV), which means they still impact upon the bandgap of CaZnBr4 to facilitate their use in solar cells.
The double peak phenomenon also appeared in the graphs CaZnBr4 with Carica Papaya extract (P5) and Ficus Exasperata extract (P6) as displayed in Fig. 2. It was observed that double peak was not present in graphs belonging to CaZnBr4 with Musa paradisiaca stem extract. CaZnBr4 with P5 has both amounts of additives resulted in largely identical peaks, with the exception of downward dip with an increase of the concentration to 0.5 g. This significant identical matching of peaks means the magnitude of possible pore filling does not substantially change with increase in the amount of P5 green-based additives. The difference between the two amounts are significantly represented in the bandgaps, where that for 0.5 g P5 is 3.924 eV, and that of 0.2 g P5 is 3.724 eV. The difference in the peaks in additives P6, i.e., 0.2 g is higher than that of 0.5 g. This observation indicates same reduction in magnitude of pore filling as that in P5, where the peak for 0.5 g was also lower. The bandgaps also follow the major trend, i.e., 0.5 g of P6 (2.230 eV) being lower than that of 0.2 g P6 (2.678).
A JV curves of simulated CaZnBr4-based solar cells with and without green-based additive P4 at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm. B JV curves of simulated CaZnBr4-based solar cells with and without green-based additive P5 at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm. C JV curves of simulated CaZnBr4-based solar cells with and without green-based additive P6 at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm. D JV curves of simulated CaZnBr4 solar cells with and without green-based additive Musa paradisiaca stem at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm
Figure 2 displays the JV characteristics as they appear in four different thicknesses, in order of 100 µm, 10 µm, 1 nm, and 10 nm, respectively. All the graphs show a positive parabolic trend, which agrees with theory [42]. A general trend in the JV graphs across the various thicknesses is the homogenization of the performance of the new material. In the nanometer thickness range, their graphs and efficiencies converge at certain exceptions, as seen below.
The difference between the JV for the green-based additives P4 (0.5 g) was higher than P4 (0.2 g) and, therefore, has a lower Jsc because it is closer to zero. This is reversed in the graph for 10 μm, although the differences are slight (Fig. 2A). P4 is also a poor green-based additives for CaZnBr4. Figure 2B has wildly differing graphs for differing amounts of P5 green-based additives, with the higher amount leading to a drastically lower Jsc and efficiency at 100 μm. The slight improvement of P5 (0.5 g) at 10 μm is negated at the nanometer scale. The slight improvement is probably due to chemical pressure or increased defect density. However, P5 is still a poor additive for optoelectrical optimization of CaZnBr4.
Figure 2C shows that P6 (0.5 g) works over a larger range of voltages at 100 μm, even if the Jsc and efficiency is not much improved from the pure sample. The voltage range of the pure sample and that of P6 (0.2 g) improves at 10 μm, and completely merges with the other graphs.
at 1 nm. The graph for 10 nm shows a slight divergence of the P6(0.2 g) graph from the rest, which have merged, breaking slightly with the trend so far. However, the efficiencies are not much improved from that of the pure sample. In fact, they are lower.
Figure 2D shows the JV graphs for the CaZnBr4 containing the Musa paradisiaca extract. Due to the high bandgap, the simulation of Musa paradisiaca (0.2 g) fails at the micrometer range, although it does run at the nanometer scale with a significant reduction in its efficiency and overall performance. Although the efficiency improves with reduced thickness, the highest efficiency was found for Musa paradisiaca (0.5 g) at 1.93%, i.e., woefully low.
Figure 3 shows the Mott Schottky curves of the various samples discussed in Fig. 2. Figure 3A shows that at 100 μm, P4 had higher defect density than pure CaZnBr4, which resulted to lower efficiency [43, 44]. The increasing amount of P4 seems to increase its defect density, which means the green-based additives is not sufficiently aiding in closing structural pores or giving greater stability. At 10 μm, the reversing of the curves is not as pronounced as in Fig. 3B, indicating a poorer performance for P4. However, the comparative performance between thicknesses still indicates a better performance for the larger thicknesses. As already observed in the trend, graphs homogenize and steepen at the nanoscale. Further corroborating that the physics of the nanoscale needs to be further investigated by considering the self-consistent field (SCF) calculations for the pure samples.
