Abstract
Background
Subjective cognitive decline (SCD) and mild cognitive impairment (MCI) may occur as preclinical stages of Alzheimer's disease (AD), ultimately leading to dementia. Glycated hemoglobin A1c (HbA1c) is a diagnostic marker for diabetes mellitus and indicates mortality risk.
Objectives
This university-based, exploratory retrospective study examined the impact of HbA1c serum level on 5-year mortality among individuals with cognitive impairment.
Methods
Included were 1076 subjects aged at least 50 years who visited the Memory Outpatient Clinic of the Medical University of Vienna due to memory problems. Participants were diagnosed with SCD, MCI, or AD subsequent to neurological examination, standard laboratory blood tests, and neuropsychological testing. Survival was compared between diagnostic subgroups and with respect to HbA1c categories using log-rank tests based on Kaplan–Meier functions. The Neuropsychological Test Battery Vienna (NTBV) was dimensionally reduced, and a principal component analysis (PCA) was performed to further analyze results. Corresponding factor scores, HbA1c values, and baseline characteristics were included in Cox proportional hazards models to assess 5-year mortality risk.
Results
During the observation period, 323 patients (30%) died at a mean age comparable between diagnostic subgroups (SCD 84.2 ± 10.1, MCI 81.2 ± 8.3, AD 82.2 ± 7.4 years). Individuals with normal serum HbA1c levels had significant advantages in survival within the MCI (12.9 ± .3 vs. 10.0 ± .8 years) and the AD subgroups (8.2 ± .4 vs. 5.5 ± .6 years), and metric HbA1c predicted 5-year mortality (HR 1.24).
Conclusion
This study demonstrates an association between abnormal HbA1c serum levels and increased mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In later life, cognitive performance declines as a part of normative aging processes [1]. In some individuals, however, a more pronounced deterioration can be detected, objectified and quantified with the aid of neuropsychological tests [2]. Due to an increasing awareness about dementia in the general population, the concept of subjective cognitive decline (SCD) has been established [3]. SCD refers to individuals' concerns about cognitive decline that cannot be objectified by neuropsychological testing [3, 4]. If cognitive deficits are manifested, but everyday life is fundamentally still manageable, the term mild cognitive impairment (MCI) is applied [5]. A more extensive cognitive decline and the resulting deficits and inabilities in everyday functions are clinical features of dementia [2], most commonly caused by Alzheimer's disease (AD) [6]. After a years-long asymptomatic preclinical phase, AD progresses toward an amnestic dementia syndrome [6]. MCI and SCD are considered preliminary stages that may occur in some, but not all, individuals suffering from AD. [3, 4, 6] Increased mortality rates among AD patients have been reported [6].
Glycated hemoglobin A1c (HbA1c) is created by non-enzymatic chemical reaction processes with glucose, whereby the resulting quantity correlates with the average blood glucose level [7]. Therefore, HbA1c serum level may be used as a diagnostic tool to detect diabetes mellitus (DM) [8]. It is considered a risk factor for all-cause mortality[9,10,11] and has been described as a predictor of cognitive decline [12,13,14,15]. Common underlying pathophysiological principles between AD and DM are being discussed [12, 13, 16]. Furthermore, HbA1c level was shown to influence both disease risk and progression [17, 18].
We hypothesize an increased 5-year mortality among cognitively impaired individuals with elevated HbA1c compared to normal serum levels. In the present study, we aim to investigate the relationship between HbA1c status and 5-year mortality in patients attending a memory outpatient clinic.
Materials and Methods
This retrospective single-center analysis was based on data collected by the Department of Neurology of the Medical University of Vienna between November 1, 2004 and March 8, 2020. The HbA1 laboratory value was measured routinely from the beginning of the observation period. The study protocol of this analysis was approved by the ethics committee of the Medical University of Vienna under EK No. 2345/2019 and followed the principles of the Declaration of Helsinki.
