Abstract
PPy-Cr2O3 hybrid flexible electrodes (HFEs) have been synthesized by successive ionic layer adsorption and reaction (SILAR) method using flexible aluminum (Al) strips (derived from cold drink cans) as substrates. For the synthesis, 0.1 M pyrrole and 0.02 M K2Cr2O7 dissolved in 0.5 M aqueous H2SO4 were used as precursors while the flexible aluminum strips derived from waste cold drink cans were used as substrates. XRD pattern shows the peak at \(50.48^\circ\) indicating the existence of rhombohedral Cr2O3 in the hybrid. FTIR spectrum corroborates the formation of hybrid. SEM image exhibits porous morphology with interconnected granules. TEM image depicts the Cr2O3 granules of average size 20 nm and PPy globules of average size 50 nm. The liquid–solid contact angle was found to be \(10^\circ 30{\prime }\) indicating the near-superhydrophilic nature of HFE. Cyclic voltammetric (CV) analyses of HFEs have been carried out in each of 20 ml, 0.5 M solutions H2SO4, Na2SO4, and K2SO4. Changes in potential window, redox behavior and hence, the specific capacitance have been observed. The pseudocapacitance of HFE is combined effect of doping–dedoping of PPy matrix with SO42− anions as well as redox reaction shown by Cr2O3. HFE shows maximum specific capacitance 4246 Fg−1 in K2SO4 as measured from CV. HFE shows appreciable stability with 58.20% retention in capacitance even after 1000 cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different materials are being discovered and used to prepare supercapacitive electrodes. Metal oxides [1,2,3,4,5], sulfides [6,7,8,9,10], conducting polymers [11], carbonaceous materials, etc., have been used till today. Conducting polymers have grabbed more attention. Polypyrrole (PPy) has also been used as electrode material in supercapacitors in pure [1, 11] and hybrid forms [12,13,14,15,16]. PPy suffers from poor elasticity of shape, poor cycling stability, and limited potential window. To overcome these limitations, co-polymerization [31], hybridization or composite formation is going on [13,14,15,16,17,18,19,20]. Chromium has multiple oxidation states. The redox transitions of Cr between different oxidation states may be useful for charge storage. Cr2O3 has been used as electrode material for supercapacitors [20,21,22], but did not receive more attention. Composite or hybrid of PPy with Cr2O3 may improve the specific capacitance and cycling stability of the electrode. Aluminum being highly conductive and cost-effective has been used in the preparation of ordinary capacitors. The electrodeposition of PPy on Al substrate has been attempted by Liu et al. [22, 23]. Shinde et al claimed successful SILAR synthesis of PPy on stainless steel [23, 24]. Various composites and hybrids of PPy have been prepared by SILAR, viz, PPy-Cu(OH)2 [13, 14], C10H9N2-PPy [5]. There are no references regarding the SILAR synthesis of PPy-Cr2O3 flexible electrodes and their electrochemical study.
In our previous work, we have studied the effect of electrolytic anions on the charge storage of Fe3O4 electrodes [24, 25]. Just like anions, charges stored by redox interaction between electrode and electrolyte are dependent on the properties cations of electrolytes, viz, ionic radius, mobility, etc. Hence present work focused on preparation of PPy-Cr2O3 hybrid flexible electrodes (HFEs) on the surface of Al substrate by simple, cost-effective SILAR method and analysis of the prepared HFEs physically using XRD, FTIR, SEM and EDX and electrochemically using cyclic voltammetry (CV). The cycling stability analysis of HFE has also been aimed.
Materials
For the present work, extra pure pyrrole (Sigma-Aldrich), K2Cr2O7 (SD Fine chemicals), and H2SO4 (SD Fine chemicals) were used. Double-distilled water was used as solvent in all the experimental works. The aluminum strips derived from waste cold drink cans (\(1\,{\text{cm }} \times 5\,{\text{cm}}\)) were used as conducting substrates. All chemicals used were of analytical grade.
