Abstract
Tin oxide (SnO2) films were electrodeposited on graphite substrates using direct and pulse current electrodeposition techniques. The influence of applied current density on the morphological properties, crystal structure, and electrochemical behavior of the resulting films were studied by scanning electron microscope, X-ray diffraction spectroscopy, Mott–Schottky analysis, cyclic voltammetry, and electrochemical impedance spectroscopy techniques. The results showed that pulse electrodeposited films have porous flower-like morphology with smaller crystallite size and high donor density in comparison with direct current electrodeposited films that include equiaxed particles in their morphologies, such characteristics give them better electrochemical performance (higher degree of reversibility, higher specific capacitance, and faster lithium-ion diffusion) than those films that were synthesized by conventional direct current electrodeposition method. Furthermore, using higher applied current densities leads to the improvement of SnO2 films’ electrochemical performance due to the formation of the films with finer morphology that include more porosity and oxygen vacancies in their respective crystal structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, considerable efforts have been taken to produce lithium-ion batteries to fulfill the needs for electrical energy storages in different industries. The synthesis and characterization of a wide range of materials have been investigated to select promising candidates as cathode and anode materials in production of such batteries [1, 2]. In this respect, one important task is to use materials with larger specific capacity and longer cycle life than those of conventional carbon-based ones [3]. Transition-metal oxides have drawn a great deal of attention as successful anode material in Li-ion batteries due to their promising electrochemical characteristics [2]. Tin oxide (SnO2) has been regarded as one of the most suitable metal oxides for the production of anode electrodes in both aqueous and nonaqueous lithium-ion batteries owing to its large storage capacity (two times more than graphite) [2, 4].
Many deposition methods have been used to produce SnO2 electrodes [5,6,7,8,9], among which electrodeposition technique would be regarded as an economical and simple method for the production of such metal oxide material [10,11,12,13]. Up to now, direct current (DC) electrodeposition technique has been employed as the most popular electrochemical technique for synthesis of SnO2 films [10, 11, 13, 14] in which it has been shown that the electrochemical properties of electrodeposited SnO2 films can be modified by controlling electrodeposition parameters. For instance, the highest diffusion rate of Li+ was obtained for highly porous ones that were synthesized by higher applied potential in the course of potentiodynamic electrodeposition of SnO2 films [14]. Moreover, it has been reported that the change in applied potential, in potentiostatic electrodeposition of SnO2 films, changes their electrochemical properties, and reasonable electrochemical performance was attributed to the films with nanopyramid-liked morphology [10]. The influence of applied current density on the microstructure of SnO2 films has been also investigated elsewhere in which SnO2 films with more porous and smaller crystallite size were obtained at higher applied current densities during galvanostatic electrodeposition process [15].
As stated above, most of the relevant published research works deal with DC electrodeposition of SnO2 films. However, pulse electrodeposition technique would lead to formation of coatings with better characteristics than those that were synthesized by DC electrodeposition method [16,17,18]. For instance, compared to DC electrodeposition method, the application of pulse current electrodeposition (PC) technique can facilitate the deposition of homogeneous Bi2Te3 films with highly crystalline structure due to longer relaxation times during such deposition technique that can pave the way for the recrystallization process [16]. Moreover, pulse electrodeposition procedure has been utilized for synthesis of CoSn4 for application as anode in lithium-ion batteries; better electrochemical performance is reported for the CoSn4 films deposited at higher applied pulse current density having flower-liked morphology [18]. Since there is not sufficient research work regarding pulse electrodeposition of SnO2 films, so the purpose of this research is to compare the structural, morphological and electrochemical properties of SnO2 films that were deposited by DC and PC methods.
Experimental
SnO2 films were electrodeposited on graphite rods by DC and PC electrodeposition techniques from an aquatic bath containing tin chloride dihydrate (SnCl2⋅2H2O) and nitric acid (HNO3) with 25 mM and 75 mM concentrations, respectively, at 65 °C. Before conducting each electrodeposition experiment, oxygen gas was flushed through the bath for 60 min. Graphite rods (with diameter of 4 mm and height of 47 mm) were pretreated ultrasonically in C3H6O and DI water baths for 15 min, respectively, then immersed in 5% HNO3 solution for 20 min and finally rinsed in deionized water to be used in electrodeposition experiments. Galvanostatic DC and PC electrodeposition processes were performed at various average current densities (i.e., 10, 20, and 40 mA cm−2) at the fixed charge of 60 C. All PC electrodeposition experiments were carried out at duty cycle of 10% and a pulse frequency of 500 mHz.