Participants
Study subjects visited the memory outpatient department due to self-reported memory problems or referrals by physicians. After undergoing neurological examination and neuropsychological testing, participants were assigned to an AD, MCI, or SCD subgroup. In accordance with Jessen et al., SCD classification criteria included (a) the presence of subjective cognitive deterioration as manifested by seeking of medical help for cognitive problems and (b) the current absence of any objectively measurable cognitive deficits (mean z-scores in each cognitive domain greater than -1.5 SD) [4, 19]. MCI was classified according to Petersen criteria, requiring a z-score of 1.5 SD below age and education-corrected norms [5, 19]. AD demented patients were diagnosed using the NINCDS-ADRDA criteria [2]. Furthermore, participants’ blood samples were obtained to measure HbA1c serum level. Those included in this study were individuals aged 50 years and above who completed at least the Mini Mental Status Examination (MMSE)[20] and had their HbA1c serum level measured from blood samples collected within ± one month of the examination date. Any conditions affecting cognition, such as stroke, head trauma, organ failure, psychiatric conditions that may cause pseudo-dementia or non-AD dementia, were exclusion criteria.
Participants’ sociodemographic data, neuropsychological testing results and diagnostic subgroup assignments were imported into an SPSS® file and listed by means of identification numbers. Participants were thus identified in the Allgemeines Krankenhaus Informations Management (AKIM), where the clinic records all services, to acquire their HbA1c blood test results. All available participant death data up to March 8, 2020, was gathered from the Research, Documentation and Analysis (RDA) system of the Medical University of Vienna. This system is updated annually and synchronized with the death data from Statistik Austria. In cases where there were no death data available, either from the RDA or the AKIM, it was concluded that the relevant participants were still alive at the end of study.
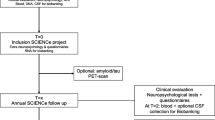
After all data were imported, 2450 protocols were registered. During data cleaning, 1351 cases were removed due to missing HbA1c data. Furthermore, 22 duplicate entries and one case aged under 50 years had to be excluded. Figure 1 displays the the data screening process to obtain 1076 valid patient protocols that met all inclusion and exclusion criteria.
HbA1c serum level
Blood samples for standard laboratory testing were provided by the Department of Neurology at the Medical University of Vienna. All samples were collected ± one month from neuropsychological assessment. HbA1c serum level was measured by the Clinical Institute for Laboratory Medicine of the General Hospital Vienna (AKH-Wien) using high-performance liquid chromatography (HPLC). The reference value in healthy subjects is 4.0–6.0 relative % and does not differ in gender; thus, reference values for men and women are not separated [21]. Values ranging from 5.7 to 6.49% indicate prediabetes [22]. Those 6.5% and above allow the diagnosis of diabetes mellitus to be established [23].
Neuropsychological instruments / inventories
Neuropsychological testing was performed in the memory outpatient department of the Medical University of Vienna on each subject in one session. The Mini Mental State Examination (MMSE) was used as a screening tool for cognitive impairment. Healthy individuals may achieve up to 30 points; reduced scores correlate with the presence of cognitive impairment [20]. Symptoms of depression were assessed using the Geriatric Depression Scale short form (GDS-15) [24, 25]. Omitting physical factors that depend on age [26], this self- rating screening tool is suitable for the elder population as well as for cognitively impaired individuals [25, 26]. While up to four points are considered normal, scores of five points and above indicate mild, moderate, or severe depression. To assess their cognitive performance across multiple domains, participants underwent the Neuropsychological Test Battery Vienna short version (NTBV-15). This standardized clinical-use test kit was developed and validated to identify patients at risk for cognitive deterioration and to detect cases of dementia early on in a clinical setting. This inventory covers psycho-motor-speed, attention, language, memory, and executive function [27, 28]. The NTBV can be obtained from www.psimistri.com. Because of redundancy, MMSE results are not being further discussed or analyzed in this study.
Statistical analysis
Descriptive and inferential statistical analyses were performed using IBM SPSS® 27 for Windows 10®. Metric variables are presented here as mean (M) ± standard deviation (SD), minimum (min) and maximum (max). In cases of skewed distribution according to the nonparametric Kolmogorov–Smirnov and Shapiro–Wilk tests [29], the alternative measure median (Mdn) and interquartile range (IQR, 25–75%) are given.
