Abstract
Dye-sensitized solar cells (DSSCs) rely heavily on the counter electrode for their performance, which is responsible for collecting and transferring electrons generated at the photoanode. While platinum (Pt) has traditionally been used as a counter-electrode material, its cost, limited availability, and environmental concerns make it an unsuitable option for large-scale implementation. Iron–nitrogen––carbon (Fe–N–C) catalysts are receiving increasing attention due to their high catalytic activity and low cost. This study aims to investigate the performance of Fe–N–C materials as counter electrodes in DSSCs and assess their potential as a sustainable alternative to currently used platinum. Two different Fe–N–C-based materials have been synthesized using different carbon and nitrogen sources, and their electrochemical behavior has been assessed using current–voltage curves and impedance spectroscopy. The catalyst comprised a higher amount of iron and nitrogen shows higher efficiency and lower charge-transfer resistance due to improved iodide reaction kinetics and proper stability under potential cycling. However, this catalyst shows lower stability under a passive ageing procedure, which requires further clarification. Results provide new insights into the performance of Fe–N–C-based materials in DSSCs and aid in the further development of this promising technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world's energy crisis is becoming increasingly pressing, with the growing demand for electricity resulting from the rapid expansion of the world's population and the depletion of traditional energy sources such as fossil fuels [1]. To address this crisis, it is essential to find new, sustainable energy sources that are both environmentally friendly and economically feasible. Solar energy has emerged as one of the most promising solutions, providing an abundant and renewable energy source that has a low carbon footprint [2,3,4]. Solar energy is clean, renewable, and widely available, making it an attractive alternative to traditional energy sources. In recent years, technology has advanced rapidly, and the cost of photovoltaic (PV) materials has declined, making solar energy increasingly accessible worldwide. One promising technology in the field of solar energy is Dye-Sensitized Solar Cells (DSSCs), which have gained significant attention as a promising alternative to conventional silicon-based solar cells [5,6,7,8,9].
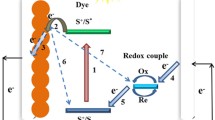
DSSCs have several advantages over conventional solar cells, including high energy conversion efficiency, low cost, and ease of fabrication. In addition, they are highly flexible, making them ideal for use in portable electronic devices and building-integrated photovoltaics (BIPVs). The performance of DSSCs is highly dependent on the performance of the counter electrode, which plays a critical role in the overall functioning of the cell. The counter electrode is responsible for collecting electrons generated during the photoexcitation of the sensitizer and transferring them to the external circuit [10, 11].
The three major types of counter electrodes are platinum-based, carbon-based, and other material-based [12].
Traditionally, platinum (Pt) has been used as the counter-electrode material in DSSCs, but its high cost, limited availability, and environmental concerns make it an unsuitable option for large-scale implementation [13,14,15].
This has led to the search for alternative materials for the counter electrode in DSSCs [16,17,18,19,20,21]. For example, to replace the use of platinum-based electrodes for the DSSC, hexagonal palladium-carbon nanocubes (H-Pd/CNCs) were synthesized in a single step utilizing a straightforward chemical process. An examination of the porous structure revealed that the H-Pd/CNC sample had a large surface area and an average pore size of about 3 nm. Using H-Pd/CNCs serving as the counter electrode, a Power Conversion Efficiency (PCE) of 4.1% was achieved [22].
To avoid noble metals, another approach involves the use of transition metal oxides as counter electrodes. Zinc oxide (ZnO) [23], titanium dioxide (TiO2) [21], and tungsten oxide (WO3) [24] have been investigated as potential Pt-free counter electrodes in DSSCs. These materials have been found to have excellent catalytic activity and stability, making them promising candidates for use as counter electrodes.
Carbonaceous materials showed good perspectives as low-cost counter electrodes with performance comparable to platinum [10, 25,26,27,28,29,30,31]. For instance, graphene nanoplatelets (GNPs), produced from natural graphite utilizing a quick and efficient liquid-phase high-shear exfoliation procedure, were employed as the counter-electrode material in DSSCs; the photoconversion efficiency was 6.23% under standard conditions [32].
A metal-free conductive catalyst, based on N-doped core–shell (N-CCS) carbon spheres, was also used, in which the doping of N atoms activated the fully linked mesoporous basal planes of carbon spheres. The PCE for the N-CCS-based DSSC was 7.89% [33]. Alegre et al. proposed carbon xerogel materials, doped with nitrogen or sulfur, demonstrating an improvement in efficiency [34].
In another work, a DSSC was created using a structured configuration (n-MWCNT-TiO2/N3/MWCNT). Here, Pt was swapped out for MWCNT, allowing for less-priced DSSC manufacturing [35].
