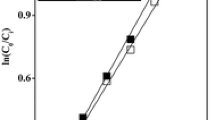
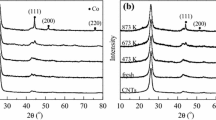
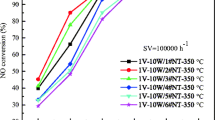
Abstract
TiO2 nanotubes supported amorphous Co-B(Co-B/TNTs) catalyst was prepared via impregnationchemical reduction procedure. The catalyst was characterized with transmission electron microscopy(TEM), ammonia temperature-programmed desorption(NH3-TPD), thermogravimetry-differential thermal analysis(TG-DTA), Fourier transform infrared spectroscopy(FTIR) and Raman spectroscopy. The effects of temperature and ratio of CO to H2 on the hydroformylation of 1-octene were studied. At an optimized reaction temperature(150 °C) and volume ratio of CO to H2(2:1), the conversion of 1-octene can reach 97.4% with a selectivity of 23.1% for total aldehydes and n/i-aldehyde molar ratio of 40:60. To obtain higher selectivity for linear aldehydes, Co-B/TNTs modified with triphenylphosphine for the hydroformylation of 1-octene were investigated. When molar ratio of P/Co was 4, the yield of total aldehydes was the highest(31.6%) with a good selectivity for linear product(n/i-aldehyde molar ratio was 70:30). In recycle use, the Co-B/TNTs catalyst modified with triphenylphosphine could be reused five times without reducing the activity and selectivity obviously. For a comparative study, all the Co-B/TNTs to catalyze the hydroformylation of other olefins exhibited high conversion under the optimized conditions.
Similar content being viewed by others
References
Piras I., Jennerjahn R., Jackstell R., Spannenberg A., Franke R., Beller M., Angew. Chem., 2011, 123(1), 294
Neves Â. C. B., Calvete M. J. F., Pinho e Melo T. M. V. D., Pereira M. M., Eur. J. Org. Chem., 2012, 32, 6309
Zhang L., Li C., Zheng X. L., Fu H. Y., Chen H., Li R. X., Catal. Lett., 2014, 144(6), 1074
Gonsalvi L., Guerriero A., Monflier E., Peruzzini M., Top Curr. Chem., 2013, 342, 1
Behr A., Reyer S., Tenhumberg N., Dalton Trans., 2011, 40(44), 11742
Zhang D. L., Fu H. Y., Zhao X. Y., Zhao H. W., Chen H., Liu Y. M., Li X. J., Chem. J. Chinese Universities, 2012, 33(8), 1835
Dabbawala A. A., Bajaj H. C., Bricout H., Monflier E., Appl. Catal. A: Gen., 2012, 413/414, 273
Cai Y., Li Z. H., Yang Y. Q., Yuan Y. Z., Chem. Res. Chinese Universities, 2002, 18(3), 311
Song K. C., Baek J. Y., Bae J. A., Yim J. H., Ko Y. S., Kim D. H., Park Y. K., Jeon J. K., Catal. Today, 2011, 164(1), 561
Wu L. P., Fleischer I., Jackstell R., Profir I., Franke R., Beller M., J. Am. Chem. Soc., 2013, 135(38), 14306
Krausová Z., Sehnal P., Bondzic B. P., Chercheja S., Eilbracht P., StaráI G., Saman D., Starý I., Eur. J. Org. Chem., 2011, 20/21, 3849
Bungu P. N., Otto S., Dalton Trans., 2011, 40(36), 9238
Vu T. V., Kosslick H., Schulz A., Harloff J., Paetzold E., Radnik J., Kragl U., Fuld G., Janiak C., Tuyen N. D., Micropor. Mesopor. Mater., 2013, 177, 135
