Abstract
The oxygen adsorption and subsequent reduction on the {100} and {110} surfaces of 25% Ba-doped LaMnO3 (LBM25) have been studied at the density functional theory (DFT) with Hubbard correction and the results compared with adsorption on 25% Ca-doped LaMnO3 (LCM25) and Sr-doped LaMnO3 (LSM25). The trend in the reduction energies at the Mn cation sites are predicted to be in the order LSM25 < LBM25 < LCM25. In addition, the trend in dissociation energies for the most exothermic dissociated precursors follow the order LBM25 < LSM25 < LCM25. The adsorption energies (− 2.14 to − 2.41 eV) calculated for the molecular O2 precursors at the Mn cation sites of LCM25, LSM25 and LBM25 are thermodynamically stable, when compared directly with the adsorption energies (Eads = − 0.56 to − 1.67 eV) reported for the stable molecular O2 precursors on the Pt, Ni, Pd, Cu and Ir {111} surfaces. The predicted Gibbs energies as a function of temperature (T = 500–1100 °C) and pressures (p = 0.2 atm) for the adsorption and dissociation on the surfaces were negative, an indication of the feasibility of oxygen reduction reaction on the {100} and {110} surfaces at typical operating temperatures reported in this work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world’s demand for energy, coupled with the finite supply of fossil fuels and the environmental and political drawbacks associated with them, has shifted the focus of energy research to more efficient and sustainable conversion and storage technologies [1].
Solid Oxide Fuel Cells (SOFCs) are electrochemical devices for converting chemical energy into electrical energy and additional heat. Intermediate conversion of heat to mechanical work required by conventional combustion techniques is largely avoided. SOFCs with electrolyte that is ionic conducting presents unique opportunities over other fuel cells, such as cheap constituents, decreased susceptibility to fuel impurities and effectiveness. SOFCs embodies the cleanest, capable and flexible chemical to electrical energy transfer system [2, 3] providing alternatives to broader consumption of hydrogen and carbon-based fuels and regenerating fuel sources [4, 5]. However, for SOFCs to be explored on a bigger commercial scale, they must be less expensive and the electrode fabricated from readily available materials. One approach to cost reduction is the drastic reduction in the operation temperatures, which suppresses cell degradation, thermal stress buildup and improvement in cell lifetimes [6]. Due to their high catalytic reactivity toward the oxygen reduction reaction, noble metal electrocatalyst [7], such as platinum (Pt), is employed as a cathode. However, the cost of platinum represents a fundamental problem for its application.
Perovskite-type oxides such as LaMnO3 have attracted attention for applications built upon their unique electronic and magnetic features. For example, La1-xSrxMnO3-δ is the cathode of choice when the electrolyte material is zirconia in SOFCs [7]. This is attributed to the good electrical conductivity, stability, low cost and efficient catalytic activity for the oxygen reduction reaction [8]. In SOFCs cathode, at dopant concentration (x = 0.2 − 0.3), La1−xSrxMnO3−δ is reported to be employed as mixed ionic–electronic conducting (MIEC) [9].
For O2 adsorption and subsequent reduction on pure and 25% Ca-doped LaMnO3 (LCM25) {100} and {110} surfaces, Aniagyei et al., [10] reported that adsorption or reduction processes are more favorable at the Mn sites than La and Ca sites at the DFT level with Hubbard correction. The adsorption energies calculated for the {110} surfaces were more favorable and stable than the {100}.
Chen et al., [11] studied the kinetic behavior of the oxygen reduction reaction and diffusion pathways on 25% Sr-doped LaMnO3 (LSM25) cathode surface. From the spin-polarized DFT and molecular dynamics (MD) calculations, O2 adsorption energies were more stable at the Mn sites compared to the Sr sites.