Torres et al. provided graphene-based materials, exhibiting better stability behavior, upon electrochemical cycling and seasoning than the conventional cells [36].
Metal-nitrogen-carbon catalysts (M–N–C, with M = Fe, Co, Ni, etc.) have recently gained attention as potential candidates for use in different electrochemical applications, including fuel cells [37,38,39], water electrolysers [40,41,42], CO2 electrochemical reduction [43], and, more recently, counter electrodes in DSSCs due to their high electrocatalytic activity and low cost [44, 45].
Yet, for the latter application, M–N–C catalysts’ investigations are limited to a few publications, mainly devoted to cobalt [46], or combined with iron-iron carbide (Fe/Fe3C) nanocomposites [44] or nickel-based nanoparticles [47]. From our previous experience in Fe–N–C catalysts [48,49,50], which showed top performance activity as cathode catalyst for the oxygen reduction reaction, the approach in this work was to investigate our developed catalysts as counter electrodes in DSSCs assessing their potential as a sustainable alternative to traditional platinum-based materials. In the present work, we have selected two Fe–N–C catalysts derived from two different nitrogen–carbon precursors (nicarbazin and aminobenzimidazole) consisted of different iron and nitrogen composition. Their performance as counter electrodes in DSSCs has been assessed using impedance spectroscopy and current–voltage curves. The results of this work provide new insights into the performance of Fe–N–C-based materials as counter electrodes in DSSCs and help to further develop this promising technology.
Experimental
Physicochemical characterization
X-ray diffraction (XRD) measurements were acquired by a Bruker D8 Advance spectrometer (Bruker Italia Srl, Milano, Italy) on the FeNC powders employing a Cu–K source operating at 40 kV and 40 mA. The analysis was carried out with Bragg-Bentano optical geometry in the range of 5–70°.
Scanning transmission electron microscopy–energy dispersive X-ray spectroscopy (S/TEM–EDX) investigations were carried out by a Jeol JEM-F200 electron microscopy (Jeol Italia S.p.A., Milano, Italy).
Catalyst preparation
Two Fe–N–C catalysts were synthesized using a sacrificial support method (SSM). The synthesis procedures are briefly described below and more details can be consulted elsewhere [49, 50].
Fe–ABZIM: A mixture of two types of commercial silica materials was used as a sacrificial support (LM150 with surface area of 150 m2 g−1 and A90 with surface area of 90 m2 g−1) [Fe(NO3)3⋅9 H2O, Sigma-Aldrich] 4 g was dissolved in 10 mL of acetone. 25 g of aminobenzimidazole (ABZIM) was dispersed in 100 mL of acetone. The mix of silica materials was added to a solution of ABZIM and a sonicated for 4 h. Iron nitrate solution was added to the SiO2/ABZIM suspension under vigorous stirring. Acetone was evaporated and the mixture was ball-milled in a planetary ball mill at 400 rpm for 2 h. The finely homogenized mixture of precursors was heat treated in an inert atmosphere of ultrahigh-purity (UHP) nitrogen at a flow rate of 150 mL min−1 at 945 °C for 60 min. The mixture of silica was removed by means of 25 wt % of HF for 7 days. The powder was washed with deionized water until neutral pH was achieved. To remove the low soluble but volatile silica compounds, a second treatment in ammonia atmosphere (10% of NH3) was performed at 975 °C for 45 min. To remove iron nanoparticles coated with graphitic layer, an additional treatment with 4 M HNO3 was performed at room temperature for 48 h. Material was washed from the remained HNO3 and heat treated a third time in an atmosphere of 7% NH3 at 975 °C for 15 min.
Fe–NCB: First, a calculated amount of silica (Cab-O-Sil® M5P, surface area 125 m2 g−1) was dispersed in water using a high energy ultrasound probe. Then, suspension of nicarbazin (Nicarbazin, Sigma-Aldrich) in acetone was added to silica and sonicated for 20 min in an ultrasound bath. Finally, a solution of iron nitrate (Fe(NO3)3*9H2O, Sigma-Aldrich) was added to SiO2–NCB solution and ultrasonicated for 8 h (the total metal loading on silica was calculated to be ~ 20wt.%). A gel of silica and Fe–NCB was dried overnight at T = 85 °C. The obtained solid was ground to a fine powder in an agate mortar and then subjected heat treatment (HT). The general conditions of HT were UHP nitrogen (flow rate of 100 cc min−1), 20 deg min−1 temperature ramp rate. The experimental variable component of heat-time trajectory was temperatures and duration of HT (900 °C, 1 h; 950 °C, 30 min and 950 °C, 1 h). After heat treatment, silica was leached using 25 wt.% HF overnight. Finally, the Fe–NCB catalyst was washed with DI water until neutral pH was achieved and then dried at T = 85 °C. A second heat treatment was performed at T = 950 °C in reactive (NH3) atmospheres.
