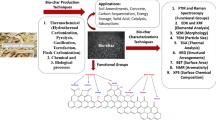
Abstract
CO2 capture is a promising approach to aid in the mitigation of the global environmental crisis caused by greenhouse gas emissions. The efficiency of adsorbents is critical to the success of this approach. Sugarcane bagasse fly ash (SBA) was used in this study as a support to increase the CO2 adsorption capacity of CaO. The physical and chemical characteristics of SBA treated with various reagents (HCl, H3PO4, CH3COOH, NaOH, NH3, and H2O2) were investigated. The CaO was then loaded at 10–50 wt% on the support surface, and the modified adsorbent was tested for its potential to adsorb CO2. According to the results of the experiments, the acidic reagent increased the surface area of SBA, whereas the base reagents provided SBA with a higher pore volume and a larger pore size. The different surface characteristics of the modified SBA had a direct impact on its CO2 adsorption capacity. The adsorbent with NaOH-pretreated SBA and 50% CaO loading had the highest CO2 adsorption capacity, which was 27% higher than that of unsupported CaO due to the decent distribution of CaO found on the NaOH-treated SBA surface. For a better understanding, a graphical model was finally proposed to describe the aforementioned changes in surface characteristics and adhesion of CaO on the SBA support. These findings show that SBA, a valueless bagasse-incinerating waste material, can be used as a support to increase the CO2 adsorption capacity of adsorbents, transforming it into a more valuable and environmentally sustainable material.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In light of the known consequences of continuing to increase CO2 emissions at such a rapid rate, a global agreement to eliminate all carbon output by the year 2050 has been reached, and the terms “net zero emission” and “carbon neutral” are trending in discussion throughout the world. Reducing greenhouse gas (GHG) emissions and increasing use of clean energy will be central to the success of the net zero emission scheme, as discussed at the COP26 conference in Glasgow, Scotland, at the end of 2021. However, some GHG emissions are inevitable as they result from activities necessary to sustain human life, such as the generation of electricity, the manufacture of consumer goods, and so on. In this respect, carbon capture and storage (CCS) is viewed as a process that could potentially play an important role.
CO2 capture techniques currently in use include chemical absorption, membrane separation, and solid-sorbent adsorption. The latter method has grown in popularity as it can be used in a wide range of temperatures, is durable, and, unlike absorption, it does not allow chemical leakage. On top of that, the adsorbent can be restored and reused. Among many materials, calcium oxide (CaO) is one of the most popular adsorbents for CO2 capture at high temperatures (greater than 400 °C), not only because it is inexpensive but also abundant [1]. Moreover, it has a high CO2 adsorption capacity (theoretically up to 17.8 mmol CO2/g of CaO). The adsorption and desorption of CO2 is a two-step process that requires carbonation and calcination, which are, respectively described by Eqs. 1 and 2 [2, 3].
Carbonation:
Calcination:
Although CaO has demonstrated promise as a CO2 adsorbent, further research is required to enhance its adsorption efficiency and stability. On the basis of previous research, Wu et al. [4] compared the effects of micro- and nano-scale CaO particles and found that the latter had greater adsorption and regeneration efficiency. In addition, the incorporation of CaO onto metal oxide supports is a technique used to increase the carbon adsorption efficiency and extend the adsorbent's life span [5]. It was revealed that the type of support has substantial effects on the interaction with CaO, which directly influences the efficiency and stability of the adsorbent. Some of the most popular supports include Al2O3, SiO2, TiO2, and ZrO2 [6,7,8,9,10,11,12,13,14,15]. Furthermore, commercial supports with a high surface area, such as SBA-15, KIT-6 [8, 16], and fly ash, are becoming popular in this area due to their satisfactory durability.