A Mott Schottky curves of simulated CaZnBr4 solar cells with and without green-based additive P4 at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm. B Mott Schottky curves of simulated CaZnBr4 solar cells with and without green-based additive P5 at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm. C Mott Schottky curves of simulated CaZnBr4 solar cells with and without green-based additive P5 at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm. D Mott Schottky curves of simulated CaZnBr4 solar cells with and without green-based additive—Musa paradisiaca at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm
The curves in Fig. 3B reveal what occurs at varying defect densities as initiated by higher concentrations of the green-base additives. At thickness of 100 μm, pure CaZnBr4 has a higher defect density than both P5-containing samples. This difference is marginal compared to the curve for 0.2 g of P5 at 10 μm. As discussed above, the science of the nanoscale has to been ascertained to understand defects passivation protocols. However, it has been shown that structural pores and stability in the micrometer scale may be unrealistic in practical science. The better performance of 0.2 g of P5 indicates a significant improvement on the physical and structural stability of the emerging materials.
Figure 3C indicates that at 100 μm thickness, a reduction in defect density at 0.2 g of P6 is established. A reversal was observed at 10 μm, where increase in green-based additives leads to decreased slope and therefore reduced defect density and better performance. However, the overall performance drops with respect to thickness.
The relatively steeper curves of Musa paradisiaca stem containing CaZnBr4 in Fig. 3D at both micrometer scales indicate higher defect density caused by the failure of the green-base additives to enhance better structural stability. It was further observed that significant homogenization and steepening still occurs at the nanoscale.
At the nanometer scale, following with the trend of the previous sets of curves, the external quantum efficiency (EQE) curves converge to a square peak between 400 and 900 nm, both above 90% as presented in Fig. 4. All the graphs displayed the possibility solar cell materials that show optimum performance at irradiance over AM 1.5 spectrum may possess the highest energies in the visible spectrum [45].
A Quantum efficiency curves of simulated CaZnBr4 solar cells with and without green-based additive—P4 at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm. B Quantum efficiency curves of simulated CaZnBr4 solar cells with and without green-based additive P5 at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm. C Quantum efficiency curves of simulated CaZnBr4 solar cells with and without green-based additive—P6 at thicknesses a 100 µm, b 10 µm, c 1 nm, d 10 nm. D Quantum efficiency curves of simulated CaZnBr4 solar cells with and without green-based additive Musa paradisiaca stem at thicknesses: a 100 µm, b 10 µm, c 1 nm, d 10 nm
The quantum efficiency (QE) (Fig. 4A) in the pure sample is not stable at micrometer scale. The QE for CaZnBr4 with 0.2 g of P5 and 0.5 g of P5 possess the same stabilities in the micrometer scale, hence, the green-base additive had showed significant stability. However, in the nanoscale, all the samples are stable. The wavelength in the micrometer scale > 400 nm. At < 400 nm, the material would show no efficiency. The instability of the pure compound terminates at 875 nm. This result confirms that the additives extends the performance of the material if structural material of the main compound is further optimized using nanoscale protocols.
The same pattern as presented in Fig. 4A is revealed in Fig. 4B. The quantum efficiency (QE) (Fig. 4B) in the pure sample is not stable at micrometer scale. CaZnBr4 with 0.2 g of P5 had higher QE peak than 0.5 g of P5. The QE of the green-based additives was seen to be significantly dependent on thickness. This was not the case in P4. The QE is extremely low However, in the nanoscale, all the samples are stable with a well-defined operational wavelength range of 380–700 nm.
The same trend in Fig. 4A can be seen in Fig. 4C with higher QE for 10 μm. The QE for green-base Musa paradisiaca collapsed in the 100 μm scale and was slightly noticeable at the 10 μm scale. The nanoscale demonstrated same feature for all green-base. This shows that if the main compound CaZnBr4, i.e., could be in the nanoscale, more interesting results may emerge.
The summary of the PCE, Bandgap for all sample is presented in Table 4. CaZnBr4 proves itself to be a decent solar cell material with its 7.52% efficiency at 100 μm, with a bandgap of 3.658 eV. As a pure lead-free inorganic perovskite, it is already higher than some of the best performing among them [46]. P4 performs even worse than P6 in this regard, having efficiencies below 6% at their best, consistent with its JV and Mott Schottky graphs. Musa paradisiaca stem raises the bandgap to the point where the lower quantities only runs when the thickness is in nanometer scale, and at that level, the efficiency is below 2%, with nothing to indicate otherwise in the graphs.