Dye-sensitized solar cell electrochemical experiments
The photoanodes as well as the counter electrodes were prepared using F-doped tin oxide glass substrates (FTO, sheet resistance: 15 Ω/square). The photoanodes were prepared by spraying two layers: a thin film of titanium dioxide aqueous solution (from 40 mM TiCl4), and subsequently, spray coating deposition of TiO2 paste onto the FTO glass, prepared from a dispersion of commercial powder (Degussa P90) and Triton surfactant in water and isopropyl alcohol (50% v/v). Electrodes were sintered at 450 °C in air for 30 min. Dye sensitization was performed by immersing the as-prepared electrodes in 0.5 mM ruthenium dye [N719, Solaronix, cis-isothiocyanate-bis (2,20-bipyridyl-4,40-dicarboxylato) ruthenium(II) bis(tetrabutylammonium)] solution in ethanol for approximately 18 h.
For the preparation of counter electrodes, Fe–N–C catalysts were mixed with a 15 wt.% of TiO2 (Degussa P90) as a binder. The powders were dispersed in ethanol. The catalyst-based slurry was sprayed onto FTO glass substrates. Afterwards, the electrodes were sintered in an inert atmosphere at 450 °C for 10 min. A platinum counter electrode was also prepared by depositing hexachloroplatinic acid aqueous solution (5 mM) on the FTO substrate, followed by thermal treatment at 450 ºC for 30 min.
The cell electrolyte consisted of 0.4 M LiI, 0.04 M I2, 0.3 M 4-tertbutylpyridine (TBP), and 0.4 M tetrabutylammonium iodide (TBAI) in acetonitrile. A spacer, thick 60 µm (Surlyn® SX1170-60, Solaronix SA) was used to allocate the electrolyte solution between the photoanode and the counter electrode.
Polarization curves (current vs. voltage) of the cells were recorded under simulated AM 1.5 solar illumination (Osram, 300W) at room temperature. The incident light intensity was adjusted to 100 mW cm−2 using a photometer (3 M Photodine Inc.). The DSSC cells were connected to an Autolab Potentiostat/Galvanostat (Metrohm) equipped with a frequency response analyser (FRA). The active area of the cells was 0.28 cm2. EIS experiments were carried out in the frequency range 100 mHz–100 kHz at open-circuit potential (OCP), with a potential pulse amplitude of 0.01 V (rms). The charge-transfer resistances (Rct) were calculated from the intercept of the Nyquist plot at zero imaginary impedance at low frequency and subtracting the series resistance (Rs) at the intercept of high frequency.
Results and discussion
XRD analysis in Fig. 1 shows that broad peaks characterize the diffractographic patterns of Fe–NCB and Fe–ABZIM. The diffraction peaks at 26.2°, 42.2°, 44.4°, 50.4°, 54.0°, and 59.4° correspond to hexagonal graphitic 2H carbon (JCPDS 75-1621), whereas peaks related to metallic iron or iron oxides are not found for both samples [51]. The shoulder on the left of the predominant graphitic peak and the peak at 34.4° indicate the presence of carbon under the carbolite mineral phase (JCPDS 50-0926). This latter peak, slightly more pronounced in Fe–ABZIM, represents the unique difference between the two counter-electrode diffraction patterns.
TEM images, in Fig. 2, were acquired to investigate the morphology and the eventual presence of iron nanoparticles. While EDX and X-ray Photoelectron Spectroscopy (XPS) [49, 50] analyses confirmed the presence of Fe (Table 1), iron nanoparticles were not clearly observed in the TEM images, suggesting that Fe particles are extremely small and homogeneously dispersed throughout the nitrogen-enriched carbon network.
DSSCs were assembled with the two iron-based catalysts herein investigated as counter electrodes. The photocurrent–voltage response is depicted in Fig. 3.
The main difference between catalysts was found in the short-circuit current density, which was found to be higher for the cell with Fe–NCB as the counter electrode. This could be attributed to a larger amount of iron (0.4 at.%) and/or nitrogen (4.7 at.%) compared to the Fe–ABZIM catalyst (0.13 and 3.1 at.% for iron and nitrogen, respectively) [49, 50] According to a previous work, nitrogen doping causes a decrease of charge-transfer resistance (Rct) due to the increase of triiodide reduction reaction kinetics. This translates into a better electrochemical behavior (larger current density in the whole window of potential).