In all analyses, the per-protocol approach was used, i.e., the available patient data were examined, and no imputations were made in case of missing values. Two-tailed statistical tests were performed, assuming an α = 5% significance level. Thus, results with p ≤ 0.05 are described as “significant”. To prevent type 1 error accumulation in the case of multiple testing, the significance level was adjusted according to Bonferroni using the equation α* = (\(\frac{\alpha }{k}\)). Kruskal–Wallis tests were used to compare dependent variables between the independent diagnostic subgroups, followed by Mann–Whitney U-tests for posthoc pairwise comparison in case of significance [29]. Survival rates and mean and median estimated survival times were calculated based on Kaplan–Meier (KM) functions, taking censored cases into account, with respect to the diagnostic subgroups and depending on the HbA1c category [30]. To address our primary research question, posthoc pairwise log-rank tests were conducted to assess differences between subgroups within the sample [31, 32]. Multivariate Cox proportional hazards models were used to calculate hazard ratios (HR) corresponding to the predictive value of metric and categorical HbA1c serum levels for 5-year survival. Covariates, including GDS-15 results, NTBV-15 factor scores, and demographic variables, were selected based on previous analysis. Predictors and covariates were added block-wise and simultaneously using the enter method [33, 34].
Results
HbA1c serum level
HbA1c % at admission was assessed close to neuropsychological testing; the mean time difference regarding cognitive testing was 0.1 ± 0.3 (min -0.9–max 1.0) months. HbA1c values showed a skewed distribution according to significant Kolmogorov–Smirnov testing in all diagnostic subgroups (ps < 0.05). Kruskal–Wallis testing revealed no significant difference in HbA1c serum level according to diagnostic subgroups, H (χ2, df 2) = 2.910, p = 0.233. However, when the ADA categories 4.0–5.69 (norm), 5.7– 6.49 (prediabetic), and ≥ 6.5% (diabetic) were applied in reference to the HbA1c serum level, Chi-square testing revealed a significant distribution difference, χ2 (df 4) = 10.853, p = 0.028, indicating heterogeneity of proportions. See Table 1 for descriptive statistics regarding metric and and categorical HbA1c level.
NTBV-15 principal component analysis
The NTBV-15 analysis comprised the data protocols of 902 individuals who completed neuropsychological testing. According to the computed KMO value of 0.90, the variance in response behavior on the NTBV-15 subscales could be explained using fewer dimensions (factors) [35]. An explorative principal component factor analysis (PCA) was employed to achieve dimensional reduction. The subsequently performed Kaiser’s Varimax orthogonal rotation converged at the 11th iteration [35]. The communality hi2 (≤ 1), the row sum of the squared loadings of an item across the extracted factors, the eigenvalue λ (≥ 1), and the column sum of the squared loadings per factor across the items are relevant indicators to interpret the results of a factor analysis [33, 36]. Based on the function of the eigenvalue λ in the scree plot, four factors describing the cognitive structure of the subjects were determined. Thus, a cumulative 77.0% of variance could be explained, while the communality hi2 reached fairly high values for all subtests [33]. The resulting factor scores were mutually uncorrelated (r = 0) and z-standardized (μ = 0, σ = 1). As they were weighted and unaffected by the polarity sign of the loadings, they were suitable for further analysis without loss of information [37]. The item loadings of the NTBV-15 subtests within the four computed factors, including the communality and eigenvalue, are displayed in Table 2.
A pattern of cognitive structure can be assumed from performances on subtests that load mainly on one factor. Considering the group of subtests loading on the same factor, it is proposed to name the dimensions F1 Attention, F2 Memory, F3 Executive functions and F4 Verbal fluency and naming, in alignment with a previous publication on the NTBV [38]. Conducting a Levene test revealed heterogeneity of variance among the four factor scores in the three diagnostic subgroups (p < 0.001). Welch ANOVAs were performed to compare the performance between the three diagnostic subgroups, taking the Bonferroni correction (α* = 0.0125) into account [34]. This revealed significant differences for all four factors (F(2, 899) = 99.654; F(2, 899) = 184.562; F(2, 899) = 19.296; F(2, 899) = 40.167; ps < 0.001), with effect sizes ranging from small (0.04; Executive functions) to large (0.29; Memory). Posthoc pairwise analyses using Games-Howell procedure[29] and considering the Bonferroni correction (α* = 0.0167), revealed a hierarchy among all four factors SCD > MCI > AD (ps < 0.01).
