Abstract
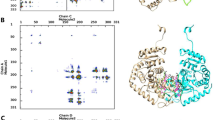
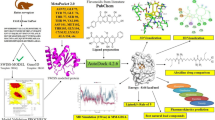
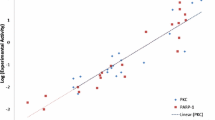
The hexokinase II enzyme is bound to the (VDAC1) channel in the form of a dimer and prevents the release of cell death factors from mitochondria to the cytoplasm. Studies have shown that blocking the binding of hexokinase II enzyme to (VDAC1) led to the initiation of apoptosis in cancer cells. No peptide has been designed so far to inhibit hexokinase II. The aim of this study was to inhibit the dimerization of enzyme subunits in order to inhibition the formation of (VDAC1) and the hexokinase II complex. In this study, the molecular dynamics simulation of the enzyme in monomer and dimer states was investigated in terms of RMSF, RMSD and radius of gyration. The following process involves extracting and designing variable-length peptides from the interacting segments of enzyme monomers. Using molecular dynamics simulation, the stability of the peptide was determined in terms of RMSD. Molecular docking was used to investigate the interaction between the designed peptides. Finally, the inhibitory effect of peptides on subunit association was measured using dynamic light scattering (DLS) technique. Our results showed that the designed peptides, which mimic common amino acids in dimerization, interrupt the bona fide form of the enzyme subunits. The result of this study provides a new way to disrupt the assembly process and thereby decreased the function of the hexokinase II.







Similar content being viewed by others
Data availability
All data created during this study are published in this article (and its supplementary files are included).
References
Abiy D, Vogel MC, W.M.A.M. Rabeh, (2017) Hexokinase II–derived cell-penetrating peptide targets mitochondria and triggers apoptosis in cancer cells. The FASEB Journal. https://doi.org/10.1096/fj.201601173R
Ahmed MC, Crehuet R, Lindorff-Larsen K (2020) Computing, analyzing, and comparing the radius of gyration and hydrodynamic radius in conformational ensembles of intrinsically disordered proteins. Intrinsically Disordered Proteins. https://doi.org/10.1007/978
Aslam PM et al (2023) Apoptosis promoting activity of selected plant steroid in MRMT-1 breast cancer cell line by modulating mitochondrial permeation pathway. Steroids. https://doi.org/10.1016/j.steroids.2022.109151
Belonoshko AB, Fu J, Smirnov G (2021) Free energies of iron phases at high pressure and temperature: molecular dynamics study. Phys Rev. https://doi.org/10.1103/PhysRevB.105.059903
Belvisi L, Domenico L, Jiménez MAS (2021) Peptides targeting protein-protein interactions: methods and applications. Mol Biosci. https://doi.org/10.3389/fmolb.2021.780106
Bessa C et al (2023) Counteracting colon cancer by inhibiting mitochondrial respiration and glycolysis with a selective PKCδ activator. Int J Mol Sci. https://doi.org/10.3390/ijms24065710
Burley SK et al (2023) RCSB Protein Data Bank (RCSB.org): delivery of experimentally-determined PDB structures alongside one million computed structure models of proteins from artificial intelligence/machine learning. Nucleic Acids Research. 51(D1): D488–D508. https://doi.org/10.1093/nar/gkac1077
Catalano G (2023) MCL1 regulates AML cells metabolism via direct interaction with HK2. Metabolic signature at onset predicts overall survival in AMLs’ patients. https://doi.org/10.1038/s41375-023-01946-5
Charih F, Biggar KK, Green JR (2022) Assessing sequence-based protein–protein interaction predictors for use in therapeutic peptide engineering. Scientific Reports. https://doi.org/10.1038/s41598-022-13227-9
Charitou V (2022) Cyclization and docking protocol for cyclic peptide-protein modeling using HADDOCK24. J. Chem. Theory Comput. 18(6):4027–4040. https://doi.org/10.1021/acs.jctc.2c00075
Ciscato F et al (2021) Analysis of the effects of hexokinase 2 detachment from mitochondria-associated membranes with the highly selective peptide HK2pep. Bio-protocol. 11(14):4087. https://doi.org/10.21769/BioProtoc.4087
Fukutani T et al (2021) G-RMSD: root mean square deviation based method for three-dimensional molecular similarity determination. CSJ. https://doi.org/10.1246/bcsj.20200258
