Abstract
Objectives
To summarize the ultrasonic characteristics of peripheral nerve damage in type 2 diabetes and to verify the diagnostic value of DCEC score for DPN.
Methods
A total of 289 patients with type 2 diabetes evaluated peripheral neuropathy with neuroultrasound and nerve conduction at the Affiliated Hospital of Guizhou Medical University from June 2016 to June 2020. According to the diagnostic criteria from 2017 guidelines of China, 289 patients with type 2 diabetes were divided into three groups: DPN group: 203 cases; subclinical group: 48 cases; and non-DPN group: 38 cases. Kruskal Wallis test was used to identify the differences and characteristics of ultrasound scores between the all groups. The best cut-off value, sensitivity and specificity of DCEC score were obtained by receiver operator characteristic curve. Taking the diagnostic standard of diabetes peripheral neuropathy as the “gold standard”, the best diagnostic threshold, sensitivity and specificity were obtained by drawing the ROC curve of DCEC score, and then the diagnostic value of DCEC score for DPN was verified
Results
Compared with non-DPN group, DCEC score in DPN group was significantly higher (P < 0.05). Otherwise,according to the ROC curve, the best cut-off value of DCEC score for DPN diagnosis was 12.5 (sensitivity 69.7%, specificity 71.1%).
Conclusions
The DCEC score system can effectively diagnose DPN with length-dependence,mainly including the increase of definition score.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
Abbreviations: DPN: Diabetic Peripheral Neuropathy; DCEC: Definition, Cross sectional area, Echo, Compression; ROC: Receiver Operating Characteristic curve; HRUS: High-resolution ultrasound; T2DM: Type 2 Diabetes; BMI: Body mass index; HbAlc: Glycosylated hemoglobin; V-R: Virchow-Robin space; UPSS: ultrasound pattern sum score; GBS: Guillain Barre syndrome.
Introduction
Diabetic Peripheral Neuropathy (DPN) is one of the chronic complications of diabetes, mainly caused by sensory, motor and autonomic neurological dysfunction. At present 463 million adults worldwide are suffering from diabetes, and are expected to jump to 700 million by 2045. 4000–6000 patients have diabetic foot and lower extremity complications among them[1]. China is one of the prone areas of the disease. The latest data show that the prevalence rate of diabetes in China has risen to 11.2%. As a common complication, DPN incidence rate has increased year by year [2]. Due to the lack of doctors’ awareness of early diagnosis and people’s low awareness, most patients may have serious adverse consequences in the later stage, affecting the quality of life. In the early stage of DPN, small fiber neuropathy is often the main manifestation, which is characterized by symmetrical abnormal temperature and pain perception[3][4]. Among them, 25% of patients mainly seek medical treatment for the first time due to neuralgia[5], and some patients even die suddenly due to autonomic nerve dysfunction, which is an important indicator reflecting the prognosis of DPN patients; When the disease involves myelinated large fiber nerves, the patient may have numbness, weakened tendon reflex, abnormal walking and gait, or even distal limb muscle atrophyup to 50% of diabetic peripheral neuropathies may be asymptomatic[6], which brings great challenges to diagnosis and treatment. Therefore, it is very important to explore the early diagnosis of DPN and take effective treatment measures to improve the quality of life of patients. The pathogenesis is related to a variety of factors, which may be related to oxidative immune stress[7], accumulation of advanced glycokinase products[8, 9], metabolic disorders[10, 11], gastrointestinal flora disorders and immune factors[12]. At present, the exact etiology is not clear.
Optimize glucose control and nutritional nerve are only to slow the progression of distal symmetric polyneuropathy in people with type 2 diabetes, but can not prevent the neurological impairment[6]. DPN is mainly diagnosed by clinical manifestations, electrophysiological testing or referral to a neurologistisrarely needed, except in situations where the clinical features are atypical or the diagnosis is unclear, due to lacking of sensitivity to small fibers[13]. With the development of ultrasound imaging, there are evidences that high-resolution ultrasound (HURS) plays a crucial role in the study of peripheral neuropathy. Many studies have confirmed that HURS has advantages in the diagnosis of early and subclinical lesions[14, 15], and makes up for the blank of morphological visualization. Nerves exposed permanently to high glucose and hypoxia environment are prone to axonal transport obstruction, resulted in insufficient nutrient supply of nerve endings[16].