Assessing 5-year survival
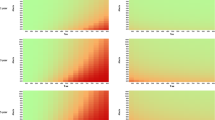
According to Kaplan–Meier (KM) survival functions, and considering the information of deceased and censored participants within the follow-up time, the 5-year survival rates of the diagnostic subgroups could be assumed to be 97.6% for SCD, 89.2% for MCI and 70.0% for AD. Overall, significant differences in survival times were observed for the three diagnostic subgroups using log-rank testing, χ2(2) = 142.608, p < 0.001 (n = 1076). Posthoc pairwise comparisons for median and mean survival regarding the three diagnostic subgroups using log-rank tests revealed for SCD (Mdn = 15.2, M = 13.9) vs. MCI (M = 12.2) years, χ2(1) = 6.764, p = 0.009, SCD vs. AD (Mdn = 7.2, M = 8.0) years, χ2(1) = 48.110, p < 0.001 and MCI vs. AD, χ2(1) = 112.681, p < 0.001 either significant differences for overall survival after study entrance. Subsequently, KM functions regarding HbA1c categories were calculated for all three diagnostic subgroups separately, as shown in Fig. 2.
KM survival functions regarding HbA1c % categories, considering the diagnostic subgroups (SCD n = 95, MCI = 542, AD = 439), taking 5- survival likelihood into account. Abbreviations: SCD, subjective cognitive decline; MCI, mild cognitive impairment; AD, Alzheimer's disease; HbA1c, glycated hemoglobin A1c; DM, diabetes mellitus
Log-rank tests and posthoc pairwise comparisons of the survival functions, taking the Bonferroni adjustment (α* = 0.0167) into account, revealed an advantage in survival time for the normal HbA1c category compared to the diabetic category within the MCI subgroup (M = 12.92 vs. 10.01 years, χ2(1) = 12.224, p < 0.001). In the AD subgroup, both normal and prediabetic HbA1c serum levels showed an advantage in median survival time over the diabetic category (Mdn = 8.06 vs. 5.62 years, χ2(1) = 16.193, p < 0.001 and 7.84 vs. 5.62 years, χ2(1) = 14.963, p < 0.001). It should be noted that this function could not be estimated principally for the SCD subgroup, and mean estimates are given here instead of median survival for the MCI subgroup, as the latter could only be calculated if the KM-function declined below 50%.
Table 3 displays the mean and median estimates for the MCI and AD groups.
To estimate the 5-year survival probability within the sample, multivariate analyses were performed using Cox proportional hazards models, taking follow-up time into account. Considering predictors and covariates, there were 859 complete records available, including 96 events and 763 censored cases, in the observation period. Predictors were added in a blockwise, hierarchical approach, using the enter method. In a first step (model I), we assessed metric HbA1c % in the first block, followed by sociodemographic variables such as age, gender, and years of education in the second block, depressiveness (GDS-15) in the third block, and the four cognitive domains according to the NTBV-15 in the fourth block as predictors for the 5-year mortality criterion. The summarized results of the final model step are shown in Table 4.
Model I results indicated significant explanatory value for metric HbA1c % (HR 1.24; p = 0.019), for age at testing, for male gender (ps < 0.001), and for GDS-15 depressiveness (p < 0.01) as predictors of 5-year mortality, but not of years of schooling. Considering the NTBV-15 factors, the inverted values (1/HR) of the hazard ratios for Attention and Memory showed an increased mortality risk in proportion to poorer test performance (ps < 0.01), whereas Executive functions and Verbal fluency and naming had no significant explanatory value predicting mortality.
In a second step (model II), the same Cox model was applied, but in block I, categorical HbA1c serum levels (HbA1c categories {0} norm, {1} prediabetic, {2} diabetic) were evaluated as dummy-coded predictors, considering norm as the reference category. According to block I analysis, diabetic HbA1c showed significant evidence (HR 2.73; p < 0.001) of increased relative risk for 5-year mortality, while no significant explanatory value could be derived for prediabetes. Subsequently, the second, third, and fourth blocks were added in the same manner as for model I. The summarized results of model II analysis, including all covariates of all four blocks, are shown in Table 5.
According to full model II analysis, diabetic long-term blood glucose HbA1c no longer showed significant explanatory value, but indicated a trend as a categorical predictor (HR 1.59), whereas significant explanatory values for the relative risk of mortality within 5 years could be assumed (ps < 0.01) for increasing age (HR 1.08), male gender (inverted HR 2.14) and GDS-15 depressiveness (HR 1.10). Furthermore, no significant contribution could be derived for school attendance. Considering the NTBV-15 domains, an inverted hazard ratio HR was determined (Attention 1.35 and Memory 1.47), showing an increasing relative risk of mortality in proportion to poorer test performance (ps < 0.01), whereas no significant relative risk of mortality was found for Executive functions and Verbal fluency and naming.