Sugarcane bagasse fly ash (SBA) is a waste material from the use of bagasse, a by-product of sugar the industry, as a fuel to produce electricity and heat. Typically, 10 to 15 wt% of SBA is discarded without being used for any benefit. Under limited disposal costs, most of which are disposed of in landfills to reduce damage to the environment [17]. In fact, bagasse ash contains primarily more than 50 wt% silicon dioxide (SiO2), an important compound with the property of being a good support material. In past research where fly ash was used as a carbon adsorbent without pretreatment, a CO2 adsorption capacity was found to vary in the range of 1.3 to 3.8 g/100 g at 650 °C, depending on the CaO content in the ash. This is in line with research by Miklová et al. [18], where three types of coal ash, including fly ash, heavy ash, and high-temperature calcined ash (1340 °C), were investigated for use as adsorbents. It was found that the carbon adsorption capacity was dependent on the content of CaO and alkali metals present in the ash. In an effort to improve the efficiency of CaO adsorbents, Chen et al. [19] explored CaO in combinations with fly ash derived from coal combustion. It was found that the addition of fly ash was able to provide an adsorption capacity of 0.27 g CO2/g sorbent, which was five times more efficient than CaO alone. Furthermore, the interaction of CaO with Al2O3 on the fly ash yields Ca3Al2O6 and CaAl2O4, which are more resistant to thermal degradation, leading to greater stability and hence a longer life span. Li et al. [20] investigated the effectiveness of CaO adsorbent mixed with silica-rich rice husk ash after 50 cycles of carbonation and calcification. It was found that the CaO-rice husk adsorbent degraded at a slower rate than CaO alone, which was presumably due to the presence of the formed Ca2SiO4. In addition, activation of ash by acid [21] and base [22] is another method that increases CO2 adsorption efficiency through an increase in adsorbent surface area.
This research aims to develop a CO2-adsorbent support from a non-valuable SBA. Herein, the physical and chemical properties of SBA and the effect of SBA surface treatment with acids, bases, and oxidizing agents on efficiency and stability in CO2 adsorption are investigated.
Materials and methods
Surface pretreatment of SBA
SBA preparation
The SBA samples used in the research were byproducts obtained from Thai Rung Ruang Industry Co., Ltd.'s sugar production process. To remove moisture, the SBA was dried for 12 h at 110 °C in a hot air oven. They were then sized with a sieve shaker to less than 90 microns and stored in a desiccant chamber for further use.
Surface pretreatment of SBA by chemical (acid–base) activation
At a concentration of 3.5 M, 80 mL of surface-improving acids or bases, including hydrochloric acid (HCl), phosphoric acid (H3PO4), acetic acid (CH3COOH), sodium hydroxide (NaOH), ammonia (NH3), and hydrogen peroxide (H2O2), were added to a beaker containing 10 g of SBA. After thoroughly stirring, the mixtures were left at room temperature for 24 h, followed by a rinse with distilled water until neutral. The treated SBA samples were then dried at 110 °C for 12 h and stored in a desiccator until use.
Development of SBA-supported CaO adsorbent by incipient wetness impregnation
A calcium solution (prepared from Ca(NO3)2.4H2O) was gently pipetted onto the pretreated SBA until saturated before left at room temperature overnight and was then dried in an oven at 110 °C. Subsequently, the re-impregnation was carried out with the same calcium solution, which was thereafter left at room temperature for 12 h before drying until its calcium content reached 10%, 30%, and 50% by weight. Finally, the calcium-treated SBA was sintered at 900 °C with a heating rate of 20 °C/min for 2 h and was then sized with a sieve shaker to less than 90 microns and stored in a desiccant chamber until used.
Analysis of physical and chemical characteristics of SBA and SBA-supported CaO adsorbents
SBA and SBA-supported CaO were analyzed for specific surface area, pore volume, and pore size by the Brunauer–Emmett–Teller (BET) technique using a BET surface area analyzer (V-Sorb 2800P, APP Application, China) and the phase analysis was carried out using an X-ray Diffractometer: (XRD) (D8 ADVANCE ECO, Bruker, Germany).
Analysis of number of basic sites on adsorbent surface by CO2 temperature-programmed desorption (CO2-TPD)
The U-shaped quartz reactor (Quantachrome Anton Paar model Autosorb iQ-C-XR (1 STAT.) PFE, USA) was charged with approximately 0.05 g of SBA-supported CaO, and then helium gas at a flow rate of 40 ml/min was flowed through the reactor while the temperature was maintained at 150 °C for 30 min. Once the reaction was complete, a steady stream of helium gas was introduced to bring the temperature down to ambient levels. Subsequently, 40 ml/min of CO2 gas was allowed to flow through the reactor for 30 min, followed by helium for another 15 min. After that, a 40 ml/min helium gas flow was enabled to raise the temperature from ambient level to 900 °C and a thermal conductivity detector was used to examine the amount of CO2 desorbed.
