Abstract
This work presents the synthesis and characterizations of a stable and well-sinterable proton conductor BaCe0.35Zr0.50Y0.15O3-δ in which a transition of conductivity occurred steeply from the order of 10−3 to 10−2 S cm−1 and activation energy changed from 0.35 to 0.22 eV in the temperature range from 350 to 400 °C, due to the dissociation of protons from the dopant-proton defect pairs. The anode composite Ni-BaCe0.35 Zr0.50Y0.15O3-δ (40:60 vol%) prepared by liquid condensation process showed comparatively very high electrical conductivity than the existing solid oxide fuel cell (SOFC) anodes, in the temperature range of 350–800 °C. Fabrication (by screen printing and co-firing processes), performance and post-mortem analysis of anode-supported protonic SOFC cell using these materials are discussed elaborately.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The solid oxide fuel cell (SOFC) has attracted a great attention since the decades as an alternate energy source for future generations. Main advantage of SOFC is the fuel flexibility, where a variety of carbohydrates can be used as fuel without requiring an expensive external fuel reformer. However, high operating temperature (i.e., 800–1,000 °C) of SOFC leads to the formation of electrode–electrolyte inter diffusion layers as well as degradation of SOFC components and sealing materials. Over the past few years, proton-conducting ceramics that show conductivity ~10−2 S cm−1 at temperatures below 700 °C have been considered as alternate electrolyte candidates for SOFC, compared to the conventionally used oxygen ion conducting yttrium stabilized zirconia (YSZ). Among the various ceramic proton conductors, perovskite oxides such as yttrium doped barium cerates (BCY) show highest proton conductivity, i.e., ~10−2 S cm−1 at 600 °C with lower activation energy [1]. Similarly, the Y- and Yb-doped barium zirconates (BZY and BZYb) show the conductivity in the order of 10−3 S cm−1 at 600 °C [2, 3]. Moreover, barium cerates are unstable in CO2 [4] and moist atmosphere [5] (which are parts of SOFC operating condition), and barium zirconates (BaZr1−xY x O3) are highly stable but offer very high grain boundary resistance, causing a reduction in conductivity. Various authors have partially replaced the Ce4+ by Zr4+ in BCY and found that the Zr rich samples are more stable but conductivity reduces with increase in Zr content [6, 7]. These oxides show pure protonic conduction in wet hydrogen atmosphere, while they exhibit protonic, oxygen ionic and electronic mixed conduction under high oxygen partial pressure at elevated temperatures [6]. Major problem in case the of BaCe1−x−yZr x YyO3-δ materials is the poor sinterability and they require very high temperature (i.e., above 1,700 °C) for well sintering with pore free microstructure [8–11].
In this work we have developed a stable barium cerate zirconate (BCZY) that exhibits very high proton conductivity at intermediate temperatures and is well sinterable at comparatively lower temperatures. A novel anode composite NiO-BCZY is prepared by liquid condensation method and its sintering and electrical properties are discussed. Fabrication process of anode-supported planar proton-conducting SOFC cell, using BCZY as electrolyte, NiO-BCZY composite as anode and La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF) as cathode, is discussed and the performance of the cell is evaluated.
Experimental
The barium cerate zirconate, i.e., BaCe0.35Zr0.50Y0.15O3-δ (BCZY) was prepared by solid-state reaction method, using the stoichiometry amounts of BaCO3, CeO2, ZrO2 and Y2O3 with purity above 99.9 %. Phase purity of BCZY and its stability in CO2 atmosphere were studied by X-ray diffraction technique (using Philips PW 3830 X-ray generator). The particle size distribution was studied by using particle size analyser (LS230, COULTER Co.) after dispersing the powder in ethanol. The powder sample was pressed into pellets (with diameter of 10 mm) by applying a pressure of 100 MPa uni-axially. Sinterability of the material was studied using dilatometer (DIL 402C, NETZSCH, Germany), by heating the pellet from room temperature to 1,500 °C in air at the rate of 3 °C/min. The green pellet was also sintered at 1,600 °C for 30 h and its density was measured by the Archimedes method. Microstructures of as-prepared powder and sintered pellet were studied by scanning electron microscope (SEM) (FEI XL-30, Philips, Netherlands). Platinum paste was painted on both sides of polished sintered pellet (with relative density of 95 %) as electrode and fired at 1,000 °C (1 h) for AC impedance study. The impedance measurements were carried out in wet hydrogen atmosphere (i.e., 3 % H2O + 4 % H2 balanced with Ar) by two probe method using Solatron 1260 Impedance/Gain phase analyser. During the measurements, an external field of 100 mV was applied varying the frequency from 0.1 Hz to 10 MHz. The impedance data were recorded in the temperature range of 250–700 °C at an interval of 50 °C, holding the furnace temperature more than 3 h at each step for equilibrium. Impedance plots were fitted with their equivalent circuits using Zview software package to get the resistance and capacitance values.