The most relevant parameters related to DSSC performance are summarized in Table 2. The low efficiency of around 2.2–2.4% can be ascribed to a non-optimized fabrication process for the photoanode. Like in previous publications, herein, we used a thick spacer (60 µm) between electrodes for the electrolyte. This configuration is convenient for reproducibility and stability experiments. Moreover, a simple photoanode preparation method is used to avoid side effects. These two aspects negatively influence the power conversion efficiency compared to typical values that can be found in the literature, but this configuration allowed us to obtain good reproducibility and stability for the experiments. Hence, our main attention was focused on the behavior related only to the counter electrode, which is the purpose of the work.
The performance was compared with a cell equipped with the same photoanode and using Pt as the counter electrode under the same conditions (Table 2) [34, 36]. The open-circuit potential (OCP) was similar in both Fe–N–C-based cells and very similar also to the cell equipped with Pt. Whereas, the short-circuit current density (jsc) was found slightly higher for the Fe–NCB catalyst, together with also a slightly higher fill factor (FF) compared to Fe–ABZIM. The FF value depends on several phenomena like the quality of the contact between the catalyst film and the FTO at the counter electrode, the series resistance, or the recombination reaction at the photoanode. Since all the cells in this investigation were built using the same electrolyte and photoanode configurations, the differences in FF could be attributed mainly to differences at the CE/FTO contact. In the electrodes investigated, the FF is relatively high, especially if compared with bare carbonaceous electrodes (0.36–0.57) [31, 34, 36].
Electrochemical impedance spectroscopy (EIS) was carried out to further evaluate the cell behavior. Figure 4 includes the Nyquist plots at open-circuit potential for the DSSC assembled with Fe–N–C catalysts as counter electrodes. Usually, the presence of various semicircles indicates the contribution of different components to the total impedance: charge transfer of CE/electrolyte interface and TiO2/electrolyte interface, and mass transport of iodide. Here, we have included all of them into a global charge-transfer resistance (Rct) that accounts main differences for CE, since the other components were equally built and assembled.
The charge-transfer resistance is found to be 24 Ω cm2 for Fe–NCB and 37 Ω cm2 for Fe–ABZIM, higher for both Fe–N–C catalysts if compared with Pt, but still lower if compared with other carbon materials like undoped carbon xerogels (90 Ω cm2) [34], graphene quantum dots (65 Ω cm2) [36], and comparable or better than graphene (35 Ω cm2) [36] or carbon nanofibers (10–30 Ω cm2) [31] under similar experimental conditions. This points out a positive effect of the presence of metallic species coordinated with nitrogen in lowering the charge-transfer resistance at the counter electrode of DSSC.
To evaluate the stability of the counter electrode, two different tests were carried out. In the first one, the electrochemical stability was assessed by continuously cycling the DSSC from open-circuit to short-circuit potentials, at 0.1 V s−1 up to 100 cycles. The polarization curves before and after this procedure are reported in Fig. 5. Upon cycling, there is a slight decrease in performance, mainly in terms of short-circuit current density. This is more evident for the Fe–NCB counter electrode, whereas the behaviour of Fe–ABZIM remains very similar to the beginning of the test. A DSSC cell with Pt as counter electrode is included also in Fig. 5, showing better performance than Fe–N–C catalysts. However, the polarization curve upon 100 cycles for the Pt-based cell exhibits a certain loss of current, especially at higher potentials, with 16% lower maximum power density than the initial one.
The second test to assess the stability consisted of the realization of polarization curves during a seasoning period. This kind of durability test is more commonly used in the literature. The DSSCs are kept in the dark at room temperature and ambient pressure and humidity. During ageing, performance can decay due to dye degradation or the reaction of the different components with the electrolyte, including the counter electrode. In Fig. 6 the polarization curves recorded on different days upon seasoning are plotted. Fe–NCB catalyst, despite presenting a better initial performance, exhibits a higher degree of sequential loss of performance with an important decay in jsc. Whereas the cell equipped with Fe–ABZIM maintains almost 80% of the initial performance after 10 days of seasoning. A comparison of the relative maximum power density for the two catalysts together with a DSSC equipped with Pt counter electrode is represented in Fig. 6c.
Individualization of the parameters influencing the performance during seasoning can also be assessed by EIS. Figure 7 shows the Nyquist plots at OCP. The charge-transfer resistance increases rapidly for Fe–ABZIM up to 77 Ω cm2, but then it does not increase much further with seasoning (82 after Ω cm2 10 days). Fe–NCB shows different behavior, with a moderate increase of charge-transfer resistance with time. The higher stability observed for the Fe–ABZIM catalyst was also evidenced in long-term durability investigation in another application. The decrease in performance under constant voltage after 100 h operation as cathode for direct methanol fuel cell was much lower for the Fe–ABZIM catalyst (50%) compared to Fe–NCB (67%). [39] A plausible explanation for this enhanced stability in the Fe–ABZIM catalyst could be derived from the triple heat treatment in the final synthetic process for this catalyst, in comparison with Fe–NCB characterized by a double treatment. Thermal treatment is well known to create more active species on this kind of catalysts for fuel cell applications [52].