Gniewek A., Trzeciak A. M., Top Catal., 2013, 56(13/14), 1239
Franke R., Selent D., Borner A., Chem. Rev., 2012, 112(11), 5675
Adint T. T., Landis C. R., J. Am. Chem. Soc., 2014, 136(22), 7943
Nairoukh Z., Blum J., J. Org. Chem., 2014, 79(6), 2397
Li X. M., Ding Y. J., Jiao G. P., Li J. W., Yan L., Zhu H. J., Chem. Res. Chinese Universities, 2009, 25(5), 738
Yang Y., Deng C., Yuan Y., J. Catal., 2005, 232(1), 108
Riisager A., Erikson K. M., Hjortkjaer J., Fehrmann R., J. Mol. Catal. A: Chem., 2003, 193(1/2), 259
Wilkes J. S., Green Chem., 2002, 4(2), 73
Makhubela B. C. E., Jardine A., Smith G. S., Green Chem., 2012, 14(2), 338
Li X., Li J. H., Li S. J., Fang X., Fang F., Chu X. Y., Wang X. H., Hua J. X., Chem. Res. Chinese Universities, 2013, 29(6), 1032
Zheng X. C., Zhang X. L., Wang S. P., Wang X. Y., Wu S. H., J. Nat. Gas Chem., 2007, 16(2), 179
Guan R. Q., Chao G. K., Ye C. P., Wang Y. X., Liu Y. M., Li H. H., Zhao Y. J., Tai Y. L., Chem. Res. Chinese Universities, 2014, 30(2), 284
Ma L., Peng Q. R., He D. H., Catal. Lett., 2009, 130(1/2), 137
Hu X. J., Shi Y. K., Zhang Y. J., Zhu B. L., Zhang S. M., Huang W. P., Catal. Commun., 2015, 59, 45
Aphairaj D., Wirunmongkol T., Niyomwas S., Pavasupree S., Ceram. Int., 2014, 40(7), 9241
Li H. X., Chen X. F., Wang M. H., Xu Y. P., Appl. Catal. A: Gen., 2002, 225(1/2), 117
Ivekovic D., Gajovic A., Ceh M., Pihlar B., Electroanal., 2010, 22(19), 2202
Zanella R., Rodríguez-Gonzalez V., Arzola Y., Moreno-Rodriguez A., ACS Catal., 2012, 2(1), 1
Gai L. G., Du G. J., Zuo Z. Y., Wang Y. M., Liu D., Liu H., J. Phys. Chem. C, 2009, 113(18), 7610
Patel N., Fernandes R., Guella G., Kale A., Miotello A., Patton B., Zanchetta C., J. Phys. Chem. C, 2008, 112(17), 6968
Birbeck J. M., Haynes A., Adams H., Damoense L., Otto S., ACS Catal., 2012, 2(12), 2512
Li B. T., Li X. H., Asami K. J., Fujimoto K. R., Energ. Fuel, 2003, 17(4), 810
Zhou G. B., Pei Y., Jiang Z., Fan K. N., Qiao M. H., Sun B., Zong B. N., J. Catal., 2014, 311, 393
Wu D., Zhang J. W., Wang Y. H., Jiang J. Y., Jin Z. L., Appl. Organometal. Chem., 2012, 26(12), 718
Wender I., Sternberg H. W., Orchin M., J. Am. Chem. Soc., 1953, 75(12), 3041
Heck R. F., Breslow D. S., J. Am. Chem. Soc., 1961, 83(19), 4023
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(Nos.21373120, 21301098, 21071086, 21271110), the National “111” Project of China’s Higher Education(No.B12015), the Applied Basic Research Programs of Science and Technology Commission Foundation of Tianjin, China(Nos.13JCQNJC02000, 12JCYBJC13100).
Rights and permissions
About this article
Cite this article
Shi, Y., Hu, X., Zhu, B. et al. Hydroformylation of 1-octene over nanotubular TiO2-supported amorphous Co-B catalysts. Chem. Res. Chin. Univ. 31, 851–857 (2015). https://doi.org/10.1007/s40242-015-5002-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-015-5002-9