The composite NiO-BCZY material (with Ni:BCZY volume ratio as 40:60) was prepared by liquid condensation process (LCP) [12, 13]. The granule formed from the LCP was characterized by dilatometer to check the sinterability. DC electrical conductivity of the composite anode sample was studied by linear four probe method in the temperature range of 350–800 °C. The bar-shaped sample was first sintered at 1,500 °C for 3 h and then reduced in H2 atmosphere at 900 °C for 3 h before using in electrical measurement. The granule was also compressed into disk form for anode substrate.
The electrolyte paste of BCZY was prepared by mixing the stoichiometry amounts of α-terpineol (as solvent), dispersing agent (KD6), binder, plasticizer and BCZY powder in planetary mill. Similarly, cathode pastes of the composite [BCZY—La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF)] (with 50:50 volume ratio) and LSCF were also prepared by planetary milling. The commercially available LSCF powder was used to prepare the cathode pastes.
The proton-conducting ceramic fuel cell (i.e., NiO-BCZY/BCZY/BCZY-LSCF/LSCF) was prepared by screen printing and co-firing processes. At first, The NiO-BCZY substrate was pre-sintered at 1,100 °C (3 h) and then the BCZY electrolyte paste was screen printed and co-fired at 1,500 °C (3 h). The cathode pastes of BCZY-LSCF (one layer) and LSCF (two layers) were screen printed and co-fired at 1,050 °C (2 h). Microstructure of the cell and various cell components were studied by SEM before and after the cell test. Performance of the button (2 × 2) cell was studied by current–voltage–power (I–V–P) characteristic and impedance measurement (at OCV) using Impedance analyser (solatron 1260 gain/phase analyser) coupled with potentiostat (Solatron 1287).
Results and discussion
Stability and sinterability of BaCe0.35Zr0.5Y0.15O3-δ (BCZY)
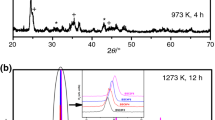
The powder X-ray diffraction pattern of BaCe0.35Zr0.5Y0.15O3-δ (Fig. 1a) confirmed the cubic perovskite phase of sample that was calcined in two steps; first at 1,300 °C (5 h) and then at 1,400 °C (15 h). To check the stability of the material against CO2, the sample was heated at 900 °C for 3 h in CO2 (which was supplied to the furnace right from the beginning of the heating process). The X-ray diffraction of the BCZY powder heat treated in CO2 (Fig. 1b) did not show any change in phase, which confirmed that the sample is stable against CO2. It has been studied that due to basic nature of barium cerates (BaCeO3 or BaCe0.9Y0.1O3-δ), they react with acidic gases such as CO2 and decompose into BaCO3 and CeO2 phases [14]. Katahira et al. [6] have studied the chemical stability of Zr-substituted BaCe0.9−xZ x Y0.1O3-δ by heat treating the samples at 900 °C (2 h) in 100 % CO2 atmosphere and observed that the material with x = 0.1 decomposed to BaCO3 and CeO2 phases, while the phase of material with x = 0.4 remained same after CO2 treatment, as untreated sample. Authors observed that, Zr act as a phase stabilizer and stability of barium cerate increases with Zr amount. Fabbri et al. [7] have also studied the similar materials and found that the chemical stability of samples in CO2 increases with zirconia content and samples with x ≥ 0.5 are more stable, as seen in the case of present material.