La1−xBaxMnO3 is a colossal magnetoresistance (CMR) classical compound [12] with a Curie temperature of 340 K [13]. Dependent on the dopant concentration, the crystal structure moves from orthorhombic through rhombohedral (x > 0.13) to cubic (x > 0.35). For the magnetic spin alignment, it shows ferromagnetic behavior at Ba concentrations of x ˃ 0.15. They are reported to undergo metal-to-insulator transition at x ≈ 0.20 [13, 14]. La1-xBaxMnO3 has received extensive investigation of its crystallographic and magnetic properties [14, 15], phase transitions [13, 16, 17], and spin dynamics [18]. For the reduction activity on Ba-doped LaMnO3 as cathode materials toward ORR, to the best of our information, no theoretical studies have been shown, which makes this theoretical study timely.
In this work, an extension to previous studies [10], Hubbard-corrected DFT approach is applied to study the Gibbs free energies of adsorption of the multiple reaction paths associated with the reduction of oxygen at 25% Ba-doped LaMnO3 {100} and {110} surfaces (LBM25). The oxygen reduction energetics on LBM25 surfaces are compared with LCM25 [10] and LSM25 [see Table S1 and Figure S1 and S2], the main cathode of preference for conventional SOFCs. This is to ascertain whether they could be used as an alternative electrocatalyst for oxygen reduction reaction (ORR) based on their adsorption/reduction energies. The adsorption studies were restricted to the {100} and {110} surfaces of LaMnO3 because they present the most stable surfaces [19, 20].
Computational details
The calculations were carried out within the Kohn–Sham DFT formalism [21], using a plane-wave basis set as implemented in the Quantum-ESPRESSO [22]. The Perdew–Burke–Ernzerhof (PBE) generalized gradient approximation (GGA) was employed for the exchange and correlation terms [23]. The plane-wave basis set cutoffs for the smooth part of the wave function and the augmented density were set to 40 and 420 Ry, respectively, which ensured convergence of the forces to within 0.01 eV/Å. The Brillouin zone for the bulk LaMnO3 was sampled using a 4 × 4 × 4 Monkhorst–Pack k-points mesh [24]. To correct the large self-interaction error inherent in standard DFT-GGA methods for mid-to-late first-row transition metal oxides [25], the DFT + U approach [26] with a Ueff value of 4.0 eV for Mn ions (mainly Mn3+ ions) provides the best results when modeling the LaMnO3 ground-state properties [27]. We have investigated the symmetric Pm3m cubic structure, because it is stable under SOFC operating conditions (above 500 °C in ambient air) [28, 29]. All calculations were spin-polarized to describe accurately the magnetic properties of the LBM25, LSM25 and the triplet ground state of oxygen.
The different possible magnetic orderings at the Mn3+ sites in the LBM25 and LSM25 surface structures were considered and found that the ferromagnetic (FM) ordering is 0.3 and 0.2 eV, respectively, more stable than the antiferromagnetic (AFM) ordering. Hence, all the structures investigated in this study have FM spin ordering.
The {100} and {110} surface structures were created from the fully optimized bulk structure using the METADISE code [30], which generates different atomic layer stackings to result in a zero-dipole moment perpendicular to the surface plane, as is required for reliable and realistic surface calculations [31]. The fully relaxed bulk structures were used to create the surfaces to eliminate the presence of fictitious forces during the surface relaxation. The surfaces were modeled using a slab model comprising of eight atomic layers with a vacuum size of 12 Å introduced in the z-direction, which is large enough to avoid any spurious interaction between the periodic slab images.
Similar to previous studies [10], all the surface calculations for the interactions between the molecular oxygen species on the LSM25 and LBM25 surfaces were performed by relaxing the top three layers while keeping the bottom five layers fixed at the bulk parameters. The adsorption energy was calculated according to the following relation:
where \({E}_{{\text{surface}}+{O}_{2}}\) is the total energy of the substrate–adsorbate system in the equilibrium state, and \({E}_{\text{surface}}\) and \({E}_{{O}_{2}}\) are the total energies of the substrate (clean surface) and adsorbate (free O2 molecule in the spin triplet state), respectively.