Based on the power conversion efficiencies (PCEs) of the compounds, the crystallographic characterization of the samples was further investigated to know the microstructural modifications that enhanced the given PCE. Due to the partial deliquescence nature of the compound after some days, rice sample was used to preserve its physical properties for longer days. The crystallographic structure of the materials was investigated as shown in Fig. 5. The scanning angle ranges from 0 to 66.68°. Under a continuous scan technique with a maximum intensity of 1400 cps, the rice powder was characterized (Fig. 5a). Nine peaks were discovered and adequately labeled. The typical rice powder is highly oxidized as presented in Fig. 6a. The peaks had the following planes, i.e., (100), (110), (111), (200), (210), (220), (300), (310), and (211). The particle size of the powder was in a nanoscale as presented in Table 5a. The d-spacing was found at the point of high intensity.
a SCF calculations of the parent compound, i.e., CaZnBr4 (i) total energy pattern at different SCF accuracy, b total energy pattern at different threshold, c Fermi energy pattern at different cutoff kinetic energies, d Fermi energy pattern at different cutoff kinetic energies for stressed samples, e total energy pattern at different cutoff kinetic energies, f total energy pattern at different cutoff kinetic energies at stressed state, g internal energy pattern at different cutoff kinetic energies, h total force at different cutoff kinetic energies
The pure CaZnBr4 compound is presented in Fig. 5b. The particle size shows that the compound, i.e., despite the framework of the rice powder (RP) is in the nanoscale. Unlike the supporting compound (rice powder), the highest intensity of the pure compound does not have the highest d-spacing. All this result shows that the RP did not influence the original compound chemically. The only constant compound in both material is the peaks at (200) and (211) planes. The reduced FWHM shows that the compound optical properties of the compound are still intact despite the RP (Table 5b).
The addition of 0.5 g of green-based additives P4 to pure compound CaZnBr4 (CaZnBr4 + 0.5 g P4) shows the extension of peaks of planes (100) and (211). In addition, intensity count at planes (220), (300), and (310) was significantly low (Fig. 5c). In addition, it was observed that the FWHM increased, showing that its optical properties were mitigated (Table 5c). This means that green-based additives P4 did not only deplete the bromine level but affected the calcium and zinc content (Fig. 5c and Table 5c). This outcome is the significant reduction of its PCE outcome as 5.36%.
Figure 5d shows reduced peaks in planes (111), (200) and (210). In addition, there was an increase in the peaks of planes (211) and (300). This result shows that the addition of 0.2 g of green-based additives P5 to pure compound CaZnBr4 (CaZnBr4 + 0.2 g P5) depleted the bromine content moderately, hence, the attainment of the CaZnBr4pseudo-perovskite as proposed by the author. Like the previous compound, i.e., CaZnBr4 + 0.5 g P4, the FHWM and the particle sizes followed the same pattern (Table 5d) but the significance of increase or decrease at certain planes make the difference between both the compounds. The oxidized nature shows that the RP content was more in content which may affect the FHWM and the particle sizes. Hence, the scientific significance in the microstructural modification of the pseudo-perovskite is based on the planar changes.
The addition of 0.5 g of green-based additives P4 to pure compound CaZnBr4 (CaZnBr4 + 0.5 g P4) shows the extension of peaks of planes (100) and (211).
The addition of 0.5 g of green-based additives P5 to pure compound CaZnBr4 (CaZnBr4 + 0.5 g P5) shows the extension of peaks at same planes, which shows a complete modification of the structures as seen in the rearrangement of the planes (Fig. 5e and Table 5e). Reduced peaks in planes (100), (210), (220), and (111) were observed. In addition, there was an increase in the peaks of planes (200) and (310). This outcome significantly differs from the CaZnBr4 + 0.2 g P5 which further shows that the increase of the concentration of the green-based additives may not affect the bromine content as earlier discussed. Unlike the CaZnBr4 + 0.2 g P5, the FHWM and the particle sizes reduced but with a lower PCE.
From the microstructural analysis of the compounds with higher PCE, it could be inferred that the green-based additives at higher concentration influence the ‘A’ and ‘B’ sites of the pseudo-perovskite sample such as CaZnBr4.