Giaquinto N et al (2022) (2022) Estimated cases and deaths in. Cancer J Clin. 72:524–541. https://doi.org/10.3322/caac.21718
Guex NAP (1997) SWISS-MODEL and the swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723. https://doi.org/10.1002/elps.1150181505
Hashemi ZS et al (2021) In silico approaches for the design and optimization of interfering peptides against protein–protein interactions. Biosci, Mol. https://doi.org/10.3389/fmolb.2021.669431
Holsbeeck PV, Martins JC, and Ballet S (2022) Downsizing antibodies: Towards complementarity-determining region (CDR)-based peptide mimetics. https://doi.org/10.1016/j.bioorg.2021.105563
Hosseini-Gerami L et al (2023) Benchmarking causal reasoning algorithms for gene expression-based compound mechanism of action analysis. BMC Bioinformatics. https://doi.org/10.1186/s12859-023-05277-1
Hosseinzadeh P et al (2021) Anchor extension: a structure-guided approach to design cyclic peptides targeting enzyme active sites. Nat Commun 12:3384. https://doi.org/10.1038/s41467-021-23609-8
Hu H et al (2022) Mitochondrial VDAC1: A potential therapeutic target of inflammation-related diseases and clinical opportunities cells. https://doi.org/10.3390/cells11193174
Huda MM, Jahan N, Rai N (2021) Effect of water models on structure and dynamics of lignin in solution. AIP Advances. DOI 10(1063/5):0047974
Islam R et al (2021) A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1761883
Juret D (2022) Designed multifunctional peptides for intracellular targets antibiotics. 11.
Ke P et al (2022) Effects of thermostats/barostats on physical properties of liquids by molecular dynamics simulations. J Mol Liq 365:120116. https://doi.org/10.1016/j.molliq.2022.120116
Laskowski RA, Chistyakov VV, Thornton JM (2005) PDBsum more: new summaries and analyses of the known 3D structures of proteins and nucleic acids. Nucleic Acids Research. 33:D266–D268. https://doi.org/10.1093/nar/gki001
Lee P et al (2022) Size distribution monitoring for chemical mechanical polishing slurries: an intercomparison of electron microscopy, dynamic light scattering, and differential mobility analysis. Powder Technol 396:395–405. https://doi.org/10.1016/j.powtec.2021.10.045
Lei Y et al (2021) A deep-learning framework for multi-level peptide–protein interaction prediction. Nat Commun. https://doi.org/10.1038/s41467-021-25772-4
Li X et al (2023) Bergenin inhibits tumor growth and overcomes radioresistance by targeting aerobic glycolysis. Am J Chin Med Online Ready. https://doi.org/10.1142/S0192415X23500842
Liang X et al (2021). Large-scale comparative review and assessment of computational methods for anti-cancer peptide identification Briefings in Bioinformatics. https://doi.org/10.1093/bib/bbaa312
Lin E, Lin C-H, Lane H-Y (2022) De novo peptide and protein design using generative adversarial networks: an update. J Chem Inf Model 64(4):761–774
Liu X et al (2022) Hexokinase 2 promotes cell proliferation and tumor formation through the wnt/β-catenin pathway-mediated cyclin d1/c-myc upregulation in epithelial ovarian cancer. J Cancer 13(8):2559–2569. https://doi.org/10.1016/j.intimp.109556
Liu L et al (2023) Network pharmacology, molecular docking and molecular dynamics to explore the potential immunomodulatory mechanisms of deer antler. Int J Mol Sci. https://doi.org/10.3390/ijms241210370
Mallet V et al (2022) InDeep: 3D fully convolutional neural networks to assist in silico drug design on protein–protein interactions. Bioinformatics 38(5):1261–1268. https://doi.org/10.1093/bioinformatics/btab849
Marie TJ et al (2021) Designing stapled peptides to inhibit protein-protein interactions: An analysis of successes in a rapidly changing field. Peptide Science. https://doi.org/10.1002/pep2.24191
Morris CJ, Corte DD (2021) Using molecular docking and molecular dynamics to investigate protein-ligand interactions. Modern Physics Letters. https://doi.org/10.1142/S0217984921300027
Paul K et al (2021) Supply chain recovery challenges in the wake of COVID-19 pandemic. J Bus Res 136:316–329. https://doi.org/10.1016/j.jbusres.07.056
Porto F et al (2022) Sense the moment: A highly sensitive antimicrobial activity predictor based on hydrophobic moment. Biochimica et Biophysica Acta (BBA). https://doi.org/10.1016/j.bbagen.130070