Mott–Schottky, cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) tests were conducted to investigate the electrochemical performance of the resulting films. All the electrochemical experiments were performed using Ivium A32700 device in a three-electrode cell containing 0.12 M LiClO4⋅3H2O aquatic solution including Ag/AgCl, platinum rod, and modified graphite rods as a reference, counter, and working electrodes, respectively. The electrolyte solution was bubbled with Ar gas for 10 min before doing each electrochemical test. Mott–Schottky tests were performed in potential range of − 0.7 to − 0.6 V vs. Ag/AgCl at a constant frequency of 1 kHz and step potential of 10 mV. Cyclic voltammetry curves were recorded in potential range of − 2 to + 2 V vs. Ag/AgCl at scan rate of 0.1 V s−1. EIS responses were obtained at open circuit potential (OCP) in frequency range of 0.1 Hz to 1 MHz with applied AC voltage having an amplitude of 0.1 V.
X-ray diffraction (XRD) technique was performed with Cu Ka radiation in 2θ range from 20° to 80° (Brucker D8-Advance). Xpert HighScore software was used to analyze the resulting spectra and Williamson Hall method (with procedure details reported elsewhere [19]) was used to calculate the average crystallite size of the SnO2 films. Scanning electron microscopy (Tescan Vega3) was used to investigate the morphologies of the resulting films. The image analysis of SEM micrographs was carried out using ImageJ software to estimate the porosity values of the SnO2 films.
Results and discussion
Morphological and microstructural characteristics
Figure 1 indicates SEM images from the surface of the films that were deposited on graphite substrates at various applied current density values in DC and PC electrodeposition modes. As can be noticed in Fig. 1, application of DC current has resulted in formation of films whose morphologies include aggregates of equi-axed particles; however, this type of morphology changes to flower-liked one for the films synthesized by PC electrodeposition technique; it may be related to the recovery of depleted zones near the cathode surface (during current-off-time) from the electroactive species (OH− and Sn+ ions) which in turn enhances multi-directional growth of forming nuclei in pulse electrodeposition mode. Such observations have been also reported in a recent study, where flower-like Pt clusters were deposited on graphene substrates by pulse electrodeposition [20]. Moreover, using higher current densities, in both DC and PC modes, has resulted in the electrodeposition of more porous films with finer morphologies and pulse electrodeposited films have more porosity than those that electrodeposited by DC method (Fig. 2). Indeed, the higher current density is accompanied with higher cathodic overpotential during the electrodeposition process, which in turn leads to higher nucleation rates (limited growth of nuclei) and formation of more porous films with finer morphologies [19, 21, 22]. Such morphological characteristics would affect films; electrochemical performance as will be discussed below.
Cross-sectional SEM micrographs of the films that were deposited on graphite substrates at different applied current densities in DC and PC electrodeposition modes are presented in Fig. 3. It can be seen that as the applied current density increases thicker films are electrodeposited due to the accumulation of more OH− and Sn+ ions (building blocks of film formation) at the vicinity of the cathode surface under such electrodeposition conditions; this phenomenon has also been reported for the electrodeposition of other types of metal oxide films [19, 21, 23]. In addition, the films that were synthesized by PC electrodeposition technique are thicker than those that were electrodeposited by DC method; it demonstrates higher electrodeposition efficiency for the PC than DC mode in the case of electrodeposition of SnO2 films. This result would be attributed to the more adequate supply of reacting ions at the electrode surface during pulse electrodeposition (with respect to Dc method) that in turn improves the electrodeposition efficiency (formation of thicker films at the same amount of charge passing through the electrodeposition cell in both PC and DC methods) [24, 25].