Results and discussion
Description of Ba-doped LaMnO 3 surfaces
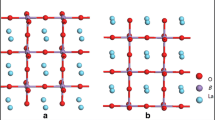
For reduction reaction on SOFC cathodes, the role played by oxygen vacancies present on the surface is critical. The vacant sites are expected to compete against adsorption and dissociation in the mobility of oxygen. To examine the role of the vacant site on the oxygen reduction reactions at the cathode, LBM25 surfaces with different layer models are shown in Fig. 1. Summarized in Table 1 are the calculated oxygen vacancy formation energies obtained by substitution of the host La3+ cation for Ba2+ in the {100} and {110} LaMnO3 surface models with different layers. The calculated oxygen vacancy formation energies located on the top layer were based on the reaction of
In previous studies of LaMnO3 with MnO2-terminated {100} surface at the GGA + U [32] and hybrid B3LYP [33] levels of theory, a calculated oxygen vacancy formation energy of 2.2 eV was reported. Surfaces model A of the MnO2 terminated {100} and model B of the LaMnO-terminated {110} with the La ion in/near the topmost layers (Fig. 1) were found to have the lowest oxygen vacancy formation energies; thus, we chose these two models for subsequent studies of O2–LBM25 interactions.
In this work, molecular or dissociative chemisorption of O2 on (1 × 1) and (2 × 1) surface models at various coverages of Θ = 0.25 ML and 0.50 ML, where a monolayer (ML) refers to an oxygen molecule per active surface cation. Shown in Fig. 2 are the top and side views of the LBM25 {100} and {110} surface models showing the different adsorption sites explored for O2 adsorption.
O 2 adsorption on the oxygen-deficient LBM25 {110} surface
For O2 adsorption on the {110} surface with coverages of Θ = 0.25 ML and 0.50 ML, molecular and dissociative scenarios for the selected (1 × 1) and (2 × 1) surface models were considered. Substitution of host La3+ with Ba2+ cation creates a defective surface with a vacant oxygen site. Similar to the undoped surface [10], different adsorption configuration and modes were exploited at the {110} and {100} surfaces, that may serve as precursors for O2 dissociation. Figure 3 shows the optimized adsorption structures, whiles the adsorption energies, Löwdin atomic charges, interatomic bond distances and vibrational stretching frequencies are presented in Table 2.
For O2 adsorbed on Mn cation site, the end-on structure is changed to a side-on structure after optimization (Fig. 3, A1), with an exothermicity of − 2.25 eV. The formed molecular precursor A1 is accompanied by significant charge transfer (− 0.34 e− from the substrate) to the O2 orbital, weakening and elongating the d(O–O) to 1.45 Å compared to gas phase and experimental d(O–O) of 1.23 and 1.21 Å [34], respectively. For A1, the shortest interatomic distance d (O–Mn) is 1.838 Å. On the LSM25 surface, O2 adsorbed end-on and side-on at the Mn sites, and exothermicities of 1.59 and 2.41 eV were calculated and the d(O–O) = 1.357 and 1.449 Å, respectively (Table S1 and Figure S1). In both molecular processes, the d(O–O) are elongated by 1.315 and 1.480 Å, respectively. The adsorption energies of − 2.14, − 2.41 and − 2.25 eV calculated at the Mn cation sites of LCM25, LSM25 and LBM25 are thermodynamically stable, when compared directly with the adsorption energies (Eads = − 0.56 to − 1.67 eV) reported for molecular O2 precursors on the Pt, Ni, Pd, Cu and Ir {111} surfaces [35, 36]. This indicates that LCM25, LSM25 and LBM25 cathode materials may be more efficient for O2 activation than the transition metal surfaces. In addition, since molecular precursors at the Mn cation sites of LCM25, LSM25 and LBM25 are associated with stronger bonds compared to LaMnO3 [10], this implies Ca, Sr and Ba as dopants in the cathode influences the O2 reduction. The trend in the reduction energies on the Mn cation sites are predicted to be in the order LSM25 < LBM25 < LCM25.