The powder sample was pressed into pellet and characterized by dilatometry to study its sintering properties. The dilatometry curve (Fig. 2a) did not show any shrinkage of pellet in the temperature range up to 1,500 °C. The pellet prepared in similar condition was sintered at 1,650 °C for 30 h and the relative density of the pellet was about 85 %. The particle size distribution curve (Fig. 3) indicates the presence of secondary agglomerations in addition to the primary particles. The mean particle size D10 was about 0.133 and 0.132 micron after dispersing the powder for 20 and 60 min in ethanol, respectively. Similarly, the value of D90 was found to be 1.594 and 1.605 micron after dispersing the powder for 20 and 60 min, respectively. The SEM image of as-prepared powder (Fig. 4a) also confirmed the agglomerating nature of particles. Therefore, the as-prepared powder was further ball milled for 48 h and corresponding pellet was also characterized by dilatometry (Fig. 2b), which showed that the shrinkage of pellet is about 12 % at 1,500 °C. Pellet made of this latest ball-milled (for 48 h) powder was sintered at 1,600 °C (30 h) and its relative density was found to be around 95 %. Pellet prepared under this condition were also used for SEM and Impedance studies. The SEM micrograph of sintered pellet (Fig. 4b) also confirmed the dense microstructure of pellet.
Electrical properties of BCZY
Electrical properties of the BCZY sample were studied by AC impedance spectroscopy. Figure 5a, b shows the complex impedance plots of BCZY in wet 4 % H2 at 300 and 500 °C. At lower temperature range (i.e., up to 400 °C), the impedance plots contain two semicircles, i.e., at higher and intermediate frequencies corresponding to the grain and grain boundary conductivities, respectively. The lower frequency tail/arc in the Fig. 5a corresponds to the electrode polarization. Above 400 °C, the semicircle for grain interior conductivity diminished and the lower frequency tail appeared as a semicircle (Fig. 5b). In that case, the high-frequency intersection of Z-plot with Z′ axis is taken as the bulk/grain interior resistance. The Z-plots were fitted with their equivalent circuit (shown in the inset of Fig. 5a) using Z view software to get the resistance and capacitance values. At higher temperatures (Fig. 5b), obtained range capacitance values, i.e., ~10−8 F for high-frequency arc (shown as inset) and ~10−6 F for lower frequency semicircles indicate that they are corresponding to the grain boundary conduction and electrode polarization, respectively. The Z″ vs. frequency plots (Fig. 6) also exhibit that, at lower temperatures, there are two peaks at high and intermediate frequencies, which correspond to relaxation of charge carriers at grain and grain boundary, respectively. The tail/peak at lower frequency range is due to the electrode polarization. At elevated temperatures the peak positions shifted to higher frequencies. Comparing to the frequency range of peaks in Fig. 6, it is observed that the peak shown in the plot at 500 °C is due to the electrode polarization which directly corresponds to low-frequency semicircle in the Fig. 5b. This confirmed that the low-frequency semicircle in the Fig. 5b is due to the electrode polarization. Comparing the frequency range in Fig. 5a and inset of Fig. 5b, it can be also clear that, the high-frequency arc shown in the inset of Fig. 5b is due to grain boundary conductivity. Moreover, the total resistance value was obtained by summation of grain and grain boundary resistances. The conductivity of the sample was calculated by using the formula σ = L/(RA), where R is the value of total resistance, L is the thickness of pellet and A is the area of cross section.