Ultrasound imaging is still unspecific and difficult to distinguish the severity of the disease. Therefore, establishing the standard for evaluation of ultrasound is very critical for the early diagnosis of diabetic peripheral neuropathy. A few studies abroad have quantified the results of HRUS, such as Bochum ultrasonic score (BUS) [17], the ultrasonic pattern sum score (UPSS) [18], and study scoring system [19]. Although the diagnostic accuracy of HURS has been improved, this studies can not thoroughly quantify the ultrasonic evaluation. Therefore, the purpose of this paper was to comprehensively quantify the diabetic peripheral neuropathy by the “DCEC” scoring system and further confirm the diagnostic value.
Materials and methods
Study participants
This study was approved by the Affiliated Hospital of Guizhou Medical University in the Department of Neurology. Participants obtained written informed consents. None of the study results have been previously reported.
The study screened 289 consecutive patients with DPN, who were referred for the Chinese guidelines for the prevention and treatment of type 2 diabetes in 2017 between June 2016 and June 2020. Nerve conduction studies and ultrasonography were used for assessing neuropathies. Inclusion criteria was according to the 2017 Chinese guidelines for the prevention and treatment of type 2 diabetes, patients aged > 18 who meet the following criteria are included: fasting plasma glucose (FPG) in the morning ≥ 126 mg/dl (7.0mmol/l), 2-hour plasma glucose (2-h PG) ≥ 200 mg/dl (11.1mmol/l), or glycosylated hemoglobin ≥ 6.5% (48mmol/mol), or patients with symptoms of “excessive drinking, eating, urination and weight loss” + random peripheral blood glucose ≥ 200 mg/dl (11.1mmol/l)[13]. Patients were excluded if they had a history of peripheral neuropathy surgery, other infectious, immune, metabolic, poisoning, tumor, alcoholism, drugs and other causes of peripheral neuropathy; Patients with foot ulcer, severe cardiopulmonary insufficiency, obvious myasthenia, pregnancy, and so on[20, 21].
Sample sizes
This study is a diagnostic retrospective study. The included patients are patients in the Department of Neurology. Therefore, most patients already have symptoms or signs, so it is difficult to obtain different grouping sample sizes according to the principle of sample size calculation. The sample size is grouped according to the actual situation of the included population.
Grouping criteria: 203 patients with DPN symptoms or signs and abnormal neuroelectrophysiology were included in DPN group; The patients without symptoms and signs of peripheral neuropathy but with abnormal electrophysiological results were included in subclinical group (48 cases); 38 patients without symptoms and signs of peripheral neuropathy but normal nerve conduction were included in the non-DPN group[13](Fig. 1).

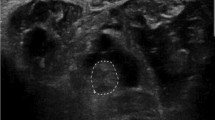
Cross sectional area of ulnar nerve in cubital tunnel under high frequency ultrasound.The cross-sectional area of normal ulnar nerve in cubital tunnel was 0.092cm2 (A); In the cross section of ulnar nerve in cubital tunnel of patients with diabetic peripheral neuropathy under high-frequency ultrasound, the internal nerve bundle structure was blurred, no hyperechoic nerve adventitia was found, and the cross-sectional area was 0. 128 cm2 (B)
There were 313 patients with type 2 diabetes mellitus, including 1 case of polymyositis (limb pain, normal nerve conduction), 2 cases of Guillain Barre syndrome, 2 cases of long-term cancer, 3 cases of varicose veins and thromboangiitis obliterans after chemotherapy, and 16 cases without neuroelectrophysiological examination, so they were excluded. Finally, 289 patients with perfect limb neuroultrasound were included and divided into 3 groups, 20 cases in DPN group There were 3 cases, 48 cases in subclinical group and 38 cases in non DPN group
Diagnostic method
Some researches have confirmed the importance of ultrasound diagnosis in peripheral neuropathy[22,23,24]. Compared to neuro-electrophysiology, ultrasound not only has improved value and efficiency for diagnosing peripheral neuropathy, but helps distinguish the type of neuropathy based on morphological and structural changes[23]. Previously, we have reviewed that high-frequency ultrasound can diagnose diabetes peripheral neuropathy from the aspects of nerve cross-sectional area, blood flow, echo and compression. As the “gold standard” of Pathology, cutaneous nerve biopsy is difficult to popularize in clinical work due to the difficulty of obtaining materials and the high requirements of pathological diagnosis[24]. Studies have shown that the morphological changes of peripheral nerves in patients with type 2 diabetes occur before the occurrence of neuropathy, that indirectly reflected the diagnostic value of high frequency ultrasound[23, 25].Therefore, we chose the clinical diagnostic standard of diabetes peripheral neuropathy as the “gold standard” to measure the diagnostic value of high-frequency ultrasound score(DCEC).