From the above results, i.e., optoelectric, optical, and electronic, there is the need to examine the parent compound to suggest further enhancements. The SCF calculations were performed as reported in Fig. 6. Figure 6a shows that the total energy trend at different smearing are almost the same. The same total energy trend was also observed in Fig. 6b with emphasis on the thresholds. Hence, the parent compound, i.e., CaZnBr4, is not significantly affected by the smearing setting. The negative Fermi energy values in Fig. 6d have no physical meaning because it is not an indication of a possible error but the choice of zero-point. In other words, at stressed state, the material losses its semiconducting properties and may not act as a solar cell. When it is not stressed, the material has a slight semiconducting property [47,48,49,50].
This result is in tandem with the PCE table. The total energy is higher for the stressed state as compared to the normal state (Fig. 6e, f). The sharp drop in the internal energy of the stressed state shows that the material may absorbed heat more at certain state. Hence, it may be better suited as thermovoltaics. The total force shows that the higher the cutoff energy, the lower the total force experienced. This is a further confirmation that the material may be better suited for thermovoltaics application at pure form. This material may find application in complex structured buildings [51,52,53].
10The scanning electron microscope (SEM) was used to determine the microstructural properties of the pure CaZnBr4 and the highest PCE of the doped sample, i.e., CaZnBr4 + P5 as presented in Fig. 7. CaZnBr4 contains sub-micrometer to -nanometer sized structures that has non-porous five colonies with certified compact surface as presented in Fig. 7A. Only a colony showed enhanced brightness at the edge that may be likely Ca2+. The doped sample, i.e., CaZnBr4 + P5 was seen to close-up the main pores in the original compound and integrated all compound as one unit. Hence, the improved optoelectric, optical, and electronic properties of CaZnBr4 + P5 was due to the component of the additive. Based on all the characterization discussed in this section, this material may have more potential for solar application than other lead-free perovskite discussed in literature [54,55,56,57,58].
Conclusion
In this study, lead-free pseudo perovskites were synthesized via solution and solid-state reaction method. Several green-based additives were used to reduce the bromine content to enhance the photoelectrical properties of the perovskite for material. The electronic characterization performed using SCAPS-1D showed improved PCE in some of the PSCs, as also evidenced in their JV curves, and in the improvements in doping density indicated by their Mott Schottky curves. All the JV graphs show a positive parabolic trend, which agrees with theory. A general trend in the JV graphs across the various thicknesses is the homogenization of the performance of the pure perovskite-based solar cell with doped perovskites. In the nanometer thickness range, their graphs and efficiencies converge with certain exceptions. At some doping concentrations (i.e., with dopants or green-based additives), it was observed that there was evidence of chemical pressure change the current orientation. The current orientation in this case may limit the voltage generation. This result means that there is the possibility that perovskite may have a better efficiency but lower tolerance limit for voltages.
The doping density was seen to agree with past research which postulate that doping density is inversely proportional to the slope of the graph. Their EQE showed that the performance of the perovskite reduced and converged due to reductions in perovskite thicknesses. Discovery shows that perovskites are not suitable for ultra-thin use if efficiency is paramount.
The efficiencies of the simulated lead-free perovskites solar cells were improved with the presence of some of its green-based additives especially include those mixed with green-based additives from Buxus Sempervirens, which raised the efficiencies of some of the perovskite solar cells to over 9%. From the microstructural analysis of the compounds with higher PCE, the green-based additives, i.e., Kola Nitida, Carica Papaya, Ficus Exasperata, Musa paradisiaca modified the structural features as well as the efficiency. However, at higher concentration, the ‘A’ and ‘B’ sites of the pseudo-perovskite are altered thereby forfeiting the prospect of pseudo-perovskite formation. The component of Carica Papaya extract was seen to be the best candidate for the pseudo-perovskite whose PCE is 7.63% which currently the inorganic perovskite with the highest PCE. The stressed state of the parent compound shows that it may be better suited for thermovoltaics application. The limitation of this study is the inability to accurately predict the concentration of bromine reduction by the green-based additives. This shortcoming is recommended for further study. The advantage of this work is the possibility of fabricating special kinds of solar cell from organo-inorganic synthesis.
Data availability
The data will be available upon request.
