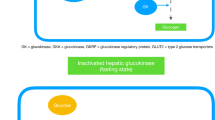
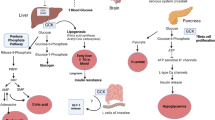
Abstract
Diabetes mellitus is a worldwide impacting disorder and the ratio through which the number of diabetic patients had increased worldwide, puts medical professionals to serious stress for its effective management. Due to its polygenic origin and involvement of multiple genes to its pathophysiology, leads to understanding of this ailment more complex. It seems that current interventions, such as dietary changes, life style changes and drug therapy such as oral hypoglycaemics and insulin, are unable to halt the trend. There are various novel and emerging targets on which the researchers are paying attention to combat with this ailment successfully. Human glucokinase (GK) enzyme is one of these novel and emerging targets for management of diabetes. Its availability in the pancreas and liver cells makes this target more lucrative. GK’s presence in the pancreatic and hepatic cells plays a very important function for the management of glucose homoeostasis. Small molecules that activate GK allosterically provide an alternative strategy for restoring/improving glycaemic regulation, especially in type 2 diabetic patients. Although after enduring many setbacks in the development of the GK activators, interest has been renewed especially due to introduction of novel dual acting GK activator dorzagliatin, and a novel hepato-selective GK activator, TTP399. This review article has been formulated to discuss importance of GK in glucose homeostasis, recent updates on small molecules of GK activators, clinical status of GK activators and challenges in development of GK activators.


Similar content being viewed by others
References
Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795.
Grewal AS, Beniwal M, Pandita D, Sekhon BS, Lather V. Recent updates on peroxisome proliferator-activated receptor δ agonists for the treatment of metabolic syndrome. Med Chem. 2016;12:3–21.
Grewal AS, Sekhon BS, Lather V. Recent updates on glucokinase activators for the treatment of type 2 diabetes mellitus. Mini Rev Med Chem. 2014;14(7):585–602.
Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren T, Shan P. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790.
Alhadramy MS. Diabetes and oral therapies: a review of oral therapies for diabetes mellitus. J Taibah Univ Med Sci. 2016;11(4):317–29.
Pal M. Recent advances in glucokinase activators for the treatment of type 2 diabetes. Drug Discov Today. 2009;14(15–16):784–92.
Rochester CD, Akiyode O. Novel and emerging diabetes mellitus drug therapies for the type 2 diabetes patient. World J Diabetes. 2014;5(3):305–15.
Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354–95.
Belay E, Abera A, Mehari A, Gebremeskel G, Endrias A, Endriset K. Achievements of diabetes goals and their determinants in type 2 diabetic patients attending outpatient diabetic clinic in northern Ethiopia. Int J Chronic Dis. 2017;2017:5713187.
Verma AK, Goyal Y, Bhatt D, Dev K, Alsahli MA, Rahmani AH, Almatroudi A. A compendium of perspectives on diabetes: a challenge for sustainable health in the modern era. Diabetes Metab Syndr Obes. 2021;2021(14):2775–87.
Sharma P, Singh S, Thakur V, Sharma N, Grewal AS. Novel and emerging therapeutic drug targets for management of type 2 diabetes mellitus. Obes Med. 2021;23:100329.
Matschinsky F. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990;39(6):647–52.
Matschinsky F, Porte D. Glucokinase activators (GKAs) promise a new pharmacotherapy for diabetics. F1000 Med Rep. 2010;2:43.
Meglasson MD, Matschinsky F. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3–4):163–214.
Matschinsky F, Liang Y, Kesavan P, Wang L, Froguel P, Velho G, Cohen D, Permutt MA, Tanizawa Y, Jetton TL, Niswender K, Magnuson MA. Glucokinase as pancreatic beta cell glucose sensor and diabetes gene. J Clin Invest. 1993;92(5):2092–8.
Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66(1):27–42.
Li W, Zhang X, Sun Y, Liu Z. Recent clinical advances of glucokinase activators in the treatment of diabetes mellitus type 2. Pharmazie. 2020;75(6):230–5.
Agius L. Lessons from glucokinase activators: the problem of declining efficacy. Expert Opin Ther Pat. 2014;24(11):1155–9.
Nakamura A, Terauchi Y. Present status of clinical deployment of glucokinase activators. J Diabetes Investig. 2015;6(2):124–32.
Agius L. Targeting hepatic glucokinase in type 2 diabetes: weighing the benefits and risks. Diabetes. 2008;58(1):18–20.
Van Schaftingen E, Vandercammen A, Detheux M, Davies DR. The regulatory protein of liver glucokinase. Adv Enzym Regul. 1992;32:133–48.
Van Schaftingen E. Short-term regulation of glucokinase. Diabetologia. 1994;37:S43–7.