Conclusion
Fe–N–C-based materials, as counter electrodes in DSSCs, have been synthesized using two different nitrogen–carbon precursors. The developed counter electrodes (Fe–NCB and Fe–ABZIM) have been assessed using impedance spectroscopy and current–voltage measurements, showing efficiencies of 2.2–2.4%, slightly lower than Pt counter electrode (3.6%), but with superior stability, in particular under ageing conditions. Among the two Fe–N–C-based catalysts, the one showing the highest amount of nitrogen and iron (Fe–NCB) performed better initially, but it was characterized by a lower stability compared to the other one (Fe–ABZIM). This aspect needs further investigation. However, the findings of this study offer novel perspectives on the appropriateness of Fe–N–C-based materials as counter electrodes for DSSCs and facilitate the advancement of this auspicious technology.
Data availability
Data will be made available on request.
References
Tian, J., Yu, L., Xue, R., Zhuang, S., Shan, Y.: Global low-carbon energy transition in the post-COVID-19 era. Appl. Energy (2022). https://doi.org/10.1016/j.apenergy.2021.118205
Sen, A., Putra, M.H., Biswas, A.K., Behera, A.K., Groβ, A.: Insight on the choice of sensitizers/dyes for dye sensitized solar cells: a review. Dyes Pigm. (2023). https://doi.org/10.1016/j.dyepig.2023.111087
Kaliramna, S., Dhayal, S.S., Chaudhary, R., Khaturia, S., Ameta, K.L., Kumar, N.: A review and comparative analysis of different types of dyes for applications in dye-sensitized solar cells. Braz. J. Phys. (2022). https://doi.org/10.1007/s13538-022-01109-4
Kumar, D.: A short review on the advancement in the development of TiO2 and ZnO based photo-anodes for the application of dye-sensitized Solar cells (DSSCs). Eng. Res. Express (2021). https://doi.org/10.1088/2631-8695/ac3b29
Alim, M.A., Repon, M.R., Islam, T., Mishfa, K.F., Jalil, M.A., Aljabri, M.D., et al.: Mapping the progress in natural dye-sensitized solar cells: materials, parameters and durability. ChemistrySelect (2022). https://doi.org/10.1002/slct.202201557
Sheela, S.E., Sekar, R., Maurya, D.K., Paulraj, M., Angaiah, S.: Progress in transition metal chalcogenides-based counter electrode materials for dye-sensitized solar cells. Mater. Sci. Semicond. Process. (2023). https://doi.org/10.1016/j.mssp.2022.107273
Teja, A.S., Srivastava, A., Satrughna, J.A.K., Tiwari, M.K., Kanwade, A., Chand Yadav, S., et al.: Optimal processing methodology for futuristic natural dye-sensitized solar cells and novel applications. Dyes Pigm. (2023). https://doi.org/10.1016/j.dyepig.2022.110997
Richhariya, G., Meikap, B.C., Kumar, A.: Review on fabrication methodologies and its impacts on performance of dye-sensitized solar cells. Environ. Sci. Pollut. Res. 29, 15233–15251 (2022). https://doi.org/10.1007/s11356-021-18049-2
Baby, R., Nixon, P.D., Kumar, N.M., Subathra, M.S.P., Ananthi, N.: A comprehensive review of dye-sensitized solar cell optimal fabrication conditions, natural dye selection, and application-based future perspectives. Environ. Sci. Pollut. Res. 29, 371–404 (2022). https://doi.org/10.1007/s11356-021-16976-8
Wu, J., Lan, Z., Lin, J., Huang, M., Huang, Y., Fan, L., et al.: Counter electrodes in dye-sensitized solar cells. Chem. Soc. Rev. 46, 5975–6023 (2017). https://doi.org/10.1039/c6cs00752j
Thomas, S., Deepak, T.G., Anjusree, G.S., Arun, T.A., Nair, S.V., Nair, A.S.: A review on counter electrode materials in dye-sensitized solar cells. J. Mater. Chem. A Mater. 2, 4474–4490 (2014). https://doi.org/10.1039/c3ta13374e
Richhariya, G., Kumar, A., Shukla, A.K., Shukla, K.N., Meikap, B.C.: Effect of different counter electrodes on power conversion efficiency of DSSCs. J. Electron. Mater. 52, 60–71 (2023). https://doi.org/10.1007/s11664-022-09973-1