Study on CO2 adsorption capacity of SBA-supported CaO adsorbents
The analysis of the CO2 adsorption efficiency of SBA-supported CaO was carried out using a gas sorption analyzer (Autosorb iQ-C-XR, 1 STAT, PFE, Quantachrome Instruments, Anton Paar, USA). A U-shaped quartz reactor tube is loaded with 0.05–0.10 g of a sorbent sample. Then, at 900 °C, helium gas was allowed to flow through the reactor at a rate of 50 ml/min for 5 min. In order for the sample to adsorb CO2, the reactor temperature was lowered to 650 °C and held there for 20 min prior to the introduction of a 50 ml/min flow of CO2 gas. Subsequently, the reactor was cooled to room temperature and then increased immediately with a heating rate of 50 °C/min until it reached 900 °C where it was held 5 min to allow for CO2 desorption, which was detected by a thermal conductivity detector and a mass spectrometer.
Results and discussion
Chemical composition of pre-treated SBA
Table 1 shows the composition of SBA before and after 24 h of surface treatment with different chemicals at room temperature, including HCl, H3PO4, CH3COOH, NaOH, NH3, and H2O2. Raw SBA was found to be primarily composed of SiO2 (64.4 wt%), Al2O3 (6.20 wt%), CaO (4.03 wt%), and approximately 9 wt% of alkali and alkaline-earth metal oxides. As treated with acid, the proportion of SiO2 increased, to the range of 73.2–81.4 wt%, due to the acid leaching of the metal oxides present in raw SBA, including Al2O3, Fe2O3, CaO, P2O5, MgO, TiO2, K2O, and Na2O [23]. This is consistent with previous research by Kongnoo et al. [21], where the SiO2 content in the palm oil mill fly ash was found to increase from 51 to 81 wt% after HCl pretreatment. Panitchakarn et al. [24] also found that acid pretreatment could remove up to 20 wt% of various metal oxides (Fe2O3, CaO, and other impurities) from coal fly ash. In addition, among the various acids tested for their efficiency in removing metal oxides, HCl contributed to the greatest increase in SiO2 composition (81.4 wt%). Similarly, Chen et al. [25] investigated the effect of acid pretreatment on rice husk ash and found that HCl was superior to CH3COOH and H2SO4 at removing various metal oxides.
In the trials where bases (NaOH and NH3) and H2O2 were used to improve the SBA surface, it was found that the increase in SiO2 content was less than that when treated with acids. After the treatment by NH3 and H2O2, the resulting SBA contained approximately 70 wt% of SiO2 constituents. However, this differs from NaOH pretreatment, which resulted in SBA having the least SiO2 composition (65.4 wt%) as a consequence of SiO2 reacting with NaOH to form a liquid Na2SiO3, which was subsequently separated along with the solution [26, 27]. Considering the removal efficiency of oxides other than SiO2 with different reagents, they can be ranked in descending order as HCl, H3PO4, CH3COOH, H2O2, NH3, and NaOH.
Effects of pretreatment on SBA surface characteristics
Considering the change in surface characteristics, the acid pretreatment had the greatest positive effect on the SBA surface area, making it increase from 21.08 to 54.80 m2/g, followed by H3PO4, and CH3COOH, with a given surface area of 48.26 and 34.24 m2/g, respectively (Fig. 1a). The surface volume, on the other hand, changed insignificantly, ranging from 0.060 to 0.075 cm3/g (Fig. 1b), while the mean pore size decreased from 11.36 to 5.35–6.55 nm (Fig. 1c). This is consistent with previous studies in which acid pretreatment was found to increase the surface area of coal fly ash by Panitchakarn et al. [24] and rice husk ash by Chen et al. [25], with strong acids resulting in a greater surface area increase than weak ones.
SBA surface treatment with bases and H2O2 solution, on the other hand, yielded different results, with SBA having slight changes in surface characteristics when treated with NH3 and H2O2. Intriguingly, when pretreated with a strong base such as NaOH, SBA showed a slight increase in surface area but a significant increase in mean pore volume and pore size to 0.110 cm3/g and 16.38 nm, respectively. Figure 2 shows the results of a pore size distribution analysis that affirmed those found in the aforementioned analyses of chemical composition and surface characteristics. In other words, acid-treated SBA has smaller pores (2–7 nm) than raw SBA, resulting in smaller mean pore sizes. This is because the minor constituents of fly ash, e.g., potassium, calcium, sodium, aluminum, and iron, were washed away by the acid solution, reducing the size of the pores and thereby increasing the surface area. In contrast to acid pretreatment, H2O2-pretreated SBA exhibited a similar pore size distribution to the untreated samples, whereas base pretreatment increased the number of 8–80 nm pores on the SBA surface, leading to an increase in the mean pore size as a consequence. This is in line with a study by Ma et al. [28], where HCl pretreatment was found to give a mean pore size of 3.34 nm on the fly ash surface, while NaOH contributed to an increase in the mean pore size of up to 10.31 nm, compared to the original of 6.53 nm.