Discussion
By the end of the study, 323 (30%) deaths had been recorded. Survivors were treated as censored cases in the context of Kaplan–Meier plots. With a median age of 72.9 years at study entry, overall median survival was 13.4 years. This is similar to the proposed median further life expectancy of 12.1 years and 14.4 years, separated for men and women among the same-aged Viennese general population, according to 2021 mortality tables [39]. The AD subgroup (Mdn 76.8 years old) showed a 7.2-year median survival, which was considerably shorter than the expected 9.8 and 11.6 further live-years in peer males and females [39]. The literature reports relatively similar survival periods among AD patients: Ganguli etl al [40]. found a mean survival of 5.9 years from the age of 80.2, while Williams et al.[41] observed a mean survival time of 8.5 years after onset at 74.6 years. Other authors observed a survival from 4 to 8 years among 65-year olds from the point of diagnosis [6]. The survival function did not decline below 50% for MCI, so no median survival time could be calculated, and mean estimates were given instead. Within the SCD subgroup, the observed median survival time was similar to further live-years expectancy among Viennese peers [39].
In pairwise comparisons regarding survival time using log rank tests, the SCD subgroup held an advantage over MCI and AD participants. However, all three diagnostic subgroups shared a similar median age at death, while the age at study entry showed a hierarchy in reverse order. This observation reflects the proposed stadial course of AD with SCD and MCI as preclinical stages that may occur years prior to the demential syndrome in comparably younger individuals [3, 4]. When survival curves related to HbA1c categories in the three diagnostic subgroups were compared separately, a survival advantage for norm over diabetic became apparent in the MCI subgroup. Among AD patients, comparisons revealed an advantage in survival time for both the norm and the prediabetic category over diabetic HbA1c values. In a finding that supported our primary hypothesis, we observed increased mortality among cognitively impaired individuals with elevated compared to normal HbA1c serum levels. Meta-analyses examining the association between HbA1c and mortality in different settings resulted in similar findings [8,9,10,11].
Furthermore, the Cox proportional hazards model assessing 5-year mortality risk indicated predictive value for metric HbA1c serum level among patients at different stages of cognitive decline. This result could be expected, as the literature reports an increased risk of mortality for both Alzheimer’s disease (AD) dementia patients and non-demented individuals with elevated HbA1c serum level [8, 42]. However, when HbA1c serum level categories norm, prediabetic and diabetic were applied, none of them predicted 5-year mortality. According to the present data, mortality generally increases with HbA1c; however, the category diabetic is not indicative. Bi-directional effects between HbA1c and mortality in diabetic individuals have been described in the literature. Accordingly, mortality risk increases with both elevated and very low HbA1c serum levels [10]. More specifically, in healthy subjects, mortality increases at an HbA1c serum level > 6%, whereas in diabetic patients, mortality increases > 7.5% [8, 10].
Cox proportional hazards model results also indicated predictive value for age, male gender, and GDS-15 severity, as well as the domains F1 Attention and F2 Memory regarding mortality. These findings are consistent with those in a previous publication on the NTBV and have been discussed there to a greater extent [38].
The retrospective design of the present study limits its informative value. Only 859 out of 1076 participants (79.8%) completed the NTBV-15 and thus were suitable for our Cox proportional hazards models. In the absence of a healthy control group, relevant study parameters were compared with regard to the three diagnostic subgroups, and survival expectancy estimates in age peers were given to put the results into perspective. Furthermore, data on participants’ intake of antidiabetic medication were not available; distorting effects on HbA1c serum levels cannot be excluded. In addition, modified target values for HbA1c, which are derived from observations regarding mortality in diabetic individuals, could not be taken into account [18, 23].
In future studies, data on participants’ metabolic status and their antidiabetic drug intake should be collected during outpatients’ visits to examine relations between HbA1c serum level and mortality considering specific target values.