High-resolution ultrasound
Ultrasound was performed using a 5-12 MHz linear array transducer (Philips iU22, Holland) by a sonographer who was blinded to the groups of participants. The measurements should be perpendicular to the skin to avoid compressing nerves. The average values were taken for three times for each part. A total of 12 nerves in the limbs were detected by ultrasound. The cross-sectional areas(CSA) of each part of nerves should be measured along the hyperechoic boundary of nerves. Meanwhile, the definition and echo of nerves should be observed from multiple angles. The nerve entrapment, observed at wrist, elbow and ankle, should be recorded and described. Based on the range of normal nerve CSA in our laboratory, each site CSA value were abnormal if they exceeded the threshold value[14,15,16,17,18].
Ultrasound scoring system
Referring to Ultrasonic scores of BUS, UPSS[19, 20], quantify the definition, cross-sectional area, echo, and compression of each nerve, and quantify the maximum value of this nerve measurement site. For detail, see the scoring system (Table 1).The Ultrasound scoring system (DCEC) is equal to total score (each score of nerve = definition score + cross-sectional score + echo score + compression score from each nerve), including the total score of both upper limbs and lower limbs; The above score was calculated by a neurologist.
Nerve conduction studies
In a constant temperature environment, the Nicoli EDX EMG inducer were.
conducted in the electromyography laboratory at The affiliated Hospital of Guizhou Medical University in the Department of Neurology by an experienced technologist (Jing Huang). According to the diagnostic criteria of electrophysiological diagnosis of diabetic peripheral neuropathy[21], at least one abnormal NCS parameter in all nerves, we can figure out which limb has pathological changes.
Statistical analysis
Statistical analyses were performed with SPSS software version 24. 0. The.
normal distribution of the data was tested with the Shapiro–Wilk test. Analysis of variance for continuous variables were used to compare variables with a normal distribution. Continuous variables were expressed as Interquartile Range due to not conform to normal distribution. Chi-square test and nonparametric Kruskal Wallis-H test was used for comparison among groups. A receiver operator characteristic curve (ROC) was generated by using the DCEC score datas (including measurements from the limbs), so that we can calculate the area under the curve (AUC) for DCEC total score, both upper limbs score, both lower limbs score, definition score,cross-sectional score, echo score, and compression score. Statistical significance was accepted at p < 0.05.
Results
Participants
313 patients with T2DM were enrolled in the study, but 24 patients were excluded, including 24 patients with incomplete datas, 16 cases without nerve conduction, and 1 patient with polymyositis, 2 patients with Guillain-Barre syndrome, 2 patients treated by cancer chemotherapy, 1 patients with polymyositis, and 3 patients with varicose veins and thromboangiitis obliterans. Thus, 289 were intended for the investigation, 153 (52.9%) male and 136 (47.1%) female, 93 (32.2%) had no symptoms of peripheral neuropathy but 196 (67.8%) had. In DPN group, 7 (3.4%) had no symptoms of peripheral neuropathy. It showed that age, gender, height, course of disease, BMI, fasting blood glucose, glycosylated hemoglobin level, smoking rate, hypertension, cerebral small vessel disease, dyslipidemia, overweight, poor blood glucose control, course of disease > 10 years and using of hypoglycemic drugs were without any statistical difference between groups(Table 2).