Wang, M., Zhao, Y., Yuan, S., Wang, Z., Ren, X., Zhang, M., et al.: High electro-catalytic counter electrode based on three-dimensional conductive grid for dye-sensitized solar cell. Chem. Eng. J. 255, 424–430 (2014). https://doi.org/10.1016/j.cej.2014.06.043
Yoon, C.H., Vittal, R., Lee, J., Chae, W.S., Kim, K.J.: Enhanced performance of a dye-sensitized solar cell with an electrodeposited-platinum counter electrode. Electrochim. Acta 53, 2890–2896 (2008). https://doi.org/10.1016/j.electacta.2007.10.074
Lee, Y.L., Chen, C.L., Chong, L.W., Chen, C.H., Liu, Y.F., Chi, C.F.: A platinum counter electrode with high electrochemical activity and high transparency for dye-sensitized solar cells. Electrochem. Commun. 12, 1662–1665 (2010). https://doi.org/10.1016/j.elecom.2010.09.022
Sharma, G., Singh, V., Dolia, S.N., Jain, I.P., Jain, P.K., Lal, C.: Present status of metal-free photosensitizers for dye-sensitized solar cells. Mater. Today Proc. (2023). https://doi.org/10.1016/j.matpr.2023.02.179
Prasanna, J.L., Goel, E., Kumar, A., Kumar, A.: Review of nanomaterials impact on improving the performance of dye-sensitized and perovskite solar cells. Opt. Quantum Electron. (2022). https://doi.org/10.1007/s11082-022-04147-z
Kabir, F., Manir, S., Bhuiyan, M.M.H., Aftab, S., Ghanbari, H., Hasani, A., et al.: Instability of dye-sensitized solar cells using natural dyes and approaches to improving stability – an overview. Sustain. Energy Technol. Assess. (2022). https://doi.org/10.1016/j.seta.2022.102196
Noorasid, N.S., Arith, F., Mustafa, A.N., Azam, M.A., Mahalingam, S., Chelvanathan, P., et al.: Current advancement of flexible dye sensitized solar cell: a review. Optik (Stuttg) (2022). https://doi.org/10.1016/j.ijleo.2021.168089
Mujtahid, F., Gareso, P.L., Armynah, B., Tahir, D.: Review effect of various types of dyes and structures in supporting performance of dye-sensitized solar cell TiO2-based nanocomposites. Int. J. Energy Res. 46, 726–742 (2022). https://doi.org/10.1002/er.7310
Dhonde, M., Sahu, K., Das, M., Yadav, A., Ghosh, P., Murty, V.V.S.: Review—Recent advancements in dye-sensitized solar cells; from photoelectrode to counter electrode. J. Electrochem. Soc. 169, 066507 (2022). https://doi.org/10.1149/1945-7111/ac741f
Vijayakumar, P., Senthil Pandian, M., Pandikumar, A., Ramasamy, P.: A facile one-step synthesis and fabrication of hexagonal palladium-carbon nanocubes (H-Pd/C NCs) and their application as an efficient counter electrode for dye-sensitized solar cell (DSSC). Ceram. Int. 43, 8466–8474 (2017). https://doi.org/10.1016/j.ceramint.2017.03.198
Yousif, Q.A., Mahdi, K.M., Alshamsi, H.A.: Enhanced photovoltaic performance of dye-sensitized solar cell based on ZnO nanoparticles and ZnO/graphene nanocomposites. J. Chin. Chem. Soc. 68, 1637–1643 (2021). https://doi.org/10.1002/jccs.202000382
Gayathri, R., Rajeswaran, P., Raja, G., Bavaji, S.R., Ameen, N., Shkir, M.: Fabrication of WO3 nanotubes/graphene oxide nanosheets hybrid structures: enhanced solar conversion efficiency in dye sensitized solar cell. Diam. Relat. Mater. (2021). https://doi.org/10.1016/j.diamond.2021.108562
Aftabuzzaman, M., Lu, C., Kim, H.K.: Recent progress on nanostructured carbon-based counter/back electrodes for high-performance dye-sensitized and perovskite solar cells. Nanoscale 12, 17590–17648 (2020). https://doi.org/10.1039/d0nr04112b
Iqbal, M.Z., Khan, S.: Progress in the performance of dye sensitized solar cells by incorporating cost effective counter electrodes. Sol. Energy 160, 130–152 (2018). https://doi.org/10.1016/j.solener.2017.11.060
Devadiga, D., Selvakumar, M., Shetty, P., Santosh, M.S.: Recent progress in dye sensitized solar cell materials and photo-supercapacitors: a review. J. Power. Sources (2021). https://doi.org/10.1016/j.jpowsour.2021.229698