Complex impedance plots for BCZY sample at a 300 °C and b 500 °C. Inset in a shows the equivalent circuit used for fitting, where, R1, R2 and R3 represent the contact, grain and grain boundary resistances, respectively. CPE1, and CPE2 are the constant phase elements in different arcs. Inset in b shows the presence of high-frequency arc corresponding to grain boundary conductivity
Figure 7 shows the Arrhenius plot of total conductivity, i.e., log σ vs. 1/T. The material shows the conductivity ~10−3 S cm−1 in the temperature range of 250–350 °C and ~10−2 S cm−1 above 350 °C. The steep increase in conductivity from the order of 10−3 to 10−2 S cm−1 and decrease in activation energy from 0.35 to 0.22 eV at the temperature range above 350 °C, appears like a super ionic type transition in the material, where state of hydrogen changes from more ordered state to disordered state, as observed in different proton-conducting materials [15]. In the present case, as Y3+ replaces Ce4+, the dopant (Y3+) associates an effective −1 charge and protons being positively charged, they make defect association. Protons are said to be trapped by dopants and state of protons become more ordered [16]. The defect pairs (Y-H) act as electric dipoles. When a thermally activated proton jump from one oxygen to another in the octahedra (YO6), maintaining its defect association with the dopant Y3+, the electric dipole appears to orient about Y3+. In presence of the external electric field, arbitrary oriented dipoles align in the direction of field and a local heat is generated. That is how the electrical energy is dissipated in terms of heat and it is called dielectric loss. Figure 8a, b shows the frequency spectra of dielectric loss tangent (tan δ) (obtained from impedance data) at various temperatures. At lower temperatures (say at 300 °C), tan δ (Fig. 8a) exhibits loss peaks due to the dipolar rotations in the bulk (high-frequency peak) [17] and space charge polarization at grain boundaries (low-frequency peak) [18]. As the temperature increases, diffusion of thermally activated protons from grains to grain boundaries can result in reduction of the space charge width and hence a decrease in space charge polarization at grain boundaries. As a result, the peak due to space charge polarization is disappeared at 350 °C (Figure 8a). However, at 350 °C, the intensity of higher frequency peak increased as the number of dipolar rotation increases due to thermal activation of charge carriers (Fig. 8a). The loss peak due to dipolar rotation is found to disappear at 400 °C (Fig. 8b), and in that case, protons are said to be dissociated from the defect pairs and perform long range order migrations where, the state of protons becomes disordered. The upturn in data points at higher frequency region, at higher temperatures, can be attributed to the conduction loss due to the migration of free charge carriers. This indicates that the transition in conductivity (i.e., about one order) between 350 and 400 °C could be due to the dissociation of protons from defect association with dopants. As the protons are almost free above 400 °C, the activation energy of conductivity is also reduced from 0.35 to 0.22 eV. Moreover, as the BCZY shows very high total conductivity (σ) i.e., around 10−2 S cm−1 above 400 °C, it increases the possibility to bring the operating temperature of SOFC down to 400 °C. A small decrease in conductivity above 650 °C (Fig. 7) could be due to the dehydration of protons from the sample.
Fabrication of anode-supported planar protonic SOFC cell
The anode composite NiO-BCZY with Ni:BZCY volume ratio of 40:60 was prepared by LCP method [12, 13]. The dilatometry study of the composite was performed to examine its sinterability. Figure 9 shows the shrinkage behavior of both anode composite (NiO-BCZY) and as-prepared BCZY (i.e., used to prepare the anode granules). It is observed that, the anode volume shrunk about 20 % in the temperature range of 50–1,500 °C. The peak around 400 °C is due to removal of phenolite that was used as binder in the processing of granule. The DC electrical conductivity of the bar-shaped anode sample was measured by liner four probe method in the wet H2 atmosphere. The bar-shaped sample was first reduced at 900 °C in H2 atmosphere for 3 h before using in conductivity measurement. Figure 10 shows the Arrhenius plot of DC conductivity in the temperature range of 350–800 °C, where the conductivity decreases with increase in temperature showing a metallic behavior, may be due to well dispersion of nickel particle in the sample. The conductivity of Ni-BCZY composite (prepared by LCP) varies between 1,050 and 1,590 S cm−1 in the measured temperature range, which is found to be much higher than that of Ni-YSZ based anodes used in conventional SOFCs [19–22] and Ni-BCY based anodes used in protonic SOFCs [23, 24]. This indicates that, as far as electrical conductivity is concerned, the Ni-BCZY composite prepared by LCP method could be a suitable anode material for the SOFC to run at lower temperatures.