Experimental work
Figure 1 shows the synthesis mechanism. It involves the following stages.
Substrate pretreatment
The cold drink cans were cut open to get rectangular strips of \(1\,{\text{cm }} \times 5\,{\text{cm}}\) area. The inner surface has Teflon coating while outer one has synthetic color hence, the strips were thoroughly polished using emery paper (grade 2500) to get rough finish and then ultrasonicated in double-distilled water for 20 min. to clean their surface. Then washed with acetone and treated with 2 mM HNO3 to improve adhesion for rapid nucleation and growth [13,14,15].
SILAR deposition
Rate of polymerization depends on the molar concentration of oxidizer. Rapid oxidative polymerization makes the material growth rapid, but films grown are non uniform and less adherent. As K2Cr2O7 is strong oxidizer, its concentration was kept minimal. Different molar concentrations were tried and uniform film formation was observed at 0.02 M of K2Cr2O7. Al strips were immersed for 10 s in each of the 20 ml aqueous solutions of 0.1 M pyrrole and 0.02 M K2Cr2O7, prepared in 0.5 M H2O4. This is followed by washing with the jets of double-distilled water. This completes one SILAR cycle. 60 such SILAR cycles have been carried to get the uniform HFE. Synthesis has been carried out at very low pH \(\sim 1.1\), to make PPy more protonated and hence, more conducting [15, 25, 26].
Characterizations
Physical characterizations of the prepared HFEs have been carried out using X-ray diffractometer (Ultima IV Rigaku D/MAX 550vb + 18kW with Cuk \(\alpha\), λ = 1.54056 Å), FTIR spectrophotometer (Nicolet iS10, Thermo Scientific, USA) and scanning electron microscope (SEM JEOL JSM-7600F HITACHI, Japan). The cyclic voltammetry (CV) of HFE has been carried out using electrochemical analyzer (CHI 408C, CH instruments USA) of standard 3-electrode set-up, comprising of HFE as working electrode, Ag/AgCl as a reference electrode and platinum wire as a counter electrode. Surface wettability analysis was carried out using contact angle meter (HO-IAD-CAM-01, Holmark opto-mechatronics, India).
Results
Film formation mechanism
The formation of HFE involves polymerization of pyrrole and formation of Cr2O3 (Scheme 1).
The loss of conjugated electron leads to the formation of polaron. Successive addition of polarons form bipolarons, trimer, quinoid structure and eventually PPy. During the growth of PPy, the reduction reaction occurs to form Cr2O3 from K2Cr2O7, as oxidation state of Cr reduces from + 6 to + 3 by engulfing the electrons released during the polymerization of pyrrole.
Physical characterization
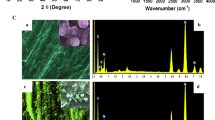
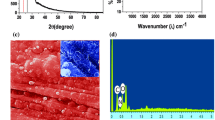
The XRD pattern of HFE shows peak at \(50.48^\circ\) indicating the formation of rhombohedral Cr2O3 (Fig. 2a). The peak at 672 cm−1 in FTIR spectrum is corresponding to metal oxygen bonding in Cr2O3 and peak at 1577 cm−1 corresponds to the characteristic pyrrole ring vibration (Fig. 2b). These peaks corroborate the formation of PPy-Cr2O3 hybrid. SEM micrograph of HFE shows porous morphology with interconnected granules of average size 50–80 nm (Fig. 2c). Peaks corresponding to the existence of C, N, Cr and O were observed in EDX spectrum confirming the formation of hybrid (Fig. 2d). TEM image depicts the Cr2O3 granules of average size 20 nm and PPy globules of average size 50 nm (Fig. 2e). The surface wettability study reveals hydrophilic nature of the HFE as the water to surface contact angle was \(10^\circ 30{\prime }\) indicating near superhydrophilic nature (Fig. 2f). This is highly desirable for the aqueous electrolyte-based supercapacitors.