Samantaray, M.R., Mondal, A.K., Murugadoss, G., Pitchaimuthu, S., Das, S., Bahru, R., et al.: Synergetic effects of hybrid carbon nanostructured counter electrodes for dye-sensitized solar cells: a review. Materials 13, 1–34 (2020). https://doi.org/10.3390/ma13122779
Muchuweni, E., Martincigh, B.S., Nyamori, V.O.: Recent advances in graphene-based materials for dye-sensitized solar cell fabrication. RSC Adv. 10, 44453–44469 (2020). https://doi.org/10.1039/d0ra08851j
Denaro, T., Baglio, V., Girolamo, M., Antonucci, V., Arico’, A.S., Matteucci, F., et al.: Investigation of low cost carbonaceous materials for application as counter electrode in dye-sensitized solar cells. J. Appl. Electrochem. 39, 2173–2179 (2009). https://doi.org/10.1007/s10800-009-9841-2
Sebastián, D., Baglio, V., Girolamo, M., Moliner, R., Lázaro, M.J., Aricò, A.S.: Carbon nanofiber-based counter electrodes for low cost dye-sensitized solar cells. J. Power. Sources 250, 242–249 (2014). https://doi.org/10.1016/j.jpowsour.2013.10.142
Nemala, S.S., Aneja, K.S., Bhargava, P., Bohm, H.L.M., Mallick, S., Bohm, S.: Novel high-pressure airless spray exfoliation method for graphene nanoplatelets as a stable counter electrode in DSSC. Electrochim. Acta 285, 86–93 (2018). https://doi.org/10.1016/j.electacta.2018.07.229
Wang, W., Yao, J., Zuo, X., Li, G.: High efficiency nitrogen-doped core-shell carbon spheres as counter electrodes for dye-sensitized solar cells. Mater. Lett. 227, 172–175 (2018). https://doi.org/10.1016/j.matlet.2018.05.066
Alegre, C., Sebastián, D., Lázaro, M.J., Girolamo, M., Aricò, A.S., Baglio, V.: Influence of nitrogen and sulfur doping of carbon xerogels on the performance and stability of counter electrodes in dye sensitized solar cells. Catalysts (2022). https://doi.org/10.3390/catal12030264
Mehmood, U., Ishfaq, A., Sufyan, M.: Nanocomposites of multi-walled carbon nanotubes and titanium dioxide (MWCNTs/TiO2) as affective counter electrode materials for platinum-free dye-sensitized solar cells (DSSCs). Sol. Energy 220, 949–952 (2021). https://doi.org/10.1016/j.solener.2021.04.018
Torres, D., Sebastián, D., Lázaro, M.J., Pinilla, J.L., Suelves, I., Aricò, A.S., et al.: Performance and stability of counter electrodes based on reduced few-layer graphene oxide sheets and reduced graphene oxide quantum dots for dye-sensitized solar cells. Electrochim. Acta 306, 396–406 (2019). https://doi.org/10.1016/j.electacta.2019.03.105
Li, J., Jaouen, F.: Structure and activity of metal-centered coordination sites in pyrolyzed metal–nitrogen–carbon catalysts for the electrochemical reduction of O2. Curr. Opin. Electrochem. 9, 198–206 (2018). https://doi.org/10.1016/j.coelec.2018.03.039
Lo Vecchio, C., Sebastián, D., Lázaro, M.J., Aricò, A.S., Baglio, V.: Methanol-tolerant m–n–c catalysts for oxygen reduction reactions in acidic media and their application in direct methanol fuel cells. Catalysts (2018). https://doi.org/10.3390/catal8120650
Berretti, E., Longhi, M., Atanassov, P., Sebastián, D., Lo Vecchio, C., Baglio, V., et al.: Platinum group metal-free Fe-based (Fe[sbnd]N[sbnd]C) oxygen reduction electrocatalysts for direct alcohol fuel cells. Curr. Opin. Electrochem. (2021). https://doi.org/10.1016/j.coelec.2021.100756
Muhyuddin, M., Zocche, N., Lorenzi, R., Ferrara, C., Poli, F., Soavi, F., et al.: Valorization of the inedible pistachio shells into nanoscale transition metal and nitrogen codoped carbon-based electrocatalysts for hydrogen evolution reaction and oxygen reduction reaction. Mater. Renew. Sustain. Energy 11, 131–141 (2022). https://doi.org/10.1007/s40243-022-00212-5
Santoro, C., Lavacchi, A., Mustarelli, P., Di Noto, V., Elbaz, L., Dekel, D.R., et al.: What is next in anion-exchange membrane water electrolyzers? Bottlenecks, benefits, and future. Chemsuschem (2022). https://doi.org/10.1002/cssc.202200027