For O2 adsorbed on top-La cation, no stable end-on configuration is obtained because it is changed to a side-on structure after optimization (A2, Fig. 3) with an exothermicity of 1.31 eV. The bound O2 molecule experiences a net charge of − 0.29 e− and d(O–O) elongation of 1.374 Å. The formation of molecular precursor A1 is energetically more favorable than A2 in terms of adsorption energies and stronger bond formation, i.e., shorter d (O–Mn) = 1.838 Å than d (O–La) = 2.324 Å showing that Mn are more active than La sites toward oxygen reduction reaction and consistent with experimental observations [37]. In this work, for the O2 molecule adsorbed end-on and side-on at the top-Mn and Sr cation sites on SrMnO-terminated {110} surface, the B (Mn) cation sites were calculated to be more active than A (Sr) cation sites toward oxygen reduction reaction (Table S1). This demonstrates that Mn cation are the favored on both oxygen-deficient LCM25 [10], LSM25 and LBM25 surfaces for adsorption, in agreement with other reported studies on perovskite structures [10, 38, 39].
Analogous adsorption trends are observed for O2 adsorbed side-on at the bridged La–Mn site (A3, Fig. 3). The adsorption energy of − 3.78 eV is released in that mode, with d (O–Mn) and d (O–La) at 1.883 and 2.377 Å, respectively. The d(O–O) is significantly elongated (1.50 Å). Compared to the LBM25, O2 adsorbed side-on at the bridge Sr–Mn site releases an energy of − 3.31 eV on the LSM25 {110} surface, whiles a net charge of − 0.51e− is transferred to the O2 orbital in the bond formation process.
When O2 adsorbate is directly incorporated into the surface oxygen vacancy (A4), an energy of 2.96 eV is released. The d(O–O) in the adsorbate are weakened significantly (1.51 Å). Löwdin population analysis shows a significant charge transfer of − 0.53 e− to the adsorbate upon incorporation at the surface oxygen vacant site in the LBM25 substrate. Löwdin population analysis shows a significant charge transfer of − 0.64 e− to the adsorbate upon incorporation at the surface oxygen vacant site in the LSM25 substrate (see Table S1) compared with a transfer of − 0.53 e− to the adsorbate on LBM25 (Table 2) and 0.76 e− on LCM25 [10] surface. The trend in charge transfer from the surfaces to the adsorbate follows in the order LCM25 < LSM25 < LBM25 while the reduction energetics at the surface vacant sites follows the order LBM25 < LCM25 < LSM25.
In all the molecular O2 precursors (A1–A4), the elongated O–O bonds were confirmed to have lower stretching vibrational frequencies: 641, 878, 514, and 490 cm−1, respectively, compared to the O2 gas-phase stretching frequencies (1558 cm−1). Similar to previous studies 10 the adsorbed molecular oxygen species (A1–A3 in Table 2) were classified based on the d(O–O) and υ(O–O) either as superoxo and peroxo-like species, which were comparable to O2− (1.33 Å) and O22− (1.44 Å) ions [40, 41]. Calculated vibrational frequencies for the superoxide and peroxide are reliable to O2–CeO2 experimental values [42].
For the dissociated oxide ions O2− on the defective surfaces, three possible pathways were exploited with O2− ions adsorbing at the oxygen vacant sites and (i) Top-Mn cation (D1), (ii) Top-La cation (D2), and (iii) Bridged Mn and La sites (D3). We found these configurations shown as D1, D2 and D3 in Fig. 3 are much more active. The dissociation energies are − 5.99, − 4.80, and − 7.83 eV, respectively, for D1, D2 and D3 modes. These energies are exothermic than those calculated for their molecular adsorbed counterparts (A1−A4 in Fig. 3). Dissociated configuration D3 has the highest exothermicity. On the SrMnO-terminated {110} surfaces, the dissociative configurations (D1, D2 and D3 in Figure S1) have been found to be more stable and have dissociation energies of − 5.98, − 3.18 and − 7.03 eV. The trend in dissociation energies for the most exothermic dissociated precursors follow the order LBM25 < LSM25 < LCM25 [10]. Hence, defective surfaces of LCM25, LSM25 and LBM25 favors dissociative over associative adsorption. Oxygen dissociation on LBM25 is the most plausible in terms of exothermicity.