The anode substrate was prepared by pressing the composite NiO-BCZY granules into disk, with diameter of 45 mm and thickness of 1.5 mm, by using a hot press. The temperature of hot press was maintained at 80 °C and a pressure of 50 MPa was applied. The anode substrate was pre-sintered at 1,100 °C (for 3 h) and then the electrolyte paste of BCZY was screen printed on the anode substrate and co-fired at 1,500 °C for 3 h. The electrolyte (BCZY) paste was screen printed three times, with each time after the screen print, the sample was air dried for 30 min and heat treated at 50 °C for 30 min. The electrolyte (BCZY) paste was prepared by using planetary ball mill, mixing appropriate ratio of BCZY powder, alpha-terpineol as solvent, organic binder and dispersing agent.
Figure 11a shows the cross-sectional image of co-fired anode-electrolyte layers. The electrolyte layer (with thickness ~18 μm) appears to be highly dense and exhibits pore free microstructure. Nickel oxide particles (gray in color in Fig. 11a) are appeared to be well dispersed. However, the anode substrate was found to be little denser than the required anode microstructure. Since the anode was already dense, the anode functional layer was not employed in this cell fabrication. The micrograph of electrolyte surface (Fig. 11b) also indicates that the electrolyte is highly dense and has pore free microstructure. The cell was then cut into 2 × 2 cm2 dimension by using diamond cutter. The cathode pastes of BCZY-LSFC (50:50 vol %) composite and LSCF were prepared by planetary milling the powders with appropriate ratio of solvent and binder. The BCZY-LSCF composite (one) layer and LSCF (two) layers were screen printed on the electrolyte surface in the area of 1 cm2. The full cell was fired at 1,050 °C for 2 h with continuous supply of air to the furnace at the rate of 200 sccm.
Performance of the proton-conducting SOFC cell NiO-BCZY/BCZY/BCZY-LSCF/LSCF was studied at 650 °C using the impedance gain/phase analyser (Solartron 1560) coupled with potentiostat. During the cell test, anode and cathode sides of the cell were separated by Pyrex glass sealing. Anode was first reduced at 650 °C by supplying the mixed gas of H2 and N2 to the anode side step by step in the (H:N) ratio of 10:90; 20:80; 40:60, 80:20; 100:0 (in sccm), and with continuous supply of air (at the rate of 100 sccm) to the cathode side. The open circuit voltage (OCV) of the cell is found to be above 1.1 V at 650 °C (with 100 % H2 supply), which implied that, the cell was well sealed and there was no electronic leakage through electrolyte. After the anode reduction process, the fuel (H2) and air was supplied to anode and cathode side, respectively, at the rate of 200 sccm and impedance measurement was carried out at OCV. The impedance plot of the cell at 650 °C is shown in Fig. 12 and it indicates that, the area specific resistance is quite high (i.e., about 100 Ω cm2). Figure 13 shows the current–voltage–power (I–V–P) characteristic curve of the cell obtained at 650 °C and it exhibits that the power density of the cell is about 6 mW/cm2. Comparing to the power density (2.35 mW/cm2 at 900 °C) of protonic SOFC based on Y-doped Barium zirconate (BZY) reported elsewhere [25], the performance of the present anode-supported cell is better but it is still much lower than that observed by Fabbri et al. [26] in case of electrolyte supported protonic SOFC cells.
Figure 14a, b shows the cross-sectional image of cell before and after test. Before the cell test, some cracks were observed in the cathode side as seen in Fig. 14a. After the cell test, the post-mortem analysis exhibits the delamination of cathode near previously existing cracks (Fig. 14b), which might be due to the thermal incompatibility of cathode (LSCF) with BZCY based proton-conducting electrolyte. So the delamination of cathode is expected be the reason for high electrode polarization observed in the impedance plot (Fig. 12). As a result, a lower power density of the cell is observed.