Cyclic voltammetric analyses
CV analysis of the HFE was carried out in 0.5 M aqueous solutions of H2SO4, Na2SO4 and K2SO4 (Fig. 3a, c, e), respectively. HFE shows large potential window \(\sim 3 V\) (0–3 V) in 0.5 M H2SO4 (Fig. 3a). This is higher than the potential window show by pristine PPy [23, 24]. Acidified water splits at − 1.23, but when it comes to aqueous solutions of salts, the potential window expands towards extremities as high as 3.2 V. The potential windows as high as 3.2 V for the device have been reported for sodium perchlorate by Hiroshi Tomiyasu et al. [31, 32]. Hence, in the present case we got the potential window of 3 V.
The gradual increase in current integral, i.e., area enclosed by the curves has been observed with increase in the potential scan rate. This is in perfect accordance with the previous results related to the scan rate variations in pseudocapacitors indicating that the charge storage takes more time as it could not follow the rapid transitions in potential due to increased scan rate [13,14,15, 23,24,25,26,27,28,29]. A sudden increase in the current at positive sweep of the applied potential at \(\sim 1.35 V\) was observed for each scan rate.
HFE shows potential window \(\sim ( - 1 V)\) (− 1.8 to − 0.8 V) in 0.5 M Na2SO4 (Fig. 3c). The potential range is same as that of the pristine PPy [23, 24], but the nature of cyclic voltammogram is entirely different. Further, the window is completely in the negative potential. The gradual increase in current integral has been observed. Both the potentials and the current were found to be negative indicating that the electrode can be used as anode. HFE shows large potential window \(\sim 0.3 V\) (− 0.8 to − 0.5 V) in 0.5 M K2SO4 (Fig. 3e). This is smaller than the potential window shown by pristine PPy [23, 24]. It was seen that the current increases slowly with the potential till − 0.75 V, thereafter it suddenly increases. This may be due to the Al substrate as some solvated ions may react with Al. The maximum current limit went on decreasing with the increasing scan rate. At higher scan rate, the voltage transitions are very rapid, hybrid electrode material is not completely utilized; hence, the increased current is not in the required proportion and thus, SC goes on decreasing with increase in scan rate [13,14,15]. Specific capacitance (SC) associated with the electrode was calculated using the Eq. 1 given below.
where V1 and V2 are potential limits, m is the mass of active electrode material, V is the net potential of the window \((V = V_{2} - V_{1} )\). The evaluated SC values at different scan rates are mentioned in the inset of Fig. 2a, c, e. Observed maximum values of SC for HFE were 435.42, 16.22 and 4246.66 Fg−1 at 5 mVs−1 in 0.5 M aqueous H2SO4, 0.5 M aqueous Na2SO4 and 0.5 M aqueous K2SO4, respectively.
When positive sweep potential is given to HFE, PPy matrix is doped with SO42− anions. The SO42− anions displace oxygen from Cr2O3 to form Cr(SO4)3, giving greenish appearance to the FE surface and nearby solution. During this process, electrons are lost by Cr as the oxidation state changes from + 3 to + 6. Cr(SO4)3 dissociates in aqueous electrolyte soon and forms Cr6+ and SO42− species. When negative sweep potential is given to HFE, the dedoping of PPy matrix occurs and SO42− anions are separated from PPy matrix. The Cr6+ species gain three electrons and react with OH- ions in the PPy matrix to form Cr(OH)3. Thus, the redox transitions of Cr during positive or negative sweep of potentials as well as intercalations and de-intercalations of SO42− anions in the matrix of HFE (i.e., doping and dedoping of PPy matrix) are responsible for the pseudocapacitive charge storage. Hence, in present case, the pseudocapacitance depends only on the rate of dissociation of electrolytes and the pH of the electrolytes. Acidic pH is favorable for the sustainability of PPy matrix. Further, the protonic conduction enhances current. Thus, large current integral (area) has been seen for CV carried in H2SO4. As the potential window was large, SC was limited to 435.42 Fg−1 at 5 mVs−1. Both the Na2SO4 and K2SO4 are salts of strong acid and strong base; hence, neutral in nature. K+ cation has greater ionic radius. Thus, charge density of K+ ions is less than that of Na+ ions. Hence K+ ions have higher mobility than Na+ cations. The Gibbs free energy of solvation depends on the ionic radius [29,30,31]. The ionization energy of Na2SO4 is 490 kJMol−1 and that of K2SO4 is 418 kJMol−1, which indicates that the ionization of K2SO4 requires less energy. Because of all these reasons, current integral and consequently the SC value of HFE was found more in 0.5 M K2SO4 among the three electrolytes [32, 33].