Similar to adsorption reactions involving the (1 × 1) surfaces, analogous adsorption trends on the (2 × 1) supercell were investigated (Fig. 4). Reported in brackets in Table 2 are the calculated adsorption energies and the optimized interatomic bond distances. For instance, adsorption energies of − 1.50, − 4.40, and − 3.34 eV on the (2 × 1) surface were calculated for O2 bound side-on at La cation (A2), bridged-LaMn (A3) and end-on at the oxygen vacant sites (A4), compared to − 1.31, − 3.78 and − 2.96 eV, respectively, reported on the (1 × 1) surface. The dissociation energies (D1 and D2) calculated were highly exothermic compared to their (1 × 1) counterparts. The more exothermic adsorption and dissociation energies calculated on the (2 × 1) compared to the (1 × 1) cells could be due to the lower O2 coverage found on the (2 × 1) cell that reduces repulsive lateral interactions between periodic images. This provides larger surface area for the diffusion of the dissociated O2− ions to locate more stable sites.
To provide atomic-level insight into the effect of Ba doping on the electronic structures of LaMnO3 surfaces and their implication for catalytic reactivity, we have plotted the projected density of states (PDOS) for the LBM25 {110} surface as shown in Fig. 5. From the projected density of states (PDOS) for the undoped defective and LCM25 {110} surface [10], La ions contribute negligible states at the Fermi level compared to the Mn ions. Since the density of states around the Fermi energy level roughly determines the availability of electrons for a given reaction [43], it can be inferred that the catalytic activity of the LaMnO3 {110} surface should be primarily linked to the surface Mn-d states. This helps to explain why the Mn sites are more active than the La sites for O2 adsorption. It was also reported that Ca doping resulted in a decrease in the Mn-d states around the Fermi level relative to the undoped surface. As the Mn-d states dictates the reactivity of the LaMnO3 {110} surface, a decrease in their intensity signifies weaker O2 binding. This helps to explain why the Ca-doped surfaces have weaker O2-binding energies than the undoped surfaces. As shown in Fig. 5, Ba doping also causes a decrease in the intensity of the Mn-d states, resulting in weaker O2-binding energies (− 2.25 eV) than the undoped defective surface (− 2.32 eV).
O 2 adsorption on defective LBM25 {100} surface
Similar to adsorption reactions involving the {110} surfaces, analogous adsorption trends on the {100} surfaces were investigated. For O2 adsorbed on top-Mn cation, no stable end-on configuration is observed because it is changed to a side-on structure after optimization. In that configuration, an adsorption energy of 0.82 eV is released. The formed molecular precursor A1 is accompanied by a slight lengthening of the d (O–O) to 1.28 Å. The d (O–Mn) is shorter at 1.850 Å for A1.
For O2 directly incorporated into the surface oxygen vacancy (A2, Fig. 6), the adsorption energy of 1.97 eV is released. The incorporation process is accompanied by a net charge gain of − 0.36e− by the adsorbate from the oxygen-deficient substrate, which weakens the calculated d(O–O) to 1.49 Å in relation to the gas phase.
On the {100} surface, configuration D1 has the highest adsorption energy, shortest interatomic distance d (Mn–O) = 1.577 Å and the d (O–O) = 2.835 Å, making this configuration the most stable.