Conclusions
The proton-conducting solid electrolyte BaCe0.35Zr0.50Y0.15O3-δ prepared by solid-state reaction method is highly stable against CO2. AC electrical study of the sample was thoroughly studied and conductivity of the sample is found to be about 10−2 S cm−1 above 400 °C with an activation energy of 0.22 eV. The transition in conductivity of the sample occurs between 350 and 400 °C due to dissociation of protons from the dopant-proton defect pairs. High conductivity of electrolyte material extends the possibility of SOFC to run at very lower temperatures (i.e., down to 400 °C). The anode composite Ni-BaCe0.35Zr0.50Y0.15O3-δ prepared by LCP method exhibits very high value of conductivity under fuel cell operating condition and at reduced temperatures. The anode-supported planar proton-conducting SOFC was successfully processed by screen printing and co-firing method and tested at 650 °C. OCV of the cell at 650 °C is found to be above 1.1 eV, which is quite promising and indicates no electronic leakage through the electrolyte. The power density of the cell at 650 °C is found to be about 6 mW/cm2. Despite high conductivity of both electrolyte and anode composite, the performance of the cell is found to be poor, due to the high cathodic polarization as a result of cathode delamination. However, the performance of the cell can be improved with better cathode materials.
References
Ma, G., Shimura, T., Iwahara, H.: Ionic conduction and nonstoichiometry in Ba x Ce0.90Y0.10O3-α. Solid State Ion. 110, 103–110 (1998)
Bohn, H.G., Schober, T.: Electrical conductivity of the high-temperature proton conductor BaZr0.9Y0.1O2.95. J. Am. Ceram. Soc. 83, 768–772 (2000)
Park, J.-S., Lee, J.-H., Lee, H.-W., Kim, B.-K.: Low temperature sintering of BaZrO3-based proton conductors for intermediate temperature solid oxide fuel cells. Solid State Ion. 181, 163–167 (2010)
Zakowsky, N., Williamson, S., Irvine, J.T.S.: Elaboration of CO2 tolerance limits of BaCe0.9Y 0.1O3-δ electrolytes for fuel cells and other applications. Solid State Ion. 176, 3019–3026 (2005)
Bhide, S.V., Virkar, A.V.: Stability of BaCeO3-based proton conductors in water-containing atmospheres. J. Electrochem. Soc. 146, 2038–2044 (1999)
Katahira, K., Kohchi, Y., Shimura, T., Iwahara, H.: Protonic conduction in Zr-substituted BaCeO3. Solid State Ion. 138, 91–98 (2000)
Fabbri, E., D’Epifanio, A., Bartolomeo, E.D., Licoccia, S., Traversa, E.: Tailoring the chemical stability of Ba(Ce0.8−xZr x )Y0.2O3-δ protonic conductors for intermediate temperature solid oxide fuel cells (IT-SOFCs). Solid State Ion. 179, 558–564 (2008)
Kreuer, K.D.: Proton conducting oxides. Annu. Rev. Mater. Res. 33, 333–359 (2003)
Ricote, S., Bonanos, N., de Lucas, M.C.M., Caboche, G.: Structural and conductivity study of the proton conductor BaCe(0.9−x)Zr x Y0.1O(3-δ) at intermediate temperatures. J. Power Sources 193, 189–193 (2009)
Azad, A.K., Irvine, J.T.S.: Synthesis, chemical stability and proton conductivity of the perovksites Ba(Ce, Zr)1−x Scx O3-δ. Solid State Ion. 178, 635–640 (2007)
Barison, S., Battagliarin, M., Cavallin, T., Doubova, L., Fabrizio, M., Mortalò, C., Boldrini, S., Malavasi, L., Gerbasi, R.: High conductivity and chemical stability of BaCe1−x−yZr x Y y O3-δ proton conductors prepared by a sol–gel method. J. Mater. Chem. 18, 5120–5128 (2008)