References
Aida, N., Ouedraogo, N., Chen, Y., Yue, Y., Meng, Q., Bao, C., Zhang, Y.: Nano energy stability of all-inorganic perovskite solar cells. Nano Energy 67(November 2019), 104249 (2020). https://doi.org/10.1016/j.nanoen.2019.104249
Assirey, R.: Perovskite synthesis, properties and their related biochemical and industrial application. Saudi Pharm. J. 27(6), 817–829 (2019). https://doi.org/10.1016/j.jsps.2019.05.003
Zhou, D., Zhou, T., Tian, Y., Zhu, X., Tu, Y.: Perovskite-based solar cells: materials, methods, and future perspectives. J. Nanomater.Nanomater. 2018, 15 (2018)
Rachedi, S., Yahia, A., Ahmed, B., Mokhtar, B., Noureddine, M., Mohamed, L., Djillali, B., Al-Douri, Y.: Electronic, elastic and piezoelectric properties calculations of perovskites materials type BiXO3(X = Al, Sc): DFT and DFPT investigations. Chem. Phys. 573, 111998 (2023)
Emetere, M. E., Bello, O. O., Afolalu, S.A.: Enhancement of novel NaZnBr3 perovskite for solar cells application. Energy Rep. 8, 4453–4460 (2022)
Tang, Y., Pang, Y., Li, X., Zong, B., Kang, B., Silva, S., Lu, G.: Interface passivation and electron transport improvement via employing calcium fluoride for polymer solar cells. J. Colloid Interface Sci. 562, 142–148 (2020). https://doi.org/10.1016/j.jcis.2019.12.025
Gonçalves Larsson, L.F., et al.: Application of zinc oxide in hybrid solar cells using a P3HT and P3OT polymer junction as charge carrier. Mater. Res.r. Res. 22, e20180820 (2019). https://doi.org/10.1590/1980-5373-MR-2018-0820
Haque F. et al.: Prospects of zinc sulphide as an alternative buffer layer for czts solar cells from numerical analysis. 8th International Conference on Electrical and Computer Engineering, 2014, pp. 504–507, https://doi.org/10.1109/ICECE.2014.7026855
Hadouchi, W., Rousset, J., Tondelier, D., Geffroy, B., Bonnassieux, Y.: Zinc oxide as a hole blocking layer for perovskite solar cells deposited in atmospheric conditions. RSC Adv. R. Soc. Chem. 6(72), 67715–67723 (2016)
Ochoa-Martinez, E., Ochoa, M., Ortuso, R.D., Ferdowsi, P., Carron, R., Tiwari, A.N., Steiner, U., Saliba, M.: Physical passivation of grain boundaries and defects in perovskite solar cells by an isolating thin polymer. ACS Energy Lett.Lett. 6(7), 2626–2634 (2021)
Raifukua, I., Ishikawa, Y., Chiang, Y.-H., Lin, P.-Y., Li, M.-H., Chen, Y.U.P.: Segregation-free bromine-doped perovskite solar cells for IoT applications. RSC Adv. Adv. 9, 32833–32838 (2019)
Zhu, J., Chen, Xi., Zhao, N., Wang, W., Du, J., Ruan, J., Xu, Z.: Bromine removal from resin particles of crushed waste printed circuit boards by vacuum low-temperature heating. J. Clean. Prod. 262, 121390 (2020). https://doi.org/10.1016/j.jclepro.2020.121390
Andy, E.: Perovskites on trial. Nature 570, 429 (2019)
Torimtubun, A.A., Augusty, A.C., Maulana, E., Ernawati, L.: Affordable and sustainable new generation of solar cells: calcium titanate (CaTiO3) - based perovskite solar cells. E3S Web Conf. (2018). https://doi.org/10.1051/e3sconf/20186701010
Jessica Christine, L.: The use of nanostructured calcium silicate in solar cells. https://researcharchive.vuw.ac.nz/xmlui/handle/10063/1053 (2009). Accessed 23 Sept 2022
Taylor, N.T., Galbiati, A., Saavedra, M., Hepplestone, S.P.: Calcium-stannous oxide solid solutions for solar devices. Appl. Phys. Lett.Lett. 117, 153901 (2020). https://doi.org/10.1063/5.0024947
Shraavya, R., Morankar, A., Verma, H., Goswami, P.: Emerging photovoltaics: organic, copper zinc tin sulphide, and perovskite-based solar cells. J. Appl. Chem. 2016, 3971579 (2016)
Jorna, A.: Copper zinc tin sulphide (CZTS) solar cells. http://large.stanford.edu/courses/2012/ph240/jorna2/ (2012). Accessed 23 Sept 2022