On the (2 × 1) supercell, the direct incorporation into the surface oxygen vacancy releases an energy of 2.88 eV (Fig. 4, A1). However, when adsorbed at the top-Mn site, either in the end-on or side-on configuration, it is dissociated into oxide ions after geometry optimization. One atomic oxygen is adsorbed on top-Mn cation whiles the other is incorporated into surface vacant site to give D1. The process releases the energy of 4.12 eV. The calculations indicates that adsorption and dissociation processes of O2 on both pure and oxygen-deficient LCM25, LSM25 and LBM25 at the {100} surface are less competitive than that at the {110} surface because of a weaker adsorption. This suggests that the {110} surface is catalytically more active for O2 reduction.
Gibbs free energies \(\Delta {\varvec{G}}^{{{\varvec{ads}}}} \left( {{\varvec{T}},{\varvec{p}}} \right)\) of the O 2 adsorption/dissociation
To assess the relevance of the calculated adsorption and dissociation energies of the most stable molecular and dissociated configurations at the typical operating temperatures of SOFCs (T = 500–1100 °C) in SOFCs, we have calculated the Gibbs free energies, \(\Delta G^{ads} \left( {T,p} \right)\) using the Gibbs free energy relation
where N represents the number of molecules adsorbed in the reaction. If the enthalpy or the entropy of the solid is not changed considerably by the presence of the adsorbates, these terms cancel out. The vibrational entropy of the adsorbates, \({S}_{\mathrm{vib}}^{\mathrm{ads}}\), and the coverage-dependent configurational entropy, \({\Delta }s_{conf}^{\theta }\), only contribute to entropy of the surface/adsorbate. The vibrational entropy of the adsorbates is given by
where \(\beta = \frac{1}{{k_{B} T}}\) and \(\varepsilon = \mathop \sum \nolimits_{i} h\nu_{i}\) is the total vibrational energy of the adsorbent obtained from normal-mode analysis DFT calculations [45], and the coverage-dependent configurational entropy may be included as
where \(\theta\) is the surface coverage. The expression for the Gibbs energy of adsorption then becomes
with the adsorption energy defined as \(\Delta E^{ads} = E^{(surf + O2)} - E^{surf} - NE^{O2}\), p = 0.2 and po = 1 atm. To account for errors in the binding energies of O2, 1.36 eV/O2 obtained from the fitting of experimental formation enthalpy and calculated oxide formation energies [44] is added to the calculated Gibbs free energies for the most stable molecular and dissociated configurations. The zero-point vibrational energy (ZPE) is calculated as the difference between the ZPE correction of the adsorbate on the surface and in the gas phase according to the following equation:
where \(h\) is the Planck constant and \(vi\) are the vibrational frequencies.
Figure 7 shows the predicted Gibbs free energies against temperature plotted for the most stable molecular and dissociative structures of O2 on the {110} and {100} surfaces of LBM25. It is evident from the plot that ΔGads (T, p) is always negative, an indication that the oxygen reduction reaction is feasible at the typical operating temperatures. In addition, it is worth stating that ΔGads (T, p) values become more negative with increasing temperature, signifying that oxygen reduction reactions are more possible at higher temperatures, partly explaining why higher temperatures are involved with SOFCs operations. In addition, the ΔGads (T, p) are more negative on the {110} compared to {100} surfaces; hence, oxygen reduction reactions are more favored on the {110} surfaces at higher temperatures.
Conclusion
We have studied the adsorption and reduction of O2 on the {110} and {100} surfaces of LSM25 and LBM25 as a SOFC cathode material using the Hubbard-corrected DFT approach. The molecular precursors at the Mn cation sites of LCM25 [10], LSM25 and LBM25 are associated with stronger bonds compared to LaMnO3 [10], this implies Ca, Sr and Ba as dopants in the cathode influences the O2 reduction. The trend in the reduction energies at the Mn cation sites are predicted to be in the order LSM25 < LBM25 < LCM25. In addition, the trend in dissociation energies for the most exothermic dissociated precursors follow the order LBM25 < LSM25 < LCM25 [10]. Thus, defective surfaces of LCM25, LSM25 and LBM25 favor dissociative over associative adsorption. The dissociated configurations on the {110} and {100} surfaces of LCM25, LSM25 and LBM25 have higher energies, showing that these adsorbed configurations are thermodynamically the most stable. The predicted ΔGads (T, p) is negative, suggesting that the oxygen reduction reactions on LCM25 [10] and LBM25 are feasible at any of the typical operating temperatures of SOFCs and consistent with the high temperatures employed in operating conditions SOFCs. The ΔGads (T, p) are more negative on the {110} compared to the {100} surfaces; hence, oxygen reduction reactions are more favored on the {110} surfaces at higher temperatures.