Ultrasonographic features of peripheral neuropathy in patients with type 2 diabetes mellitus
Statistical comparisons among groups by one-way ANOVA. This difference in DCEC total score, clarity score, cross-sectional area score, radial nerve, ulnar nerve, sciatic nerve, tibial nerve, common peroneal nerve, total score of both upper limbs and total score of both lower limbs among groups was statistically significant (p < 0.05). The results revealed that there was no statistically significant difference between DPN group and Subclinical group after adjustment for multiple comparisons, but significantly higher than the non-DPN group (p < 0.05). The CSA score of subclinical group was higher than the DPN group (Table 3). However, echo and compression score were no significant difference among groups(Table 3). Then, we combined the DPN group and subclinical group into one group because no significant difference in multiple scores between the DPN group and subclinical group. Finally, results showed that the total score, definition score, CSA score, total score of upper extremity and total score of lower limbs from DCEC scoring system were higher than the non-DPN group. Among them, the definition score accounted for the highest proportion in the total score. Otherwise, the results of nerve and limb score were consistent with above among the groups. In DPN and subclinical group, the total scores of both lower limbs were higher than the total scores of both lower limbs. Among them, there were significant differences in ulnar nerve score, radial nerve score, sciatic nerve score, tibial nerve score and common peroneal nerve score among the groups (Table 4).
Diagnostic value of DCEC scoring system in DPN
Based on the diagnostic criteria of diabetic peripheral neuropathy as “gold standard”, the ROC curves from the ultrasound score according to Fig. 2. The cut-off values of DCEC total score, total score of both upper limbs, total score of both lower limbs and clarity score for DPN were 12.5 (sensitivity 69.7%, specificity71.1%). 8.5 (sensitivity 43.4%, specificity 86.8%), 4.5 (sensitivity 78.9%, specificity 65.8%), 10.5 (sensitivity 53.4%, specificity 89.5%), 2.5 (sensitivity 72.9%, specificity 47.4%) respectly. In addition, Their corresponding cross-sectional areas are 0.755(95% confidence intervals:67.5%, 83.6%), 0.687(95% confidence intervals:60.1%, 84.9%), 0.763(95% confidence intervals:67.7%, 84.9%), 0.783(95% confidence intervals:71.0%, 85.6%), 0.623(95% confidence intervals:52.6%, 72.0%)(Table 5).
Discussion
In order to improve the value of HRUS in the quantitative diagnosis of DPN, and avoid the error from “clinical electrophysiological separation” phenomenon of neurophysiology in the early stage of DPN[22], we diagnose DPN by clinical manifestations and neurophysiology, and analyzed DCEC scoring system in patients with T2DM. Finally, we found that there was no significant difference between DPN group and subclinical group. Compared with non-DPN group, DCEC score of DPN group / subclinical group was significantly higher. The results were consistent in several sensitivity analyses and subgroup analyses. We observed definition score is the most representative score in all group, and the cut-off value of DCEC score was 12.5, specially, the cut-off value of definition score was 10.5. This shows that we have made a new breakthrough in the diagnosis of diabetes peripheral neuropathy by high-frequency ultrasound, and improved the value of ultrasound diagnosis of diabetes peripheral neuropathy.
With respect to the ultrasound score between groups, definition scores were higher in all groups. The internal echo and definition of the nerves of the patients changed in varying degrees. The “cribriform or honeycomb” structure disappeared, with blurring development and boundary, and increase or decrease internal echoes. Difference in the scores between the subclinical group and the DPN group was insignificant, which indicates that the degree of nerve damage in type 2 diabetic patients may not be consistent with the clinical manifestations. Some patients may have peripheral neuropathy before the onset of diabetes or typical symptoms or signs. There was no significant difference between the neurological impairment in asymptomatic patients and patients with DPN. Even in some patients, the severity of peripheral neuropathy is similar to that of early diabetic neuropathy in prediabetes[23]. This may be determined by the severity of the neuropathy [24], which didn’t exclude that subclinical patients may even have more severe neuropathy.
In this study, the score of both lower limbs was higher than the both upper limbs, which was consistent with the “length dependence” of DPN, indicating that DPN patients had early involvement and severe damage of both lower limbs.
Several reports have shown that DCEC scoring system has high diagnostic value for peripheral neuropathy[25, 26]. The diagnostic value for diabetic peripheral neuropathy in this trial was as anticipated and was similar to that in previous studies. Using 12.5 points as the diagnostic cut-off value, the sensitivity and specificity of DPN were 69.7% and 71.1% respectively in this study, which isn’t higher than the diagnostic value presented by Qian OuYang et al[25]. But the definition score had a higher specificity (89.5%) in the diagnosis of DPN. We demonstrated that the definition score measured by HRUS could reflect the main nerve damage. The mechanism is that a large number of advanced glycation end products (ages) through protein kinase C (PKC) and transcription nuclear factor signaling pathway and other oxidative stress mechanisms in nerve tissue in hyperglycemia environment, causing excessive accumulation of sorbitol, increasing intracellular osmotic pressure, leading to edema of nerve tissue, demyelination or axonal degeneration [27, 28].