Plausible charge storage mechanisms
See Scheme 2.
Cycling stability study
The potential transitions occur with bigger steps; hence, voltammetric cycles occur rapidly at higher scan rate. Therefore, cycling stability analyses of the HFE have been carried out at potential scan rate of 100 mVs−1. In H2SO4, the SC value of HFE goes on decreasing rapidly in first 100 cycles as it decreases by 36.20%, then it goes on decreasing slowly by steps of \(\sim 1\%\) per 100 cycles till 400th cycle thereafter by 0.5% till 800th cycle. Thereafter, it remains unaffected. Thus, the HFE shows appreciably good cycling stability with 58.20% retention of SC (Fig. 3b). In Na2SO4, the SC value of HFE goes on decreasing rapidly in first 100 cycles as it decreases by 34.46%, then it goes on decreasing slowly by steps of \(\sim 1.2 \%\) per 100 cycles till 400th cycle. Thereafter, it remains same even after 1000 cycles. Thus, the HFE shows appreciably good cycling stability with 62.02% retention of SC (Fig. 3d). In K2SO4, the SC value of HFE goes on decreasing rapidly in first 100 cycles as it decreases by 39.45%, then it goes on decreasing slowly by steps of \(\sim 0.6 \%\) per 100 cycles till 400th cycle. Thereafter, it remains same even after 1000 cycles. Thus, the HFE shows appreciably good cycling stability with 57% retention of SC (Fig. 3f).
The rapid decrease observed during the initial cycles can be explained. During the initial cycles, the loosely bound material is active and adds to the SC, but with increase in the number of cycles, this material peels off resulting in the decrease in SC.
The decrease may also be due to the fact that the adsorbed ions are not completely desorbed, but few ions remain in the matrix of the composite. These ions may prohibit the adsorption of other ions by repelling them. With increase in number of cycles, such ions in the matrix may increase reducing the number of adsorption–desorption mechanism sites.
Conclusion
PPy-Cr2O3 HFEs have been synthesized by SILAR on flexible Al substrates derived from the waste cold drink cans. The HFEs show porous morphology with interconnected granules of average size 50–80 nm. XRD, FTIR and EDX analysis confirms the formation of PPy-Cr2O3 hybrid. HFE exhibits surprising transitions in potential window for different electrolytes. For aqueous H2SO4, it was \(\sim 3 V\) lying in positive region. For Na2SO4, it was entirely negative and was \(\sim 1 V\) exhibiting negative current. While for K2SO4, though the potential window was negative, it was very small \(\sim 0.3 V\). The observed maximum SC was 4246.66 Fg−1 at 5 mVs−1. HFE shows appreciable cycling stability with 57% retention of SC after 1000 cycles. Thus, K2SO4 providing small potential window gives maximum specific capacitance and is suitable for the microelectronic applications needing potential difference of 0.3 V. H2SO4 is applicable for high potential applications providing potential window as high as 3.5 V. The HFEs are applicable as an anodic material in Na2SO4.