Availability of data and material
The coordinate of the optimized structures generated in the manuscript is available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Minh, N.Q., Takahashi, T.: Science and technology of ceramic fuel cells. Elsevier, Amsterdam (1995)
Singhal, S.C.: Solid oxide fuel cells for stationary, mobile, and military applications. Solid State Ionics 152, 405–410 (2002)
Singhal, S.C.: Advances in solid oxide fuel cell technology. Solid State Ionics 135, 305 (2000)
Yang, L., Wang, S., Blinn, K., Liu, M., Liu, Z., Cheng, Z.: Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2-xYbxO3-δ. Science 326, 126–129 (2009)
Yang, L., Choi, Y., Qin, W., Chen, H., Blinn, K., Liu, M., Liu, P., Bai, J., Tyson, T., Liu, M.: Promotion of water-mediated carbon removal by nanostructured barium oxide/nickel interfaces in solid oxide fuel cells. Nat. Commun. 2, 357 (2011)
Xia, C., Rauch, W., Chen, F., Liu, M.: Sm0.5Sr0.5CoO3 cathodes for low-temperature SOFCs. Solid State Ionics 149, 11–19 (2002)
Debe, M.K.: Nature 486, 43–51 (2012)
De Souza, R. A., Kilner, J. A.: Solid State Ionics 106, 175–187 (1998)
Fleig, J.: Advanced ceramics: processing and their applications, vol. 2. Elsevier, Amsterdam (2003)
Aniagyei, A., Dzade, N.Y., Tia, R., Adei, E., Catlow, C.R.A., De Leeuw, N.H.: Ab initio investigation of O2 adsorption on Ca-doped LaMnO3 cathodes in solid oxide fuel cells. Phys. Chem. Chem. Phys. 45, 28685–28698 (2018)
Chen, H.T., Raghunath, P., Liu, M.: Langmuir 27, 6787–6793 (2011)
Von Helmolt, R., Wecker, J., Holzapfel, B., Schultz, L., Samwer, K.: Phys. Rev. Lett. 71, 2331 (1993)
Ju, H.L., Nam, Y.S., Lee, J.E., Shin, H.S.: J. Magn. Magn. Mater. 219, 1 (2000)
Dabrowski, B., Rogacki, K., Xiong, X., Klamut, P.W., Dybzinski, R., Shaffer, J., Jorgensen, J.D.: Phys. Rev. B 58, 2716 (1998)
Roy, C., Budhani, R.C.: J. Appl. Phys. 85, 3124 (1999)
Murugavel, P., Lee, J.H., Yoon, J.G., Noh, T.W., Chung, J.S., Heu, M., Yoon, S.: Appl. Phys. Lett. 82, 587 (2003)
Mandal, P., Ghosh, B.: Phys. Rev. B 68, 014422 (2003)
Chatterji, T., Regnault, L.P., Schmidt, W.: Phys. Rev. B 66, 214408 (2002)
Jiang, S.: J. Solid State Electrochem. 11, 93–102 (2007)
Jena, H., GovindanKutty, K.V., Kutty, T.R.: J. Alloys Compd. 350, 102 (2003)
Kohn, W., Becke, A.D., Parr, R.G.: J. Phys. Chem. 100, 12974–12980 (1996)
Giannozzi, P., Baroni, S., Bonini, N., Calandra, M., Car, R., Cavazzoni, C., Ceresoli, D., Chiarotti, G.L., Cococcioni, M.: J. Phys.: Condens. Matter. 21, 395502 (2009)
Perdew, J.P., Burke, K., Ernzerhof, M.: Phys. Rev. Lett. 77, 3865 (1996)
Monkhorst, H.J., Pack, J.D.: Phys. Rev. B. 13, 5188 (1976)
Carter, E.A.: Science 321, 800–803 (2008)