This study confirmed that the ultrasound score has certain advantages in reflecting the nerve damage in patients with diabetes peripheral neuropathy. Compared with the ultrasound pattern sum score, DCEC score focused more on the neuropathy quantification in limbs by HRUS, while the UPSS score focused on the quantification of motor, sensory and autonomic nerves[20]; In the measurement index, this study is not only limited to the nerve CSA, but also more comprehensive to evaluate the nerve definition, echo and compression.
Few studies paid attention to HRUS quantification, mainly in inflammatory immune related peripheral neuropathy recently. It suggested that CSA is a more consistent index for measurement as a quantitative index in most studies. In this study, we expanded the sample size to diagnose DPN in DPN, combined with nerve conduction to evade the “clinical electrophysiological separation” phenomenon of electrophysiology at the early stage of DPN[29]. It is more accurate and reliable to reflect the nerve lesions by HRUS.
The study has some limitations for the following reasons: (1) the proportion of patients without peripheral nerve symptoms is low because the study population mainly comes from the Department of Neurology, which caused the sample size is too small in subclinical and non-DPN group. There is no significant difference in the baseline data of patients, which can ensure the reliability of the results to a certain extent. Future long-term studies including larger cohorts of patients with type 2 diabetes mellitus from multiple departments and centers are needed, so that we can verify the reliability of DCEC score for DPN diagnosis.
Conclusions
In conclusion, our results show that high frequency ultrasound can reflect the severity of peripheral neuropathy in diabetes and reliably diagnose DPN by DCEC score. Specially, the definition score is the most representative.
Other information
The research project has been reviewed and approved by the ethics committee of the Affiliated Hospital of Guizhou Medical University, and registered with China Clinical Trial Registration Center (Registration No. chictr190021110).
Data Availability
All data used to support the findings of this study are included within the article.
References
Patterson CC, Karuranga S, Salpea P, Saeedi P, Dahlquist G, Soltesz G, Ogle GD. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition[J]. Diabetes Res Clin Pract. 2019 Nov;157:107842.
Li Y, Teng D,Shi X, et al. Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, Shi B, Sun H, Ba J, Chen B, Du J, He L, Lai X, Li Y, Chi H, Liao E, Liu C, Liu L, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Ning G, Mu Y, Zhao J, Teng W, Shan Z. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study.[J].BMJ, 2020, 369: p. m997.
Chan AC, Wilder-Smith EP.Small fiber neuropathy:Getting bigger![J]Muscle Nerve. 2016 May;53(5):671 – 82.6.
Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93.
Hicks CW, Selvin E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes[J]. Curr Diab Rep. 2019 Aug 27;19 (10):86.
Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association[J]. Diabetes Care. 2017 Jan;40(1):136–54.
Ferenc, Sztanek,ágnes,等..[The role of oxidative stress in the development of diabetic neuropathy].[J].Orvosi hetilap.2016,(49).1939–1946.
Cristiane L,,de la Hoz,Chu,等.A model of chronic diabetic polyneuropathy: benefits from intranasal insulin are modified by sex and RAGE deletion.[J]American Journal of Physiology: Endocrinology & Metabolism.2017,312(5).E407-E419.
Saleh A,Smith,等.Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons[J].Experimental Neurology.2013.249149 ~ 159.
Kamesh Gupta,Anand Jain,Anurag Rohatgi.An observational study of vitamin b12 levels and peripheral neuropathy profile in patients of diabetes mellitus on metformin therapy[J].Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2018,12(1).51–58.
Jamwal, Shalini,Sharma,等.Vascular endothelium dysfunction: a conservative target in metabolic disorders[J].Inflammation research: Official journal of the European Histamine Research Society.2018,67(5).391 ~ 405.
Xiaosong Meng,Patrice Maurel,Isabel Lam,等.Necl-4/Cadm4 recruits Par-3 to the Schwann cell adaxonal membrane[J].Glia.2019,67(5).884–895.