Characteristics of SBA-supported CaO adsorbents
SBA samples treated with different reagents were analyzed using the XRD technique to determine their properties for use as CaO-based sorbent supports for CO2 capture. As shown in Fig. 3, the XRD graphs of CaO loaded on modified SBA reveal three distinct crystal structure patterns, including quartz (α-SiO2), larnite (Ca2SiO4), and lime (CaO). Here, SBA treated with different reagents had a different peak intensity of quartz. In line with the results obtained from the previous analysis of chemical composition, the HCl-treated SBA showed the highest peak intensity at the position of 2θ = 26.6˚, indicating the presence of a substantial amount of SiO2. Taking the effect of CaO loading into account, it was found that at 10 wt% CaO, the structural peaks of larnite and lime were observed only on HCl-treated SBA. Other reagent-treated SBAs initially showed structural peaks of larnite (2θ = 32.66˚ and 34.33˚) and lime (2θ = 32.20˚, 37.35˚ and 53.86˚) at the CaO loading of 30 and 50 wt%. Noticeably, the number and the intensity of peaks both increased with increasing CaO proportion, suggesting that more Ca2SiO4 and CaO were distributed and covered the surface of the SBA [29] (Supplementary Information, Fig. S1 – S6). According to previous research, the CO2 adsorption capacity and adsorbent lifetime are directly related to the Ca2SiO4 and CaO content in the samples. Wang et al. [30] synthesized Ca2SiO4 by an 800 °C calcination of CaCO3 and SiO2 and found that the resulting Ca2SiO4 phase could rapidly adsorb CO2 at temperatures between 500 and 800 °C. In addition, Ca2SiO4 possesses excellent chemical and high-temperature resistance [31] and acts as an anti-sintering support for CaO, thereby extending its life span [32].
The CO2-TPD technique was used to analyze the basicity and the number of base sites found on the adsorbents. Based on Fig. 4, modified SBA containing 50 wt% of CaO exhibits similar CO2-TPD desorption profiles over two temperature ranges. The first peak, between 350 and 500 °C, indicates a Lewis acid–base site with moderate strength, while the second peak, between 550 and 750 °C, indicates a low-coordination oxygen anion type with high strength [33]. Calculating the number of base sites from the area under the CO2-TPD desorption curve, it was found that SBA treated with various chemicals shows varying levels of basicity after CaO loading. Here, the SBA treated with NaOH exhibits the highest degree of basicity, followed by that treated with H2O2, HCl, H3PO4, NH3, and CH3COOH. Based on these results, it can be inferred that the surface treatment with NaOH enables CaO and Ca2SiO4 to be highly dispersed on the surface, resulting in a favorable carbon adsorption capacity.
CO2 adsorption capacity of SBA-supported CaO adsorbents
Figure 5 demonstrates the CO2 adsorption capacity of CaO and SBA-supported CaO adsorbent. Certainly, because CaO is the one playing a role in adsorbing CO2, the adsorption capacity varied with the amount of CaO loaded. In particular, the CO2 adsorption capacity of the adsorbents increases by 25–143% when the amount of CaO loaded onto the treated SBA increased from 10 to 50 wt%. This conforms to a study by Yan et al. ([34], which revealed an increase in the CO2 adsorption capacity of composite CaO-fly ash as the CaO loading increased from 50 to 90 wt%. Remarkably, in this study, the increased adsorption capacity is divided into two phases. As observed for all samples, the adsorption capacity increased only slightly when the proportion of CaO increased from 10 to 30 wt%, but rose exponentially from 30 to 50 wt% of CaO loading. This can be explained by the results of the surface characterization analysis using the BET technique, which detected no porous surface area when 10 and 30 wt% of CaO were loaded onto the treated SBA (Supplementary Information, Table S.1). This may be because the loaded CaO penetrates the pore, blocking most of the pore surface and thus preventing CO2 from interacting with the CaO in the pores. As a result, the sample has a low CO2 adsorption capacity. This is consistent with the results obtained from XRD crystal structure analysis, in which low intensity larnite and lime structure peaks were observed, indicating a similarly poor distribution of Ca2SiO4 and CaO on the surface of SBA. At a CaO loading of 50 wt%, the excess CaO from pore infiltration settles on the support's outer surface, which is the area where CO2 can only be taken up. The adsorption capacity thus increased exponentially by 50 wt% loading of CaO. Considering the surface characteristics of the modified SBA, as loaded with 50 wt% CaO, only three samples were detected for porous areas, including HCl-treated SBA, CaO, NaOH-treated SBA, and H2O2-treated SBA, with total pore volumes of 0.01, 0.024, and 0.021 cm3/g and mean pore sizes of 55.68, 115.78, and 25.36 