References
Journals
Thakur, A.V., Lokhande, B.J.: Source molarity affected surface morphological and electrochemical transitions in binder-free FeO(OH) flexible electrodes and fabrication of symmetric supercapacitive device, Chemical Papers, 1–9, 2018
Ingole, R.S., Lokhande, B.J.: Electrochemically synthesized mesoporous architecture of vanadium oxide on flexible stainless steel for high performance supercapacitor. J. Mater. Sci. Mater. Electron. 28(15), 10951–10957 (2017)
Ingole, R.S., Lokhande, B.J.: Effect of pyrolysis temperature on structural, morphological and electrochemical properties of vanadium oxide thin films. J. Anal. Appl. Pyrol. 120, 434–440 (2016)
Fugare, B.Y., Lokhande, B.J.: Spray pyrolysed titanium oxide thin films using different ingredients for supercapacitive charaterizations. J. Mater. Sci.: Mater. Electron. 27(6), 5788–5795 (2016)
Khavale, S.V., Bharadwaj, S.R., Lokhande, B.J.: Effect of molar concentration on supercapacitive parameters of MnO2. Invertis J. Renew. Energy 2(2), 106–113 (2012)
Karade, S., Dwivedi, P., Mujumder, S., Pandit, B., Sankapal, B.R.: First report on FeS based 2V operating flexible solid state symmetric supercapacitor device. Sustain. Energy Fuels 1, 1366–1375 (2017)
Hsu, Y.K., Chen, Y.C., Lin, Y.G.: Synthesis of copper sulfide nanowire arrays for high-performance supercapacitors. Electrochim. Acta 139, 401–407 (2014)
Karade, S., Dubal, D.P., Sankapal, B.R.: MoS2 ultrathin nanoflakes for high performance supercapacitors: room temperature chemical bath deposition (CBD). RSC Adv. 6, 39159–39165 (2016)
Karade, S., Dubal, D., Sankpal, B.R.: Decoration of ultrathin MoS2 nanoflakes over MWCNTs : enhanced supercapacitive performance through electrode to symmetric all solid state device. Chem. Select 2(32), 10405–10412 (2017)
Pandit, B., Karade, S., Sankapal, B.R.: Hexagonal VS2 anchored MWCNTs; First approach to design flexible solid state supercapacitor device. ACS Appl. Mater. Interf. 27 9(51), 44880–44891 (2017)
Snook, G.A., Best, A.S.: Review on Conducting polymer based supercapacitor devices and electrodes. J. Pow. Sour. 196, 1–12 (2011)
Brown, M.P., Austin, K.: Appl. Phys. Lett. 85, 2503–2504 (2004)
Thakur, A.V., Lokhande, B.J.: Effect of dip time on the electrochemical behavior of PPy-Cu(OH)2 hybrid electrodes synthesized using pyrrole and CuSO4. e-polymer 17(2), 167–173 (2016). https://doi.org/10.1515/epoly-2016-0160
Thakur, A.V., Lokhande, B.J.: Dip time dependent SILAR synthesis and electrochemical study of highly flexible PPy:Cu(OH)2 hybrid electrodes for supercapacitors. J. Solid State Electrochem. (2016). https://doi.org/10.1007/s10008-016-3502-2
Thakur, A.V., Lokhande, B.J.: C10H8N2-PPy hybrid flexible electrode: SILAR synthesis and electrochemical study. J. Mater. Sci. Mater. Electron. https://doi.org/10.1007/s10854-017-8074-0
Xuefei, G., Shaohuia, L., See, L.P.: Fiber asymmetric supercapacitor based on FeOOH/PPy on carbon fibers as anode electrode with high volumetric energy density for wearable applications. https://doi.org/10.1039/x0xx00000x
Sharma, R.K., Rastogi, A.C., Desu, S.B.: Manganese oxide embedded polypyrrole nanocomposites for electrochemical supercapacitor. Electrochim. Acta 53, 7690 (2008)
Sivakkumar, S.R., MyounKo, J., Kim, D.Y., Kim, B.C., Wallace, G.G.: Performance evaluation of CNT/polypyrrole/MnO2 hybrid electrodes for electrochemical capacitors. Electrochim. Acta 52, 7377 (2007)