Lee, J.-H., Heo, J.-W., Lee, D.-S., Kim, J., Kim, G.-H., Lee, H.-W., Song, H.S., Moon, J.-H.: The impact of anode microstructure on the power generating characteristics of SOFC. Solid State Ion. 158, 225–232 (2003)
Lee, H.W., Kim, J.S., Lee, J.H., Sung, H.S., You, J.Y., Lee, D.S., Heo, J.W., Shin, H.I.: United States Patent, Patent No. US 7,169,336, B2, (2007)
Kreuer, K.D.: On the development of proton conducting materials for technological applications. Solid State Ion. 97, 1–15 (1997)
Pavlenko, N.I., Stasyuk, I.: The superionic phase transition in hydrogen-bonded systems of the M3H(XO4)2 class: the effect of nearest-O(2) distortions on the proton dynamics. J. Phys. Condens. Matter 13, 4081–4095 (2001)
Yamazaki, Y., Blanc, F., Okuyama, Y., Buannic, L., Lucio-Vega, J.C., Grey, C.P., Haile, S.M.: Proton trapping in yttrium-doped barium zirconate. Nat. Mater. 12, 647–651 (2013)
Kamishima, O., Abe, Y., Ishii, T., Kawamura, J., Hattori, T.: Dielectric relaxation of protonic defects in hydrated and dehydrated SrZrO3:Yb. Solid State Ion. 177, 2375–2379 (2006)
Kjølseth, C., Fjeld, H., Prytz, Ø., Dahl, P.I., Estournès, C., Haugsrud, R., Norby, T.: Space–charge theory applied to the grain boundary impedance of proton conducting BaZr0.9Y0.1O3-δ. Solid State Ion. 181, 268–275 (2010)
Madsen, L.G., Larsen, P.H., Bonanos, N., Engell, J., Linderoth, S.: Mechanical strength and electrical conductivity of Ni-YSZ cermets fabricated by viscous processing. J. Mater. Sci. 41, 1097–1107 (2006)
Kim, S.-D., Moon, H., Hyun, S.-H., Moon, J., Kim, J., Lee, H.-W.: Performance and durability of Ni-coated YSZ anodes for intermediate temperature solid oxide fuel cells. Solid State Ion. 177, 931–938 (2006)
Fonseca, F.C., de Florio, D.Z., Esposito, V., Traversa, E., Muccillo, E.N.S., Muccilloa, R.: Mixed ionic-electronic YSZ/Ni COMPOSITE for SOFC anodes with high electrical conductivity. J. Electrochem. Soc. 153, A354–A360 (2006)
Zhu, W.Z., Deevi, S.C.: A review on the status of anode materials for solid oxide fuel cells. Mater. Sci. Eng. A 362, 228–239 (2003)
Chevallier, L., Zunic, M., Esposito, V., Bartolomeo, E.D., Traversa, E.: A wet-chemical route for the preparation of Ni–BaCe0.9Y0.1O3-δ cermet anodes for IT-SOFCs”. Solid State Ion. 180, 715–720 (2009)
Zunic, M., Chevallier, L., Radojkovic, A., Brankovic, G., Brankovic, Z., Bartolomeo, E.D.: Influence of the ratio between Ni and BaCe0.9Y0.1O3-δ on microstructural and electrical properties of proton conducting Ni–BaCe0.9Y0.1O3-δ anodes. J. Alloys Comp. 509, 1157–1162 (2011)
Shimada, H., Li, X., Hagiwara, A., Iharaa, M.: Proton-conducting solid oxide fuel cells with yttrium-doped barium zirconate for direct methane operation. J. Electrochem. Soc. 160, F597–F607 (2013)
Fabbri, E., Bi, L., Pergolesi, D., Traversa, E.: Towards the next generation of solid oxide fuel cells operating below 600 °C with chemically stable proton-conducting electrolytes. Adv. Mater. 24, 195–208 (2012)
Acknowledgments
This research was supported by the Fusion Research Program for Green Technologies through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0019297) and institutional program of KIST.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Baral, A.K., Choi, S., Kim, B.K. et al. Processing and characterizations of a novel proton-conducting BaCe0.35Zr0.50Y0.15O3-δ electrolyte and its nickel-based anode composite for anode-supported IT-SOFC. Mater Renew Sustain Energy 3, 35 (2014). https://doi.org/10.1007/s40243-014-0035-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-014-0035-4