nm, respectively. Interestingly, with higher CO2 adsorption capacity than the raw CaO loaded with 50% CaO, NaOH-treated SBA, and H2O2-treated SBA were the only two samples with the highest total pore volumes in this study (Supplementary Information, Table S.1). This indicates that the CO2 adsorption capacity was directly proportional to the total pore volume, consistent with the mechanism of the carbonation reaction of CaO where, initially, under a kinetically controlled regime, the reaction occurs rapidly. As the CaO on the surface reacts, it is converted to CaCO3. However, because the molar volume of CaCO3 is 2.2 times greater than that of CaO (36.9 vs. 16.7 cm3/g, respectively), the second phase of the reaction is slowed down, resulting in what is known as a diffusion-controlled regime [35]. Certainly, the lower the sample's total pore volume, the less its adsorption capacity is limited.
However, the CO2 adsorption capacity of CaO on the modified-SBA support (NaOH-treated SBA, loaded with 50% CaO, and H2O2-treated SBA, loaded with 50%) was 23–27% higher than that of the unsupported CaO. This is because the distribution of CaO on the SBA involves in the reduction of the agglomeration of CaO during CO2 adsorption. This result is in line with Lee et al.'s [36] study on the CO2 adsorption efficiency of CaO adsorbents mixed with NaOH. Therein, CO2 retention in fly ash-blended adsorbent was found to increase by up to 9%, as a consequence of better CaO distribution caused by the support of fly ash. In a comparative study on the adsorption capacity at a high temperature range of different adsorbents by Sreenivasulu et al. [37], the mixtures of CaO, MgO, and fly ash at a ratio of 5:1:4 possessed the highest CO2 capture capacity and stability. Due to its high heat resistance, fly ash prevents the sintering and aggregation of CaCO3 during calcinations. In this mixture, fly ash thus contributed to the adsorbent's increased stability, allowing it to be reused for up to 15 cycles. However, it should be noted that the excessive addition of fly ash could negatively cause a reduction in CO2 adsorption capacity. A review by Ge et al. [38] concluded that the adsorption capacity of the fly ash is dependent on the surface area, pore structure, CaO percentage, and surface functional groups of the fly ash. With this regard, the surface treatment of the fly ash thus has a substantial effect on its adsorption capacity. In a study by Kongnoo et al. [21], a Zeolite 13X adsorbent was prepared from palm oil mill fly ash treated with 4–8 M HCl for 4 h at a low temperature (32 °C). As a result, CO2 adsorption capacity increased by 22% in comparison to untreated adsorbents and was 11% higher than that of the commercial Zeolite13X due to an increase in mesopore and the total pore volume from the surface treatment. Moreover, loading an alkali metal with a high electropositivity could lead to an increase in the basicity and, consequently, improve the CO2 adsorption capacity of CaO [39], for example, from 3.26 to 6.27 mmol/g [40].
All of the above analyses show that the reagents used in the SBA surface treatment were an important factor that determines the properties and CaO distribution characteristics on the support surface, which directly reflect the potential of a sorbent in adsorbing CO2. From all the results obtained in this study, a graphical model describing the surface characteristic alteration and the adhesion of CaO to the SBA support is derived. As depicted in Fig. 6 (HCl and NaOH represent acid and base pretreatment, respectively), when acid is applied to raw SBA, certain metal oxides are leached to form microscopic pores, resulting in an increase in surface area and a reduction in the mean pore size. As CaO is loaded at 10 and 30 wt%, it penetrates into the pores and forms a surface coating, rendering the sample's porosity and surface undetectable during analysis. Recalling the analysis by XRD, where low intensity of the larnite and lime peaks indicate the presence of less Ca2SiO4 and CaO on the surface, these are represented in the model by red and orange dots, respectively. Then, when CaO is loaded at 50 wt%, it begins to adhere to the support, forming additional surface area and expanding the pore volume. However, the adhesion behavior of CaO on SBA treated with HCl was different from that treated with NaOH. Bases, in particular, offer SBA a greater pore volume and pore size than acid, allowing CaO to adhere more firmly and distribute more effectively over the surface of the support. As a result, it has the ability to adsorb a greater amount of CO2. The findings of this study indicate that SBA can be used as a support for CO2 adsorbents in high temperature environments. This can be viewed as an option to convert common fly ash, which is not only valueless but also difficult to dispose of, into a more valuable and environmentally sustainable material.