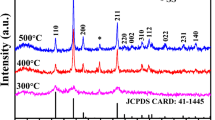
Figure 4 shows the XRD patterns of the films that were deposited at various applied current density values in DC and PC electrodeposition modes. As seen, the characteristic diffraction peaks indexed to the orthorhombic crystal structure of SnO2 [14, 26] are observed in the XRD patterns of all the films. The estimated average crystallite size for all samples (obtained from such XRD data) are provided in Fig. 5. As seen, those experimental conditions that lead to formation of films with finer morphologies have also resulted in electrodeposition of films with finer crystallite sizes, i.e., as the applied current density increases, the average crystallite size of the resulting films decreases; moreover, pulse electrodeposited films have also finer crystallite size than those that were electrodeposited by DC method. It would be related to the enhanced nucleation during electrosynthesis of films at such experimental conditions as discussed above. It has been reported that crystal defects can influence electrochemical performance of electroactive oxide films [27]. Such characteristics would be investigated by Mott–Schottky analysis that will be discussed below.
Electrochemical characteristics
Mott–Schottky (MS) plots attributed to SnO2 films that were synthesized by different electrodeposition conditions are illustrated in Fig. 6. As seen, all the MS responses show positive slopes which reveals n-type semiconducting behavior of the electrodeposited films; the donor density (ND) (that represents the oxygen vacancy concentration in the microstructure of SnO2 films [11, 28]) for the respective films could be obtained from the slope of MS curves \((\frac{1}{{C}_{\mathrm{sc}}^{2}} \mathrm{vs} V)\) according to equation (Eq. 1):
where Csc is the space charge layer capacitance (F cm−2), \(\varepsilon\) is dielectric constant of SnO2 (which may take the value of 10 as has been reported previously [29]), \({\varepsilon }_{0}\) is the permittivity of free space (8.854 × 10–12 F m−1), e is the electron charge (1.6022 × 10–19 C), V is the applied potential (V vs Ag/AgCl), \({V}_{\mathrm{FB}}\) is the flat band potential (V vs Ag/AgCl), kB is Boltzmann constant (1.3806 × 10–23 m2 kg s−2/K−1) and T is temperature (K) [11, 21]. The estimated donor densities attributed to the synthesized films are presented in Table 1. As can be seen, the films that were deposited by PC method have higher amount of crystal defects (NDs) than those that were electrodeposited with DC method. Moreover, more defective films will be electrodeposited (ND increases) as the applied current density increases in both DC and PC electrodeposition methods. In fact, such experimental conditions (application of high applied current density) favors hydrogen reduction at the cathode surface which in turn would cause formation of more defective films by enhancing oxygen vacancy creation during oxide films formation [11]
Figure 7 represents CV responses of the films that were deposited at various applied current density values in DC and PC electrodeposition modes. As seen, two pairs of redox peaks are appeared in all the CV curves in a way that the first redox peaks (I and II) and second redox peaks (III and IV) demonstrate the reduction–oxidation of SnO2 and alloying-dealloying of SnO2 with Li, respectively [30]. The successive reduction peaks (labeled as III) in CV responses of the samples that were electrodeposited by DC current are attributed to formation of stoichiometric Li-Sn alloys with different chemical compositions, respectively, as has been reported elsewhere [31, 32]. Such distinctive reduction peaks are not presents in CV responses of pulse electrodeposited samples which would be related to much more disordered crystal structure of latter films (in comparison with DC electrodeposited ones) preventing the formation of Li–Sn alloys with distinctive chemical compositions. Similar observations have been also reported in the case of CV responses of SnO2 films that were electrodeposited at other experimental conditions [14]. The difference between redox potentials (ΔV which resembles degree of reversibility for redox reactions) and specific charge capacitance (Cs which resembles electrochemical performance of electroactive films) would be estimated based on Eqs. 2 and 3, respectively:
where S is the scan rate (V s−1), m is related to the mass of the electroactive material (g), \(\int {I\left( V \right){\text{d}}V}\) is the enclosed surface of cyclic voltammetry curve, V1 and V2 are the lower and upper limit of the potentials, respectively [12]. The estimated values of ΔV and Cs are presented in Table 1. It can be seen that SnO2 films that were synthesized by PC mode exhibit smaller ΔV value (more degree of reversibility for redox reactions) and higher Cs values in comparison with those that were electrodeposited by DC method. Similar trends (decrease in ΔV and increase in Cs values) are observed by increasing applied current density in both DC and PC electrodeposition modes. In other words, application of PC electrodeposition method and using higher applied current density result in the deposition of films with better electrochemical performance. These results could be attributed to the formation of the SnO2 films with more oxygen vacancies and porosity at such experimental conditions; the first character makes diffusion process of Li ions into the structure of the films easy [27, 33] and the second one decreases the probability of mechanical collapse of electroactive films as a result of volumetric changes in alloying–dealloying reactions [1, 2].