Zang, J., Bao, S.J., Li, C.M., Bian, H., Cui, X., Bao, Q., Sun, C.Q., Guo, J., Lian, K.: Well-aligned cone-shaped nanostructure of polypyrrole/RuO2 and its electrochemical supercapacitor. J. Phys. Chem. C 112, 14843 (2008)
Bai, M.H., Bian, L.-J., Song, Y., Liu, X.: Electrochemical codeposition of vanadium oxide and polypyrrole for high performance supercapacitor with high working voltage. ACS Appl. Mater. Interf. 6(15), 12656–12664 (2014). https://doi.org/10.1021/am502630g
Xiaoyang, X., Jinze, W., Yang, N., Na, H., Li, L., Gao, Jinping: Cr2O3 an novel supercapacitor electrode material with high capacitive performance. Mater. Lett. (2014). https://doi.org/10.1016/j.matlet.2014.12.022
Chen, B., Wang, Y., Li, C., Fu, L., Liu, X., Zhu, Y., Zhang, L., Wu, Y.: A Cr2O3/MWCNTs composite as a superior electrode material for supercapacitor. RSC Adv. 7, 25019–25024 (2017). https://doi.org/10.1039/C7RA01954H
Liu, A.S., Oliveira, M.A.S.: Electrodeposition of polypyrrole films on aluminum from tartrate aqueous solution. J. Braz. Chem. Soc. 18(1), 143–152 (2007)
Shinde, S.S., Gund, G.S., Kumbhar, V.S., Patil, B.H., Lokhande, C.D.: Novel chemical synthesis of polypyrrole thin film electrodes for supercapacitor application. Eur. Polym. J. 49, 3734–3739 (2013)
Thakur, A.V., Lokhnade, B.J.: Electrolytic anion affected charge storage mechanism of Fe3O4 flexible thin film electrodes in KCl and KOH: a comparative study by cyclic voltammetry and galvanostatic charge discharge. J. Mater. Sci.: Mater. Electron. 28(16), 11755–11761 (2017). https://doi.org/10.1007/s10854-017-6980-9
Pei, Q., Quin, R.: Protonation and deprotonation of polypyrrole chain in aqueous solutions. Synth. Met. 45(1), 35–48 (1991)
Bazant, M.Z., Thornton, K., Ajdari, A.: Diffuse-charge dynamics in electrochemical systems. Phys. Rev. E 70(1), 021506 (2004)
Wang, H., Pilon, L.: Intrinsic limitations of impedance measurements in determining electric double layer capacitances. Electrochim. Acta 63, 55–63 (2012). https://doi.org/10.1016/j.electacta.2011.12.051
van Soestbergen, M., Biesheuvel, P.M., Bazant, M.Z.: Diffuse-charge effects on the transient response of electrochemical cells. Phys. Rev. E 81(1), 021503 (2010)
Marcus, Y.: Ionic radii in aqueous solutions. Chem. Rev. 88, 1475–1498 (1988)
Tomiyasu, H., Shikata, H., Tokao, K., Asanuma, N., Truta, S., Park, Y.Y.: An aqueous electrolyte of widest potential window and its superior capability for capacitors. Sci. Rep. 7, 45048 (2017). https://doi.org/10.1038/srep45048
Books
Park, Y.H., Shin, H.C., Lee, Y., Son, Y., Baik, D.H.: Formation of polypyrrole copolymer in PSPMS precursor film by electrochemical polymerization. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 327(1), 320–327 (1999)
Zhong, C., Deng, Y., Hu, W., Sun, D., Zhang, J.: Electrolytes and electrochemical supercapacitors. CRC press, Taylor and Francis group, Boca Raton (2016)
Acknowledgements
Authors are thankful to Solapur University, Solapur for making provision of DRF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Thakur, A.V., Lokhande, B.J. Synthesis and electrolytic cation-dependent cyclic voltammetric study of SILAR deposited PPy-Cr2O3 in equimolar aqueous solutions of H2SO4, Na2SO4, and K2SO4. Mater Renew Sustain Energy 7, 17 (2018). https://doi.org/10.1007/s40243-018-0125-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-018-0125-9