Conclusions
This research utilizes SBA, a byproduct of bagasse incineration for fuel, as a feedstock for the production of a support for a high-temperature CaO-based adsorbent used for CO2 capture. The effect of the surface treatment reagents, i.e., HCl, H3PO4, CH3COOH, NaOH, NH3, and H2O2, on the SBA characteristics, including appearance, chemical composition, surface area, pore volume, pore size, crystal and chemical structure, basicity, and CO2 adsorption capacity, was examined. According to the findings, the reagents used for surface treatment affected the distribution characteristics of CaO on the SBA surface, thereby significantly affecting its adsorption capacity. In particular, the NaOH-treated SBA possessed the highest CO2 adsorption capacity, which increased by 27% when loaded with CaO at 50 wt% due to the greater pore volume and the larger pore size of the NaOH-treated SBA. This allowed CaO, which plays a direct role in CO2 adsorption, to distribute properly on the SBA surface, as evidenced by the detected larnite and lime crystal structures. Additionally, this NaOH-treated SBA also showed the highest base strength. In contrast to HCl-treated SBA, despite the increased surface area, the smaller pore size causes CaO to enter the pores and glaze the surface, making it difficult for CO2 to penetrate and react. Consequently, the capacity to adsorb CO2 is hindered. The results of this study are an important starting point for understanding how pretreatment alters the characteristics of SBA, thereby affecting its CO2 adsorption capacity. This is a guideline to improve the quality of fly ash and transform it into a valuable, high-performance material suitable for use as a CO2 adsorbent for the environment and a sustainable future.
Data availability
All of the findings presented in this article and its supplementary information were generated from the study’s original contributions. All information from other sources used in this article is properly referenced.
References
Kumar, S., Saxena, S.K.: A comparative study of CO2 sorption properties for different oxides. Mater. Renew. Sustain. Energy. (2014). https://doi.org/10.1007/s40243-014-0030-9
Sun, H., Wu, C., Shen, B., Zhang, X., Zhang, Y., Huang, J.: Progress in the development and application of CaO-based adsorbents for CO2 capture—a review. Mater. Today Sustain. (2018). https://doi.org/10.1016/j.mtsust.2018.08.001
Hu, Y., Lu, H., Liu, W., Yang, Y., Li, H.: Incorporation of CaO into inert supports for enhanced CO2 capture: a review. Chem. Eng. J. (2020). https://doi.org/10.1016/j.cej.2020.125253
Wu, S.F., Li, Q.H., Kim, J.N., Yi, K.B.: Properties of a nano CaO/Al2O3 CO2 sorbent. Ind. Eng. Chem. Res. (2008). https://doi.org/10.1021/ie0704748
Dindi, A., Quang, D. V., Vega, L. F., Nashef, E., Abu-Zahra, M. R.: Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. (2019). https://doi.org/10.1016/j.jcou.2018.11.011
Aihara, M., Nagai, T., Matsushita, J., Negishi, Y., Ohya, H.: Development of porous solid reactant for thermal-energy storage and temperature upgrade using carbonation/decarbonation reaction. Appl. Energy. (2001). https://doi.org/10.1016/S0306-2619(00)00072-6
Li, Z.S., Cai, N.S., Huang, Y.Y., Han, H.J.: Synthesis, experimental studies, and analysis of a new calcium-based carbon dioxide absorbent. Energy Fuels (2005). https://doi.org/10.1021/ef0496799
Huang, C.H., Chang, K.P., Yu, C.T., Chiang, P.C., Wang, C.F.: Development of high-temperature CO2 sorbents made of CaO-based mesoporous silica. Chem. Eng. J (2010). https://doi.org/10.1016/j.cej.2010.04.045
Wu, S.F., Zhu, Y.Q.: Behavior of CaTiO3/nano-CaO as a CO2 reactive adsorbent. Ind. Eng. Chem. Res. (2010). https://doi.org/10.1021/ie900900r