Figure 8 shows Nyquist plots attributed to SnO2 films that were deposited at various applied current density values in DC and PC electrodeposition modes. As can be seen, all the plots include a semicircle (related to the charge transfer at the interface of electrolyte and electrode) followed by Warburg type impedance response (that indicates the diffusion of Li ions into the electroactive anode material) at high and low frequency regions, respectively. The equivalent circuit model that was employed to fit such Nyquist plots is presented in Fig. 9 in which Rs is the solution resistance, Rct the charge transfer resistance, CPE is the constant phase element related to double layer at the electrode surface and W0 is the Warburg impedance that can be used to estimate diffusion coefficient of Li ions (D) into the electroactive films’ structure as discussed elsewhere [34]. In fact, fitted values to W0 parameter are used to estimate diffusion coefficient of Li ions (D) within the SnO2 films in accordance with Eq. 4:
where l is diffusion length which is assumed to be equal to film’s thickness [35]. The results of such calculations are provided in Table 1. As seen, in the case of both PC and DC methods, the SnO2 films that were electrodeposited using higher applied current densities exhibit better electrochemical performance (having less Rct and more D values) than the other ones. Moreover, PC method has resulted in the deposition of films with better electroactivity than those that were electrodeposited by DC method (the former has less Rct and more D values than the latter). Last but not least, although SnO2 films that were electrodeposited by DC mode are thinner than those films that were prepared by PC mode, Li ions have lower diffusion coefficient values in them than the latter ones. This result would be attributed to the higher Warburg Impedance (W0) values fitted to the AC responses of the films that were electrodeposited with DC mode that results to estimate smaller diffusion coefficient values for Li ions within them. Such results are in consistence with CV responses as discussed above.
Equivalent circuit model used for the analysis of EIS results provided in Fig. 8
Conclusion
The crystal structure, morphological and electrochemical properties of SnO2 films deposited at various applied current density values in DC and PC electrodeposition modes were investigated. The results showed that as the applied direct current density increases from 10 to 40 mA cm−2, the percentage of porosity and thickness of the SnO2 films increases from 30 to 41% and 4.5 to 7.5 μm, respectively. Similar trend is observed in the case of the same variation in average current density (from 10 to 40 mA cm−2) during pulse electrodeposition of the films, however, such films were more porous (45–57%) and thicker (8.1–17.5 μm) than those that were electrodeposited under direct current conditions. Application of pulse electrodeposition technique led to the formation of the SnO2 films in which the lithium-ions can diffuse easier than in the SnO2 films that electrodeposited direct current method; it would be related to porous flower-liked morphology of the former films that include more oxygen vacancies in their structure than the latter ones. Last but not least, the electrochemical performance of electrodeposited SnO2 films improved (high specific charge capacitance, electrochemical reversibility, and Li-ion diffusion coefficient value) using higher applied current densities and application of pulse current technique because of the formation of more porous SnO2 films with finer morphology, smaller crystallite size that includes more oxygen vacancies.
Data availability
The data generated and/or analysed during the present study are available from the corresponding author on reasonable request.