Roy ST et al (2022) 3-Bromopyruvate inhibits pancreatic tumor growth by stalling glycolysis, and dismantling mitochondria in a syngeneic mouse model. Am J Cancer Res 12(11):4977–4987
Senadheera TRL et al (2022) In silico analysis of bioactive peptides produced from underutilized sea cucumber by-products—a bioinformatics approach. Mar Drugs. https://doi.org/10.3390/md20100610
Shegay PV et al (2023) Moonlight functions of glycolytic enzymes in cancer. Protein Biochemistry for Basic and Applied Sciences. https://doi.org/10.3389/fmolb.2023.1076138
Świrniak P, Mroczka J (2022) Forward and inverse analysis for particle size distribution measurements of disperse samples. Measurement. https://doi.org/10.1016/j.measurement.110256
Tiberti M, Mutate X et al (2022) an automated pipeline for in silico saturation mutagenesis of protein structures and structural ensembles. Briefings in Bioinformatics. https://doi.org/10.1093/bib/bbac074
Tina KG, Bhadra RAS (2007) PIC: protein interactions calculator. Nucleic Acids Res. https://doi.org/10.1093/nar/gkm423
Vangone A et al (2011) COCOMAPS: a web application to analyse and visualize contacts at the interface of biomolecular complexes. Bioinformatics 27:2915–2916. https://doi.org/10.1093/bioinformatics/btr484
William F, PortoabÁllan S, Octavio PALF (2017) Antimicrobial activity predictors benchmarking analysis using shuffled and designed synthetic peptides. J Theor Biol. 426(7):96–103. https://doi.org/10.1016/j.jtbi.2017.05.011
Wolf P, Edlich F, Edlich F (2022) How do hexokinases inhibit receptor-mediated apoptosis? Biology. https://doi.org/10.3390/biology11030412
Wu S et al (2021) Effects of SARS-CoV-2 mutations on protein structures and intraviral protein–protein interactions. JMV. https://doi.org/10.1002/jmv.26597
Xian P et al (2023) Hexokinase inhibitor 2-deoxyglucose coordinates citrullination of vimentin and apoptosis of fibroblast-like synoviocytes by inhibiting HK2 /mTORC1-induced autophagy. Int Immunopharmacol. https://doi.org/10.1016/j.intimp.109556
Xu Q et al (2023) Projections of cancer mortality by 2025 in central China: A modeling study of global burden of disease 2019. Heliyon. https://doi.org/10.1016/j.heliyon.2023.e13432
Xue Y-N et al (2019) Zinc and p53 disrupt mitochondrial binding of HK2 by phosphorylating VDAC1. Exp Cell Res 374(1):249–258. https://doi.org/10.1016/j.yexcr.2018.12.002
Xue L et al (2022) Hexokinase 2 Is a pivot for lovastatin-induced glycolysis-to-autophagy reprogramming in triple-negative breast cancer cells. J Cancer 13(12):3368–3377. https://doi.org/10.7150/jca.71592
Xue LC et al (2016) PRODIGY: a web server for predicting the binding affinity of protein–protein complexes Bioinformatics. 32(23): 3676–3678. https://doi.org/10.1093/bioinformatics/btw514
Zhang F, Angelova A, N.a.Z. Li, (2021) Mitochondrial voltage-dependent anion channel 1–hexokinase-ii complex-targeted strategy for melanoma inhibition using designed multiblock peptide amphiphiles. ACS Publ 13(30):35281–35293. https://doi.org/10.1021/acsami.1c04385
Zhang S et al (2022) Bioinformatics and computer simulation approaches to the discovery and analysis of bioactive peptides. Curr Pharm Biotechnol 23(13):1541–1555. https://doi.org/10.2174/1389201023666220106161016
Zheng M et al (2021) Novel selective hexokinase 2 inhibitor Benitrobenrazide blocks cancer cells growth by targeting glycolysis. Pharmacol Res. https://doi.org/10.1016/j.phrs.2020.105367
Zheng P et al (2022) Kaempferol impairs aerobic glycolysis against melanoma metastasis via inhibiting the mitochondrial binding of HK2 and VDAC1. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2022.175226
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors:1. Faranak Karamifard 2. Mahta Mazaheri 3. Ali Dadbinpour have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
Corresponding author
Ethics declarations
Conflict of interest
This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue. The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karamifard, F., Mazaheri, M. & Dadbinpour, A. Abatement of the binding of human hexokinase II enzyme monomers by in-silico method with the design of inhibitory peptides. In Silico Pharmacol. 12, 30 (2024). https://doi.org/10.1007/s40203-024-00201-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40203-024-00201-8