Broda, M., Müller, C.R.: Synthesis of highly efficient, Ca-based, Al2O3-stabilized, carbon gel-templated CO2 sorbents. Adv. Mater. (2012). https://doi.org/10.1002/adma.201104787
Radfarnia, H.R., Iliuta, M.C.: Development of zirconium-stabilized calcium oxide absorbent for cyclic high-temperature CO2 capture. Ind. Eng. Chem. Res. (2012). https://doi.org/10.1021/ie301287k
Valverde, J.M., Perejón, A., Perez-Maqueda, L.A.: Enhancement of fast CO2 capture by a nano-SiO2/CaO composite at Ca-looping conditions. Environ. Sci. Technol. (2012). https://doi.org/10.1021/es3002426
Xu, P., Xie, M., Cheng, Z., Zhou, Z.: CO2 Capture performance of CaO-based sorbents prepared by a sol–gel method. Ind. Eng. Chem. Res. (2013). https://doi.org/10.1021/ie401600e
Broda, M., Müller, C.R.: Sol–gel-derived, CaO-based, ZrO2-stabilized CO2 sorbents. Fuel (2014). https://doi.org/10.1016/j.fuel.2013.08.004
Sanchez-Jimenez, P.E., Perez-Maqueda, L.A., Valverde, J.M.: Nanosilica supported CaO: a regenerable and mechanically hard CO2 sorbent at Ca-looping conditions. Appl. Energy. (2014). https://doi.org/10.1016/j.apenergy.2013.12.024
Sun, H., Parlett, C.M., Isaacs, M.A., Liu, X., Adwek, G., Wang, J., Wu, C.: Development of Ca/KIT-6 adsorbents for high temperature CO2 capture. Fuel (2019). https://doi.org/10.1016/j.fuel.2018.07.044
Sinyoung, S., Asavapisit, S., Kunchariyakun, K.: Investigation of bagasse ash as an alternative raw material in clinker production. J. Mater. Civ. Eng. (2022). https://doi.org/10.1061/(ASCE)MT.1943-5533.0004073
Miklová, B., Staf, M., Kyselová, V.: Influence of ash composition on high temperature CO2 sorption. J. Environ. Chem. Eng. (2019). https://doi.org/10.1016/j.jece.2019.103017
Chen, H., Khalili, N., Li, J.: Development of stabilized Ca-based CO2 sorbents supported by fly ash. Chem. Eng. J. (2018). https://doi.org/10.1016/j.cej.2018.03.162
Li, Y., Zhao, C., Ren, Q., Duan, L., Chen, H., Chen, X.: Effect of rice husk ash addition on CO2 capture behavior of calcium-based sorbent during calcium looping cycle. Fuel Process. Technol. (2009). https://doi.org/10.1016/j.fuproc.2009.03.013
Kongnoo, A., Tontisirin, S., Worathanakul, P., Phalakornkule, C.: Surface characteristics and CO2 adsorption capacities of acid-activated zeolite 13X prepared from palm oil mill fly ash. Fuel (2017). https://doi.org/10.1016/j.fuel.2016.12.087
Dindi, A., Quang, D.V., Nashef, E., Zahra, M.R.A.: Effect of PEI impregnation on the CO2 capture performance of activated fly ash. Energy Procedia (2017). https://doi.org/10.1016/j.egypro.2017.03.1361
Zhang, Q., Liang, X., Peng, D., Zhu, X.: Development of a fly ash derived Li4SiO4-based sorbent for CO2 capture at high temperatures. Thermochim. Acta (2018). https://doi.org/10.1016/j.tca.2018.09.002
Panitchakarn, P., Laosiripojana, N., Viriya-Umpikul, N., Pavasant, P.: Synthesis of high-purity Na-A and Na-X zeolite from coal fly ash. J. Air Waste Manag. Assoc. (2014). https://doi.org/10.1080/10962247.2013.859184
Chen, P., Gu, W., Fang, W., Ji, X., Bie, R.: Removal of metal impurities in rice husk and characterization of rice husk ash under simplified acid pretreatment process. Environ. Prog. Sustain. Energy (2017). https://doi.org/10.1002/ep.12513
Shoppert, A., Loginova, I., Valeev, D.: Kinetics study of Al extraction from desilicated coal fly ash by NaOH at atmospheric pressure. Materials (2021). https://doi.org/10.3390/ma14247700
Wang, R.C., Zhai, Y.C., Ning, Z.Q.: Kinetics of SiO2 leaching from Al2O3 extracted slag of fly ash with sodium hydroxide solution. T. Nonferr. Metal. Soc. (2014). https://doi.org/10.1016/S1003-6326(14)63273-8