References
Yuan, L., Guo, Z.P., Konstantinov, K., Liu, H.K., Dou, S.X.: Nano-structured spherical porous SnO2 anodes for lithium-ion batteries. J. Power Sources 159, 345–348 (2006)
Yin, X., Chen, L., Li, C., Hao, Q., Liu, S., Li, Q., Zhang, E., Wang, T.: Synthesis of mesoporous SnO2 spheres via self-assembly and superior lithium storage properties. Electrochim. Acta 56, 2358–2363 (2011)
Tirado, J.L.: Inorganic materials for the negative electrode of lithium-ion batteries: State-of-the-art and future prospects. Mater. Sci. Eng. R Rep. 40, 103–136 (2003)
Vasanth Raj, D., Ponpandian, N., Mangalaraj, D., Viswanathan, C.: Electrochemical behavior of nanostructured SnO2 thin films in aqueous electrolyte solutions. Mater. Sci. Semicond. Process. 26, 55–61 (2014)
Wang, Z., Zhou, L., Lou, X.W.: Metal oxide hollow nanostructures for lithium-ion batteries. Adv. Mater. 24, 1903–1911 (2012)
Okude, M., Ueno, K., Itoh, S., Kikuchi, M., Ohtomo, A., Kawasaki, M.: Effect of in situ annealed SnO2 buffer layer on structural and electrical properties of (0 0 1) SnO2/TiO2 heterostructures. J. Phys. D. Appl. Phys. 41, 125309 (2008)
Pinheiro, M.A.L., Pineiz, T.F., de Morais, E.A., Scalvi, L.V.A., Saeki, M.J., Cavalheiro, A.A.: Schottky emission in nanoscopically crystallized Ce-doped SnO2 thin films deposited by sol-gel-dip-coating. Thin Solid Films 517, 976–981 (2008)
Bagheri-Mohagheghi, M.M., Shokooh-Saremi, M.: The influence of Al doping on the electrical, optical and structural properties of SnO2 transparent conducting films deposited by the spray pyrolysis technique. J. Phys. D. Appl. Phys. 37, 1248–1253 (2004)
Li, Y., Qiao, L., Wang, L., Zeng, Y., Fu, W., Yang, H.: Synthesis of self-assembled 3D hollow microspheres of SnO2 with an enhanced gas sensing performance. Appl. Surf. Sci. 285, 130–135 (2013)
Chen, X., Liang, J., Zhou, Z., Duan, H., Li, B., Yang, Q.: The preparation of SnO2 film by electrodeposition. Mater. Res. Bull. 45, 2006–2011 (2010)
Daideche, K., Azizi, A.: Electrodeposition of tin oxide thin film from nitric acid solution: the role of pH. J. Mater. Sci. Mater. Electron. 28, 8051–8060 (2017)
Raj, D.V., Ponpandian, N., Viswanathan, C.: Influence of supporting electrolytes on the structure of electrodeposited SnO2 thin films for energy storage applications. Ionics 22, 1837–1846 (2016)
Chang, S.T., Leo, I.C., Liao, C.L., Yen, J.H., Hon, M.H.: Electrochemical behavior of nanocrystalline tin oxide electrodeposited on a Cu substrate for Li-ion batteries. J. Mater. Chem. 14, 1821–1826 (2004)
Raj, D.V., Ponpandian, N., Mangalaraj, D., Viswanathan, C.: Electrodeposition of macroporous SnO2 thin films and its electrochemical applications. Mater. Focus. 4, 245–251 (2015)
Chang, S.T., Leu, I.C., Hon, M.H.: Preparation and characterization of nanostructured tin oxide films by electrochemical deposition. Electrochem. Solid-State Lett. 5, 71–74 (2002)
Lee, J., Farhangfar, S., Lee, J., Cagnon, L., Scholz, R., Gösele, U., Nielsch, K.: Tuning the crystallinity of thermoelectric Bi2Te3 nanowire arrays grown by pulsed electrodeposition. Nanotechnology 19, 365701 (2008)
Gu, Y.E., Su, X., Du, Y., Wang, C.: Preparation of flower-like Cu2O nanoparticles by pulse electrodeposition and their electrocatalytic application. Appl. Surf. Sci. 256, 5862–5866 (2010)
Javadian, S., Kakemam, J., Gharibi, H., Kashani, H.: Flower-like architecture of CoSn4 nano structure as anode in lithium ion batteries. Int. J. Hydrogen Energy. 42, 13136–13149 (2017)
Hessam, R., Najafisayar, P.: The effects of applied current density and bath concentration on the morphology, crystal structure and optical properties of electrodeposited hematite thin films. Thin Solid Films 692, 137633 (2019)
Hsieh, C., Wei, J., Hsiao, H., Chen, W.: Fabrication of flower-like platinum clusters onto graphene sheets by pulse electrochemical deposition. Electrochim. Acta. 64, 177–182 (2012)