Chinese Diabetes Society. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition)[J]. Chin J Practical Intern Med. 2018;10(1):4–67.
Kong XJ. The value of high frequency ultrasonography in the diagnosis of diabetic peripheral neuropathy[J]. Zhejiang Clin Med J. 2019;21(1):29–31.
Emril DR. Zakaria I,Amrya M,Agreement Between High-Resolution Ultrasound and Electro-Physiological Examinations for Diagnosis of Carpal Tunnel Syndrome in the Indonesian Population[J]. Front Neurol. 2019;10:888.
Liu MS, Hu BL, Cui LY, Tang XF, Du HL. B H. Clinical and neurophysiological features of 700 patients with diabetic peripheral neuropathy[J]. Chinese Journal of Internal Medicine,2005,44 (3):173–176.
Pitarokoili K, Kerasnoudis A,Behrendt V, et al. Facing the diagnostic challenge: Nerve ultrasound in diabetic patients with neuropathic symptoms[J]. Muscle Nerve. 2016;54:18–24.
Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Nerve ultrasound score in distinguishing chronic from acute inflammatory demyelinating polyneuropathy[J]. Clin Neurophysiol. 2014;125(3):635–41.
Grimm A, Décard BF, Axer H, Fuhr P. The Ultrasound pattern sum score - UPSS. A new method to differentiate acute and subacute neuropathies using ultrasound of the peripheral nerves[J]. Clin Neurophysiol. 2015;126(11):2216–25.
Breiner A, Qrimli M, Ebadi H, Alabdali M, Lovblom LE, Abraham A, Albulahi H, Perkins BA, Bril V. Peripheral nerve high-resolution ultrasound in diabetes[J]. Muscle Nerve. 2017;55:171–8.
Pelosi L, Mulroy E. Diagnostic sensitivity of electrophysiology and ultrasonography in ulnar neuropathies of different severity[J]. Clin Neurophysiol. 2019;130:297–302.
Jiang WX, Huang SR, Teng H, et al. Diagnostic performance of two-dimensional shear wave elastography for evaluating tibial nerve stiffness in patients with diabetic peripheral neuropathy[J]. Eur Radiol. 2019;29:2167–74.
Gonzalez NL, Hobson-Webb LD. The Role of Imaging for Disorders of Peripheral Nerve[J]. Clin Geriatr Med. 2021 May;37(2):223–39.
Kerasnoudis A, Tsivgoulis G. Nerve Ultrasound in Peripheral Neuropathies: A Review[J]. J Neuroimaging. 2015 Jul-Aug;25(4):528–38.
Ishibashi Fukashi,Taniguchi Miki,Kojima Rie. et al. Morphological changes of the peripheral nerves evaluated by high-resolution ultrasonography are associated with the severity of diabetic neuropathy, but not corneal nerve fiber pathology in patients with type 2 diabetes.[. J] J Diabetes Investig. 2015;6:334–42.
Mellgren SI, Nolano M, Sommer C. The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol. 2013;115:171–88.
Ishibashi Fukashi,Taniguchi Miki,Kojima Rie. et al. Elasticity of the tibial nerve assessed by sonoelastography was reduced before the development of neuropathy and further deterioration associated with the severity of neuropathy in patients with type 2 diabetes.[. J] J Diabetes Investig. 2016;7:404–12.
Chen J, Liu J, Zeng J, Wu S, Ren J. Ultrasonographic Reference Values for Assessing Normal Sciatic Nerve Ultrasonography in the Normal Population. J Med Ultrasound. 2018 Apr-Jun;26(2):85–9.
Chen J, Wang CL, Wu S, He S, Ren J. The feasibility of using high-resolution ultrasonography to assess ulnar nerve in patients with diabetes mellitus. J Ultrason. 2017 Sep;17(70):160–6.
Acknowledgements
We thank the staff from the Department of Neurology, the Affiliated Hospital of Guizhou Medical University for their help.
Funding
The study was supported by National Key R&D Program of China (NO. 2018YFC1312901) and the Guiyang science and technology plan project. No. [2019]9-1-7. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, H., Tang, C., Li, M. et al. Sensitivity and specificity of high frequency ultrasound score (DCEC) in diabetic peripheral neuropathy. J Diabetes Metab Disord 21, 1459–1467 (2022). https://doi.org/10.1007/s40200-022-01080-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01080-6