To conclude, the observed survival time among AD diagnosed individuals is consistent with previous studies and reduced compared to peers. Kaplan–Meier functions indicate an survival advantage for normal over diabetic HbA1c serum level in patients with MCI and AD in the present study. Cox proportional hazards model results show predictive value indicating 5-year mortality for metric but not categorized HbA1c % serum level among patients with cognitive decline. Female gender is associated with higher survival probability, while age, depressive symptoms, and poor results in neuropsychological testing increase risk of mortality. Our findings underscore the potential importance of monitoring HbA1c levels as a part of comprehensive care for individuals with cognitive deterioration.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Steinerman JR, Hall CB, Sliwinski MJ, Lipton RB. Modeling cognitive trajectories within longitudinal studies: a focus on older adults. J Am Geriatr Soc. 2010;58(Suppl 2):S313–8.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurol. 1984;34(7):939–44.
Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271–8.
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844–52.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94.
Alzheimer's Assocation. 2020 Alzheimer's disease facts and figures. Alzheim Dementia. 2020;16(3):391–460.
De Rosa MC, Sanna MT, Messana I, Castagnola M, Galtieri A, Tellone E, et al. Glycated human hemoglobin (HbA1c): functional characteristics and molecular modeling studies. Biophys Chem. 1998;72(3):323–35.
Cavero-Redondo I, Peleteiro B, Alvarez-Bueno C, Rodriguez-Artalejo F, Martinez-Vizcaino V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7(7): e015949.
Pan W, Lu H, Lian B, Liao P, Guo L, Zhang M. Prognostic value of HbA1c for in-hospital and short-term mortality in patients with acute coronary syndrome: a systematic review and meta-analysis. Cardiovasc Diabetol. 2019;18(1):169.
Arnold LW, Wang Z. The HbA1c and all-cause mortality relationship in patients with type 2 diabetes is J-shaped: a meta-analysis of observational studies. Rev Diabet Stud. 2014;11(2):138–52.
Schöttker B, Rathmann W, Herder C, Thorand B, Wilsgaard T, Njølstad I, et al. HbA1c levels in non-diabetic older adults - No J-shaped associations with primary cardiovascular events, cardiovascular and all-cause mortality after adjustment for confounders in a meta-analysis of individual participant data from six cohort studies. BMC Med. 2016;14:26.
Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14(3):168–81.
Chornenkyy Y, Wang WX, Wei A, Nelson PT. Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol. 2019;29(1):3–17.
Ekblad LL, Rinne JO, Puukka P, Laine H, Ahtiluoto S, Sulkava R, et al. Insulin Resistance Predicts Cognitive Decline: An 11-Year Follow-up of a Nationally Representative Adult Population Sample. Diabetes Care. 2017;40(6):751–8.
Ramirez A, Wolfsgruber S, Lange C, Kaduszkiewicz H, Weyerer S, Werle J, et al. Elevated HbA1c is associated with increased risk of incident dementia in primary care patients. J Alzheimers Dis. 2015;44(4):1203–12.
Kellar D, Craft S. Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19(9):758–66.
Feil DG, Zhu CW, Sultzer DL. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. J Behav Med. 2012;35(2):190–9.
Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–64.
Tahmasebi R, Zehetmayer S, Pusswald G, Kovacs G, Stogmann E, Lehrner J. Identification of odors, faces, cities and naming of objects in patients with subjective cognitive decline, mild cognitive impairment and Alzheimer s disease: a longitudinal study. Int Psychogeriatr. 2019;31(4):537–49.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. 2013;33(6):393–400.
Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, Davidson MB, DeFronzo R, Genuth S, Holman RR, Ji L, Kirkman S, Knowler WC, Schatz D, Shaw J, Sobngwi E, Steffes M, Vaccaro O, Wareham N, Zinman B, Kahn R. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32(7):1327.
American Diabetes Association. Standards of Medical Care in Diabetes—2013. Diabetes Care. 2013;36(Supplement 1):S11.
Chiang KS, Green KE, Cox EO. Rasch Analysis of the Geriatric Depression Scale-Short Form. Gerontologist. 2009;49(2):262–75.
Yesavage JA, Sheikh JI. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1–2):165–73.
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49.
Lehrner J. Neuropsychologische Testbatterie Vienna. NTBV 1.0 Vienna, Available from: https://kpfg.meduniwien.ac.at/fileadmin/kpfg/pdf/NTBV_GESAMT_kopiergeschützt.pdf. Accessed 24 May 2022.