Hessam, R., Najafisayar, P.: The effects of applied current density, bath concentration and temperature on the morphology, crystal structure and photoelectrochemical properties of electrodeposited hematite films. Int. J. Hydrogen Energy. 44, 22851–22862 (2019)
Sharma, A., Bhattacharya, S., Das, S., Das, K.: A study on the effect of pulse electrodeposition parameters on the morphology of pure tin coatings. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 45, 4610–4622 (2014)
Wu, M.-S., Lee, R.-H.: Electrochemical growth of iron oxide thin films with nanorods and nanosheets for capacitors. J. Electrochem. Soc. 156, A737 (2009)
Sharma, A., Das, S., Das, K.: Pulse electroplating of ultrafine grained tin coating. In: Electroplating Nanostructures (2015)
Chandrasekar, M.S., Pushpavanam, M.: Pulse and pulse reverse plating—conceptual, advantages and applications. Electrochim. Acta. 53, 3313–3322 (2008)
Jang, D.M., Jung, H., Hoa, N.D., Kim, D., Hong, S.K., Kim, H.: Tin oxide-carbon nanotube composite for NOX sensing. J. Nanosci. Nanotechnol. 12, 1425–1428 (2012)
Ma, D., Li, Y., Zhang, P., Lin, Z.: Oxygen vacancy engineering in tin(IV) oxide based anode materials toward advanced sodium-ion batteries. Chemsuschem 1, 3693–3703 (2018)
Diaz, R., Díez-Pérez, I., Gorostiza, P., Sanz, F., Joiret, S., Allongue, P.: Electrochemistry of tin in borate buffer solutions: an in-situ Raman study. Mater. Res. Soc. Symp. Proc. 781, 12–17 (2003)
Gurgul, M., Gawlak, K., Syrek, K., Kozieł, M., Sulka, L.Z.G.-D., Zaraska, L.: The influence of water-induced crystallization on the photoelectrochemical properties of porous anodic tin oxide film. J. Ind. Eng. Chem. 90, 159–165 (2020)
Zhao, S., Sewell, C.D., Liu, R., Jia, S., Wang, Z., He, Y., Yuan, K., Jin, H., Wang, S., Liu, X., Lin, Z.: SnO2 as advanced anode of alkali-ion batteries: inhibiting Sn coarsening by crafting robust physical barriers, void boundaries, and heterophase interfaces for superior electrochemical reaction reversibility. Adv. Energy Mater. 10, 1902657 (2020)
Zoller, F., Böhm, D., Bein, T., Fattakhova-Rohlfing, D.: Tin oxide based nanomaterials and their application as anodes in lithium-ion batteries and beyond. Chemsuschem 12, 4140–4159 (2019)
Ferraresi, G., Villevieille, C., Czekaj, I., Horisberger, M., Novák, P., El Kazzi, M.: SnO2 model electrode cycled in li-ion battery reveals the formation of Li2SnO3 and Li8SnO6 phases through conversion reactions. ACS Appl. Mater. Interfaces 10, 8712–8720 (2018)
Ye, L., Zhou, Y., Zhao, Y., Feng, L., Wen, Z., Zhao, L., Jiang, Q.: Engineering oxygen vacancy on iron oxides/hollow carbon cloth electrode toward stable lithium-ion batteries. Chem. Eng. J. 388, 124229 (2020)
Ng, V.M.H., Wu, S., Liu, P., Zhu, B., Yu, L., Wang, C., Huang, H., Xu, Z.J., Yao, Z., Zhou, J., Que, W., Kong, L.B.: Hierarchical SnO2-graphite nanocomposite anode for lithium-ion batteries through high energy mechanical activation. Electrochim. Acta. 248, 440–448 (2017)
Najafisayar, P., Bahrololoom, M.E.: Pulse electrodeposition of Prussian Blue thin films. Thin Solid Films 542, 45–51 (2013)
Acknowledgements
Authors would like to appreciate Shiraz University for providing Graduate Studies Grant 99GCB2M192010.
Funding
The research leading to these results received funding from Shiraz University under Grant Agreement No. 99GCB2M192010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hessam, R., Najafisayar, P. & Rasouli, S.S. A comparison between growth of direct and pulse current electrodeposited crystalline SnO2 films; electrochemical properties for application in lithium-ion batteries. Mater Renew Sustain Energy 11, 259–266 (2022). https://doi.org/10.1007/s40243-022-00218-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40243-022-00218-z