Abstract
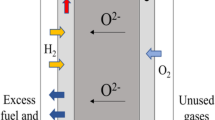
Perovskite oxide Ba0.5Sr0.5Fe0.9Nb0.1O3−δ (BSFN) as a cobalt-free cathode for intermediate-temperature solid oxide fuel cells(IT-SOFCs) on the Ce0.8Sm0.2O1.9(SDC) and La0.9Sr0.1Ga0.8Mg0.2O3−δ (LSGM) electrolytes was prepared and investigated. The single phase BSFN oxide with a cubic perovskite structure and relatively high electrical conductivities was obtained after sintering at 1250 °C for 10 h in air. The BSFN cathode exhibited excellent chemical stability on the SDC and LSGM electrolytes at temperatures below 950 °C. The area specific resistance of the BSFN cathode on the SDC and LSGM electrolytes were 0.024 and 0.021 Ω·cm2 at 800 °C, respectively. The maximum power densities of the single cell with BSFN cathode in 300 μm-thick SDC and LSGM electrolytes achieved 414 and 516 mW/cm2 at 800 °C, respectively. These results show that the BSFN material is a promising cobalt-free cathode candidate to be used in IT-SOFCs. A combination of the BSFN cathode and LSGM electrolyte is preferred owing to its excellent electrochemical performance.
Similar content being viewed by others
References
Xu L., Ding L., Dai X. F., Zhang J., Tian B. L., Liu S. Y., Qiao J. L., Chem. J. Chinese Universities, 2013, 34(1), 149
Steele B. C., Heinzel A., Nature, 2001, 414, 345
Jiang S. P., J. Mater. Sci., 2008, 3, 6799
Tsipis E. V., Kharton V. V., J. Solid State Electrochem., 2011, 15, 1007
Wei B., Lü Z., Huang X. Q., Liu M. L., Li N., Su W. H., J. Power Sources, 2008, 176, 1
Simner S. P., Bonnett J. F., Canfield N. L., Meinhardt K. D., Sprenkle V. L., Stevenson J. W., Electrochem. Solid State Lett., 2002, 5, A173
Zhao L., He B. B., Zhang X. Z., Peng R. R., Meng G. Y., Liu X. Q., J. Power Sources, 2010, 195, 1859
Niu Y., Zhou W., Sunarso J., Ge L., Zhu Z., Shao Z., J. Mater. Chem., 2010, 20, 9619
Zhou Q. J., Zhang L. L., He T. M., Electrochem. Commun., 2010, 12, 285
Ling Y. H., Zhang X. Z., Wang S. L., Liu X. Q., J. Power Sources, 2010, 195, 7042
Niu Y., Sunarso J., Liang F., Zhou W., Zhu Z., Shao Z., J. Electrochem. Soc., 2011, 158, B132
Xiao G. L., Liu Q., Wang S. W., Komvokisb V. G., Amiridisb M. D., Heydenb A., Ma S. G., Chen F. L., J. Power Sources, 2012, 202, 63
Jiang S. S., Zhou W., Niu Y. J., Zhu Z. H., Shao Z. P., ChemSusChem, 2012, 5, 2023
Yu X. L., Long W., Jin F. J., He T. M., Electrochim. Acta, 2014, 123, 426
Shao Z. P., Xiong G. X., Tong J. H., Dong H., Yang W. S., Sep. Purif. Tech., 2001, 25, 419
Wang S. L., Ionics, 2012, 18, 777
Huang S. G., Wang G. J., Sun X. H., Lei C. M., Li T., Wang C. C., J. Alloys Compd., 2012, 543, 26
Stevenson J. W., Armstrong T. R., Carneim R. D., Pederson L. R., Weber W. J., J. Electrochem. Soc., 1996, 143, 2722
Zhou Q. J., He T. M., He Q., Ji Y., Electrochem. Commun., 2009, 11, 80
Leng Y. J., Chan S. H., Khor K. A., Jiang S. P., Int. J. Hydrogen Energy, 2004, 29, 1025
Ling Y. H., Zhao L., Lin B., Dong Y. C., Zhang X. Z., Meng G. Y., Liu X. Q., Int. J. Hydrogen Energy, 2010, 35, 6905
Steele B. C. H., Solid State Ionics, 1996, 86–88, 1223
Yahiro H., Eguchi K., Arai H., Solid State Ionics, 1989, 36, 71
Huang K. Q., Tichy R. S., Goodenough J. B., J. Am. Ceram. Soc., 1998, 81, 2565
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(No.10974065) and the Applied Basic Research Programs of Science and Technology Development of Jilin Province, China(No.20130102011JC).
Rights and permissions
About this article
Cite this article
Long, W., Xu, H. & He, T. Preparation and electrochemical performance of cobalt-free cathode material Ba0.5Sr0.5Fe0.9Nb0.1O3−δ for intermediate-temperature solid oxide fuel cells. Chem. Res. Chin. Univ. 30, 806–810 (2014). https://doi.org/10.1007/s40242-014-4130-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-014-4130-y