Lee, W.H., Ko, Y.J., Kim, J.Y., Min, B.K., Hwang, Y.J., Oh, H.S.: Single-atom catalysts for the oxygen evolution reaction: recent developments and future perspectives. Chem. Commun. 56, 12687–12697 (2020). https://doi.org/10.1039/d0cc04752j
Da Silva Freitas, W., D’Epifanio, A., Mecheri, B.: Electrocatalytic CO2reduction on nanostructured metal-based materials: challenges and constraints for a sustainable pathway to decarbonization. J. CO2 Util. (2021). https://doi.org/10.1016/j.jcou.2021.101579
Wang, W., Zuo, X., Yang, Q., Yang, Q., Tang, H., Zhang, H., et al.: Constructing Fe/Fe3C nanocrystals with Fe-Nx sites in Fe–N–C electrocatalyst to achieve high performance for solar cells. Appl. Catal. B (2022). https://doi.org/10.1016/j.apcatb.2021.120726
Zhang, T., Lv, C., Wang, X., Wang, S., Xie, Y., Yu, M., et al.: Surface active-site engineering in NiCoSe2/nitrogen-doped carbon dodecahedrons for efficient triiodide reduction in photovoltaics. Appl. Surf. Sci. (2023). https://doi.org/10.1016/j.apsusc.2022.155483
Yang, W., Li, Z., Xu, X., Hou, L., Tang, Y., Deng, B., et al.: Atomic N-coordinated cobalt sites within nanomesh graphene as highly efficient electrocatalysts for triiodide reduction in dye-sensitized solar cells. Chem. Eng. J. 349, 782–790 (2018). https://doi.org/10.1016/j.cej.2018.05.139
Yun, S., Zhang, Y., Zhang, L., Liu, Z., Deng, Y.: Ni and Fe nanoparticles, alloy and Ni/Fe-Nx coordination co-boost the catalytic activity of the carbon-based catalyst for triiodide reduction and hydrogen evolution reaction. J. Colloid Interface Sci. 615, 501–516 (2022). https://doi.org/10.1016/j.jcis.2022.01.192
Sebastián, D., Serov, A., Matanovic, I., Artyushkova, K., Atanassov, P., Aricò, A.S., et al.: Insights on the extraordinary tolerance to alcohols of Fe-N-C cathode catalysts in highly performing direct alcohol fuel cells. Nano Energy 34, 195–204 (2017). https://doi.org/10.1016/j.nanoen.2017.02.039
Sebastián, D., Serov, A., Artyushkova, K., Gordon, J., Atanassov, P., Aricò, A.S., et al.: High performance and cost-effective direct methanol fuel cells: Fe-N-C methanol-tolerant oxygen reduction reaction catalysts. Chemsuschem 9, 1986–1995 (2016). https://doi.org/10.1002/cssc.201600583
Serov, A., Artyushkova, K., Niangar, E., Wang, C., Dale, N., Jaouen, F., et al.: Nano-structured non-platinum catalysts for automotive fuel cell application. Nano Energy 16, 293–300 (2015). https://doi.org/10.1016/j.nanoen.2015.07.002
Wang, X., Yang, C., Guo, P., Li, Y., Gao, N., Liang, H.P.: Construction of nitrogen-doped porous carbon nanosheets decorated with Fe-N4and iron oxides by a biomass coordination strategy for efficient oxygen reduction reaction. New J. Chem. 45, 14570–14579 (2021). https://doi.org/10.1039/d1nj02769g
Chen, Y., Huang, Y., Xu, M., Asset, T., Yan, X., Artyushkova, K., et al.: Catalysts by pyrolysis: Direct observation of transformations during re-pyrolysis of transition metal-nitrogen-carbon materials leading to state-of-the-art platinum group metal-free electrocatalyst. Mater. Today 53, 58–70 (2022). https://doi.org/10.1016/j.mattod.2022.01.016
Acknowledgements
The authors acknowledge the project NAUSICA (PON “R&S 2014–2020”, grant n. ARS01_00334); project leader NAVTEC cluster. AS gratefully acknowledges financial support from the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE) under the Hydrogen and Fuel Cells Technologies Office (HFTO), FY2018 Hydrogen and Fuel Cell R&D FOA, Award Number DE-EE0008419 and US DOE ElectroCat consortium.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sebastián, D., Trocino, S., Lo Vecchio, C. et al. Dye-sensitized solar cells based on critical raw material-free Fe–N–C counter electrodes. Mater Renew Sustain Energy 12, 209–218 (2023). https://doi.org/10.1007/s40243-023-00241-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-023-00241-8