Rathod, K., Kamble, P., Sanadi, K., Kamble, G., Guar, M., Garadkar, K.: Photovoltaic application study of zinc telluride thin films grown by chemical bath deposition method. Adv. Mater. Phys. Chem. 11, 131–144 (2021). https://doi.org/10.4236/ampc.2021.118013
Shanmugam, A., Rathanasamy, R., Kaliyannan, G., Nagarajan, N., Palanisamy, M.: Spinel zinc ferrite nanostructured thin-films for enhanced light-harvesting in polycrystalline solar cells. Mater. Sci.-Poland 39(1), 24–32 (2021)
Mfon, R.E., Usman, A.: Fabrication of zinc oxide-cuprous oxide photovoltaic cell for teaching purpose using locally available materials. Scientia Africana 13, 31 (2014)
Stutz, E.Z., Zamani, M., Damry, D.A., Buswell, L., Paul, R., Escobar Steinvall, S., Leran, J.B., Boland, J.L., Dimitrievska, M., Fontcuberta, I., Morral, A.: Showcasing the optical properties of monocrystalline zinc phosphide thin films as an earth-abundant photovoltaic absorber. Mater. Adv. 3(2), 1295–1303 (2021). https://doi.org/10.1039/d1ma00922b
Liu, Y., Kim, B.J., Wu, H., Yuan, L., Zhu, H., Liu, A., Johansson, E.M.: Flexible lead bromide perovskite solar cells. ACS Appl. Energy Mater. 3(10), 9817–9823 (2020). https://doi.org/10.1021/acsaem.0c01473
Ghasemi, M., Zhang, L., Yun, J., Hao, M., He, D., Chen, P., Bai, Y., Lin, T., Xiao, M., Du, A., Lyu, M., Wang, L.: Dual-ion-diffusion induced degradation in lead-free Cs 2 AgBiBr 6 double perovskite solar cells. Adv. Funct. Mater.Funct. Mater. 30(42), 2002342 (2020). https://doi.org/10.1002/adfm.202002342
Fan, P., Peng, H., Zheng, Z., Chen, Z., Tan, S., Chen, X., Luo, Y., Su, Z., Luo, J., Liang, G.: Single-source vapor-deposited Cs2AgBiBr 6 thin films for lead-free perovskite solar cells. Nanomaterials (2019). https://doi.org/10.3390/nano9121760
Greul, E., Petrus, M., Binek, A., Docampo, P., Bein, T.: Highly stable, phase pure Cs2AgBiBr 6 double perovskite thin films for optoelectronic applications. J. Mater. Chem. A 5(37), 19972–19981 (2017). https://doi.org/10.1039/c7ta06816f
Igbari, F., Wang, R., Wang, Z.K., Ma, X.J., Wang, Q., Wang, K., Zhang, Y., Liao, L., Yang, Y.: Composition stoichiometry of Cs 2 AgBiBr 6 films for highly efficient lead-free perovskite solar cells. Nano Lett. Lett. 19(3), 2066–2073 (2019). https://doi.org/10.1021/acs.nanolett.9b00238
Fang, D., Tong, Y., Xu, F., Mi, B., Cao, D., Gao, Z.: Preparation of CsSnBr 3 perovskite film and its all-inorganic solar cells with planar heterojunction. J. Solid State Chem. 294, 121902 (2021). https://doi.org/10.1016/j.jssc.2020.121902
Song, T., Yokoyama, T., Stoumpos, C.C., Logsdon, J., Cao, D.H., Wasielewski, M.R., Kanatzidis, M.G.: Importance of reducing vapor atmosphere in the fabrication of Tin-based perovskite solar cells. J. Am. Chem. Soc. 139(2), 836–842 (2017). https://doi.org/10.1021/jacs.6b10734s
McMeekin, D.P., Sadoughi, G., Rehman, W., et al.: A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells”. Science 351, 151–155 (2016). https://doi.org/10.1126/science.aad5845
Emetere, M.E., Ahiara, I.M.: Synthesis and characterization of aluminium coated Syzygium Samarangense extracts. Chem. Data Collect. 28, 100418 (2020). https://doi.org/10.1016/j.cdc.2020.100418
Emetere, M.E., Ahiara, I.M.: Synthesis and characterization of aluminum coated Carica papaya extracts. Chem. Data Collect. 28, 100381 (2020)
Burgelman, M., Decock, K., Niemegeers, A., Verschraegen, J., Degrave, S.: SCAPS manual. https://scaps.elis.ugent.be/SCAPSinstallatie.html (2020). Accessed 23 Sept 2022
Niemegeers, A., Burgelman, M.: Numerical modelling of ac-characteristics of cdte and cis solar cells. Proc. 25nd IEEE Photovoltaic Specialists Conference (Washington D.C., April 1996), 901–904. (1996).