Liechtenstein, A.I., Anisimov, V.I., Zaanen, J.: Phys. Rev. B. 52, R5467 (1995)
Dudarev, S.L., Botton, G.A., Savrasov, S.Y., Humphreys, C.J., Sutton, A.P.: Phys. Rev. B. 57, 1505 (1998)
Kovaleva, N.N., Gavartin, J.L., Shluger, A.L., Boris, A.V., Stoneham, A.M.: J. Exp. Theor. Phys. 94, 178 (2002)
Ravindran, P., Kjekshus, A., Fjellvag, H., Delin, A., Eriksson, O.: Phys. Rev. B. 65, 064445 (2002)
Watson, G.W., Kelsey, E.T., de Leeuw, N.H., Harris, D.J., Parker, S.C.: J. Chem. Soc. Faraday Trans. 92, 433–438 (1996)
Tasker, P.W.: J. Phys. C Solid State Phys. 12, 4977–4984 (1979)
Lee, Y.L., Kleis, J., Rossmeisl, J., Morgan, D.: Phys. Rev. B. 80, 224101 (2009)
Piskunov, S., Heifets, E., Jacob, T., Kotomin, E.A., Ellis, D.E., Spohr, E.: Phys. Rev. B 78, 121406 (2008)
Bielanski, A., Haber, J.: Oxygen in catalysis. Marcel Dekker, New York (1991)
Eichler, A., Mittendorfer, F., Hafner, J.: Phys. Rev. B 62, 4744 (2000)
Xu, Y., Mavrikakis, M.: Surf. Sci. 494, 131 (2001)
Takeda, Y., Kanno, R., Noda, M., Tomida, Y., Yamamoto, O.: J. Electrochem. Soc. 134, 2656 (1987)
Choi, Y.M., Lin, M.C., Liu, M.: Angew. Chem. Int. Ed. 46, 7214 (2007)
Read, M.S., Islam, M.S., Watson, G.W., Hancock, F.E.: Surface structures and defect properties of pure and doped La2NiO4. J. Mater. Chem. 11, 2597–2602 (2001)
Sit, P.H.L., Cohen, M.H., Selloni, A.: J. Phys. Chem. Lett. 3, 2409–2414 (2012)
Aschauer, U., Chen, J., Selloni, A.: Phys. Chem. Chem. Phys. 12, 12956–12960 (2010)
Choi, Y.M., Abernathy, H., Chen, H.T., Lin, M.C., Liu, M.: ChemPhysChem 7, 1957–1963 (2006)
Hammer, B., Nørskov, J.K.: Adv. Catal. 45, 71–129 (2000)
Wang, L., Maxisch, T., Ceder, G.: Phys. Rev. B: Condens. Matter Mater. Phys. 73, 195107 (2006)
Atkins, P.W.: Physical chemistry, 6th edn., p. 582. Oxford University Press, Oxford (1998)
Funding
The authors gratefully acknowledge the UK Royal Society and the Leverhulme Trust for a research grant under the Royal Society-Leverhulme Africa Award Scheme.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally to the preparation of the manuscript. AA performed the theoretical calculations. The initial draft of the manuscript was written by AA with input and suggestions from all the co-authors. All the authors commented on previous versions and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest whatsoever regarding the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aniagyei, A., Kwawu, C., Kwakye, R. et al. Oxygen (O2) reduction reaction on Ba-doped LaMnO3 cathodes in solid oxide fuel cells: a density functional theory study. Mater Renew Sustain Energy 10, 15 (2021). https://doi.org/10.1007/s40243-021-00200-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-021-00200-1