Lehrner J, Maly J, Gleiß A, Auff E, Dal-Bianco P. The Vienna Neuropsychological Test Battery (VNTB) for detecting Alzheimer´s Dementia: Standardization, norms, and validation. Psychologie in Österreich. 2007;358–65.
Field A. Discovering statistics using SPSS. Sage publications. 2009.
Ziegler A, Lange S, Bender R. Überlebenszeitanalyse: Eigenschaften und Kaplan-Meier Methode. Dtsch med Wochenschr. 2007;132(S 01):e36–8.
Ziegler A, Lange S, Bender R. Survival analysis: log rank test. Dtsch Med Wochenschr. 2007;132(Suppl 1):e39-41.
Bühl A. SPSS 20: Einführung in die moderne Datenanalyse. München: Pearson Studium ein Imprint von Pearson Deutschland; 2012.
Moosbrugger H, Kelava A. Testtheorie und Fragebogenkonstruktion. 3rd ed. Springer Medizin; 2020.
Kubinger K, Rasch D, Moder K. Zur Legende der Voraussetzungen des t Tests für unabhängige Stichproben. Psychologische Rundschau - Psychol Rundsch. 2009;60:26–7.
Hatzinger R, Nagel H. SPSS Statistics - Statistische Methoden und Fallbeispiele. 1st ed. Pearson Education Deutschland GmbH; 2009.
Kubinger KD, Jäger RS. Schlüsselbegriffe der Psychologischen Diagnostik. 1st ed. Weinheim, Basel, Berlin: Beltz Verlag; 2003.
Backhaus K, Erichson B, Plinke W, Weiber R. Multivariate Analysemethoden. Eine anwendungsorientierte Einführung. 14th ed. Gabler Verlag; 2016.
Dapic B, Schernhammer E, Haslacher H, Stogmann E, Lehrner J. No effect of thyroid hormones on 5-year mortality in patients with subjective cognitive decline, mild cognitive disorder, and Alzheimer's disease. J Neuroendocrinol. 2022;e13107.
STATISTIK AUSTRIA. Sterbetafeln_2021_nach_Bundeslaendern. In: Demographische Indikatoren und Tafeln. Bundesanstalt Statistik Österreich. 2023. https://www.statistik.at/fileadmin/pages/413/Sterbetafeln_2021_nach_Bundeslaendern.ods. Accessed 14 Aug 2023.
Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Arch Neurol. 2005;62(5):779–84.
Williams MM, Xiong C, Morris JC, Galvin JE. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67(11):1935–41.
Strand BH, Knapskog AB, Persson K, Edwin TH, Amland R, Mjorud M, et al. Survival and years of life lost in various aetiologies of dementia, mild cognitive impairment (MCI) and subjective cognitive decline (SCD) in Norway. PLoS ONE. 2018;13(9): e0204436.
Acknowledgements
The present manuscript was part of the thesis of JD.
Funding
Open access funding provided by Medical University of Vienna. No funding was received for the study. The authors declare that they have no conflict of interest except that JL is CEO of psimistri GmbH, which is the owner of www.psimistri.com.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to the manuscript.
Dreier, J. (study conception, statistical analysis, writing the manuscript)
Schernhammer, E. (supervision, critical reading of the manuscript)
Haslacher, H. (supervision, critical reading of the manuscript)
Stögmann, E. (supervision, critical reading of the manuscript)
Lehrner, J. (study conception, supervision, statistical analysis, critical reading of the manuscript)
Corresponding author
Ethics declarations
Ethics
The Ethics Committee of the Medical University of Vienna gave a positive vote on the conduct of the present study under EK No. 2345/2019 on February 11, 2020.
Patient informed consent was given.
All materials were sourced from the Medical University of Vienna, and therefore we did not require further permission.
Due to the retrospective, observational nature of the present study, it did not require clinical trial registration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights.
We found predictive value indicating 5-year mortality for metric HbA1c % serum level among patients with cognitive decline. Furthermore, female gender was associated with higher survival probability, while age, depressive symptoms, and poor results in neuropsychological testing increased risk of mortality.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dreier, J., Schernhammer, E., Haslacher, H. et al. Hemoglobin A1c Serum Level Predicts 5-year Mortality in Patients with Cognitive Impairment. J Diabetes Metab Disord 22, 1705–1714 (2023). https://doi.org/10.1007/s40200-023-01303-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01303-4