Rahman, M.S., Miah, S., Marma, M.S.W., Sabrina, T.: Simulation based investigation of inverted planar perovskite solar cell with all metal oxide inorganic transport layers. 2nd International Conference on Electrical, Computer and Communication Engineering, ECCE 2019. Institute of Electrical and Electronics Engineers Inc. (2019). https://doi.org/10.1109/ECACE.2019.8679283
Li, X.D., Zhai, Q.Z.: Spectrophotometric determination of calcium with dibromo-p-methylsulfonazo. J. Chem.Chem.. (2020). https://doi.org/10.1155/2020/9232385
Silambarasan, M., Saravanan, S., Soga, T.: Mn-doped ZnO nanoparticles prepared by solution combustion method. E-J. Surf. Sci. Nanotechnol. 12, 283–288 (2014). https://doi.org/10.1380/EJSSNT.2014.283
Vasilyev, A.V., Lindeman, S.V., Kochi, J.K.: Molecular structures of the metastable charge-transfer complexes of benzene (and toluene) with bromine as the pre- reactive intermediates in electrophilic aromatic bromination. New J. Chem. 26(5), 582–592 (2002). https://doi.org/10.1039/B110169M
Wang, J., Ran, Q., Xu, X., Zhu, B., Zhang, W.: Preparation and optical properties of TiO2-SiO2 thin films by sol-gel dipping method. IOP Conf. Ser.: Earth Environ. Sci. 310(4), 042029 (2019). https://doi.org/10.1088/1755-1315/310/4/042029
Ramola, B., Joshi, N.C., Ramola, M., Chhabra, J., Singh, A.: Green synthesis, characterisations and antimicrobial activities of CaO nanoparticles. Orient. J. Chem.. Chem. 35(3), 1154–1157 (2019). https://doi.org/10.13005/OJC/350333
Liu, S., Guan, Y., Sheng, Y., Hu, Y., Rong, Y., Mei, A., Han, H.: A review on green-based additives for halide perovskite solar cells. Adv. Energy Mater. 10(13), 1902492 (2020). https://doi.org/10.1002/AENM.201902492
Honsberg and Bowden.: IV curve | PVEducation. Retrieved September 5, 2021. https://www.pveducation.org/pvcdrom/solar-cell-operation/iv-curve (2021). Accessed 23 Sept 2022
Gelderman, K., Lee, L., Donne, S.W.: Flat-band potential of a semiconductor: using the Mott-Schottky equation. J. Chem. Educ. 84(4), 685–688 (2007). https://doi.org/10.1021/ED084P685
Kirchartz, T., Gong, W., Hawks, S.A., Agostinelli, T., MacKenzie, R.C.I., Yang, Y., Nelson, J.: Sensitivity of the mott-schottky analysis in organic solar cells. J. Phys. Chem. C 116(14), 7672–7680 (2012). https://doi.org/10.1021/JP300397F
Ananda, W.: External quantum efficiency measurement of solar cell. QiR 2017–2017 15th International Conference on Quality in Research (QiR): International Symposium on Electrical and Computer Engineering, 2017-December, pp. 450–456 (2017). https://doi.org/10.1109/QIR.2017.8168528
Chen, M., Ju, M., Garces, H., Carl, A., Ono, L., Hawash, Z., Padture, N.: Highly stable and efficient all-inorganic lead-free perovskite solar cells with native-oxide passivation. Nat. Commun.Commun. 10(1), 1–8 (2019). https://doi.org/10.1038/s41467-018-07951-y
Olusola, O.I., Salim, H.I., Dharmadasa, I.M.: One-sided rectifying p–n junction diodes fabricated from n-CdS and p-ZnTe: Te semiconductors. Mater. Res. Express 3(9), 095904 (2016). https://doi.org/10.1088/2053-1591/3/9/095904