Ma, Z., Zhang, X., Guo, Y., Cheng, F.: Extraction of valuable metals and preparation of mesoporous materials from circulating fluidized bed-derived fly ash via an acid–alkali-based alternate method. ACS Omega (2020). https://doi.org/10.1021/acsomega.0c04737
Liu, T.H., Liu, H.Q., Ge, C.H.U., Bi, S.N., Feng, Y.U., Pan, D.H., Li, R.F.: Preparation of CaO/KIT-6 solid base catalyst and its catalytic performance in transesterification. J. Fuel Chem. Technol. (2021). https://doi.org/10.1016/S1872-5813(21)60024-5
Wang, M., Lee, C.G., Ryu, C.K.: CO2 sorption and desorption efficiency of Ca2SiO4. Int. J. Hydrog. Energy. (2008). https://doi.org/10.1016/j.ijhydene.2008.07.114
Zhao, M., Shi, J., Zhong, X., Tian, S.C., Blamey, J., Jiang, J.G., Fennell, P.S.: A novel calcium looping absorbent incorporated with polymorphic spacers for hydrogen production and CO2 capture. Energy Environ. Sci. (2014). https://doi.org/10.1039/C4EE01281J
Yan, F., Jiang, J., Li, K., Liu, N., Chen, X., Gao, Y., Tian, S.: Green synthesis of nanosilica from coal fly ash and its stabilizing effect on CaO sorbents for CO2 capture. Environ. Sci. Technol. (2017). https://doi.org/10.1021/acs.est.7b00320
Aziz, M.A.A., Jalil, A.A., Wongsakulphasatch, S., Vo, D.V.N.: Understanding the role of surface basic sites of catalysts in CO2 activation in dry reforming of methane: a short review. Catal. Sci. Technol. (2020). https://doi.org/10.1039/C9CY01519A
Yan, F., Jiang, J., Li, K., Tian, S., Zhao, M., Chen, X.: Performance of coal fly ash stabilized, CaO-based sorbents under different carbonation–calcination conditions. ACS Sustain. Chem. Eng. (2015). https://doi.org/10.1021/acssuschemeng.5b00355
Krödel, M., Landuyt, A., Abdala, P.M., Müller, C.R.: Mechanistic understanding of CaO-based sorbents for high-temperature CO2 capture: advanced characterization and prospects. Chem. Sus. Chem. (2020). https://doi.org/10.1002/cssc.202002078
Lee, J., Han, S.J., Wee, J.H.: Synthesis of dry sorbents for carbon dioxide capture using coal fly ash and its performance. Appl. Energy. (2014). https://doi.org/10.1016/j.apenergy.2014.06.009
Sreenivasulu, B., Sreedhar, I., Reddy, B.M., Raghavan, K.V.: Stability and carbon capture enhancement by coal-fly-ash-doped sorbents at a high temperature. Energy Fuels (2017). https://doi.org/10.1021/acs.energyfuels.6b02721
Ge, J.C., Yoon, S.K., Choi, N.J.: Application of fly ash as an adsorbent for removal of air and water pollutants. Appl. Sci. (2018). https://doi.org/10.3390/app8071116
Reddy, E.P., Smirniotis, P.G.: High-temperature sorbents for CO2 made of alkali metals doped on CaO supports. J. Phys. Chem. B (2004). https://doi.org/10.1021/jp031245b
Huang, L., Zhang, Y., Gao, W., Harada, T., Qin, Q., Zheng, Q., Wang, Q.: Alkali carbonate molten salt coated calcium oxide with highly improved carbon dioxide capture capacity. Energy Technol. (2017). https://doi.org/10.1002/ente.201600628
Acknowledgements
This work was funded by Thailand Science Research and Innovation (TSRI), Thailand, through Program Management Unit for Competitiveness (PMU C), contract number C10F630266.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical statement
The authors assure that the results presented in this paper are of original work and have not been published elsewhere, nor have they been submitted simultaneously for consideration for publication elsewhere.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sukkathanyawat, H., Bampenrat, A., Jarunglumlert, T. et al. Modification of waste sugarcane bagasse fly ash for CO2 capture application. Mater Renew Sustain Energy 11, 267–276 (2022). https://doi.org/10.1007/s40243-022-00219-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-022-00219-y