Rahman, S.I., Faisal, S., Ahmed, S., Dhrubo, T.I.: A comparative study on different HTMs in perovskite solar cell with ZnOS electron transport layer. 5th IEEE Region 10 Humanitarian Technology Conference 2017, R10-HTC 2017, 2018-January, 546–550. Institute of Electrical and Electronics Engineers Inc. (2018). https://doi.org/10.1109/R10-HTC.2017.8289019
Roman-Vazquez, M., Vidyasagar, C., Munoz-Flores, B., Jimenez-Perez, V.: Recent advances on synthesis and applications of lead- and tin-free perovskites. J. Alloys Compd. (2020). https://doi.org/10.1016/j.jallcom.2020.155112
Rothemund, R., Kreuzer, S., Umundum, T., Meinhardt, G., Fromherz, T., Jantsch, W.: External quantum efficiency analysis of Si solar cells with II-VI nanocrystal luminescent down-shifting layers. Energy Procedia 10, 83–87 (2011). https://doi.org/10.1016/J.EGYPRO.2011.10.157
Roy, A., Ghosh, A., Bhandari, S., Sundaram, S., Mallick, T.K.: Perovskite solar cells for BIPV application: a review. Buildings 10, 129 (2020). https://doi.org/10.3390/buildings10070129
Martin, R.: Cuurent density, physics libretexts. https://phys.libretexts.org/Bookshelves/University_Physics/Book%3A_Introductory_Physics_-_Building_Models_to_Describe_Our_World_(Martin_Neary_Rinaldo_and_Woodman)/19%3A_Electric_Current/19.07%3A_Summary (2020). Accessed 5 Sep 2021
Sahli, F., Werner, J., Kamino, B.A., Bräuninger, M., Monnard, R., Paviet-Salomon, B., Ballif, C.: Fully textured monolithic perovskite/silicon tandem solar cells with 25.2% power conversion efficiency. Nat. Mater. 17(9), 820–826 (2018). https://doi.org/10.1038/s41563-018-0115-4
Shao, Z., Mercier, T.L., Madec, M.B., Pauporté, T.: AgBi2I7 layers with controlled surface morphology for solar cells with improved charge collection. Mater. Lett. 221, 135–138 (2018). https://doi.org/10.1016/j.matlet.2018.03.085
Swain, G., Sultana, S., Moma, J., Parida, K.: Fabrication of hierarchical two- dimensional MoS 2 nanoflowers decorated upon cubic CaIn 2 S 4 micro flowers : facile approach to construct novel metal-free p − n heterojunction Sem. (August). (2018). https://doi.org/10.1021/acs.inorgchem.8b01221
Wang, M., Zeng, P., Bai, S., Gu, J., Li, F., Yang, Z., Liu, M.: High-quality sequential-vapor-deposited Cs 2 AgBiBr 6 thin films for lead-free perovskite solar cells. Solar RRL 2(12), 1800217 (2018). https://doi.org/10.1002/solr.201800217
Xing, G.: Facile deposition of high-quality Cs2AgBiBr 6 films for efficient double perovskite solar cells. Sci. China Mater. 63(8), 1518–1525 (2020). https://doi.org/10.1007/s40843-020-1346-0
Zhang, Z., Wu, C., Wang, D., Zhang, Q., Guo, X., Lao, Y., Qu, B., Xiao, L., Chen, Z.: Efficient non-lead double perovskite solar cell with multiple hole transport layers. ACS Appl. Energy Mater. 10, 9594–9599 (2020). https://doi.org/10.1021/acsaem.0c01066
Acknowledgements
The authors wishes to appreciate the IEREK International Experts for Research Enrichment and Knowledge Exchange (EUR) for paying the APC of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emetere, M.E., Bello, O.O. Green-based modifiable CaZnBr4 for solar cells application. Mater Renew Sustain Energy 12, 219–234 (2023). https://doi.org/10.1007/s40243-023-00242-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-023-00242-7