Baltrusch S, Francini F, Lenzen S, Tiedge M. Interaction of glucokinase with the liver regulatory protein is conferred by leucine-asparagine motifs of the enzyme. Diabetes. 2005;54(10):2829–37.
Agius L. Targeting hepatic glucokinase in type 2 diabetes: weighing the benefits and risks. Diabetes. 2009;58(1):18–20.
Gomis RR, Favre C, García-Rocha M, Fernández-Novell JM, Ferrer JC, Guinovart JJ. Glucose 6-phosphate produced by gluconeogenesis and by glucokinase is equally effective in activating hepatic glycogen synthase. J Biol Chem. 2003;278(11):9740–6.
Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanné-Chantelot C, Ellard S, Gloyn AL. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009;30(11):1512–26.
Rines AK, Sharabi K, Tavares CD, Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov. 2016;15(11):786–804.
Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48(3):e219.
Perseghin G. Exploring the in vivo mechanisms of action of glucokinase activators in type 2 diabetes. J Clin Endocrinol Metab. 2010;95:4871–3.
Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13(10):572–87.
Choi JM, Seo MH, Kyeong HH, Kim E, Kim HS. Molecular basis for the role of glucokinase regulatory protein as the allosteric switch for glucokinase. Proc Natl Acad Sci U S A. 2013;110(25):10171–6.
Raimondo A, Rees MG, Gloyn AL. Glucokinase regulatory protein: complexity at the crossroads of triglyceride and glucose metabolism. Curr Opin Lipidol. 2015;26(2):88–95.
Matschinsky F, Wilson DF. The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in islets of langerhans. Front Physiol. 2019;10:148.
Toulis KA, Nirantharakumar K, Pourzitaki C, Barnett AH, Tahrani AA. Glucokinase activators for type 2 diabetes: challenges and future developments. Drugs. 2020;80:467–75.
Walker DG, Rao S. The role of glucokinase in the phosphorylation of glucose by rat liver. Biochem J. 1964;90(2):360–8.
Al-Hasani H, Tschöp MH, Cushman SW. Two birds with one stone: novel glucokinase activator stimulates glucose-induced pancreatic insulin secretion and augments hepatic glucose metabolism. Mol Interv. 2003;3(7):367–70.
Grewal AS, Lather V, Charaya N, Sharma N, Singh S, Kairys V. Recent developments in medicinal chemistry of allosteric activators of human glucokinase for type 2 diabetes mellitus therapeutics. Curr Pharm Des. 2020;26(21):2510–52.
Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE, Marcus L, Qi L, Spence CL, Tengi J, Magnuson MA, Chu CA, Dvorozniak MT, Matschinsky F, Grippo JF. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301(5631):370–3.
Behera P, Behera D, Satpati S, Agnihotri G, Nayak S, Padhi P, Dixit A. Molecular modeling and identification of novel glucokinase activators through stepwise virtual screening. J Mol Graph Model. 2015;57:122–30.
Park K, Lee BM, Hyun KH, Han T, Lee DH, Choi HH. Design and synthesis of acetylenyl benzamide derivatives as novel glucokinase activators for the treatment of T2DM. ACS Med Chem Lett. 2015;6:296–301.
Paczal A, Bálint B, Wéber C, Szabó ZB, Ondi L, Theret I, De Ceuninck F, Bernard C, Ktorza A, Perron-Sierra F, Kotschy A. Structure-activity relationship of azaindole-based glucokinase activators. J Med Chem. 2016;59:687–706.
Cheruvallath Z, Gwaltney SL, Sabat M, Tang M, Wang H, Jennings A, Hosfield D, Lee B, Wu Y, Halkowycz P, Grimshaw CE. Discovery of potent and orally active 1,4-disubstituted indazoles as novel allosteric glucokinase activators. Bioorg Med Chem Lett. 2017;27(12):2678–82.
Zhang L, Hu S, Lei L, Zhang Y, Zhang L, Song H, Shen Z, Feng Z. Design, synthesis and evaluation of novel derivatives of orotic acid amide as potent glucokinase activators. Lett Drug Des Discov. 2017;14(3):252–61.
Singh R, Lather V, Pandita D, Judge V, Arumugam KN, Grewal AS. Synthesis, docking and antidiabetic activity of some newer benzamide derivatives as potential glucokinase activators. Lett Drug Des Discov. 2017;14(5):540–53.
Charaya N, Pandita D, Grewal AS, Lather V. Design, synthesis and biological evaluation of novel thiazol-2-yl benzamide derivatives as glucokinase activators. Comput Biol Chem. 2018;73:221–9.
Bano S, Khan AU, Asghar F, Usman M, Badshah A, Ali S. Computational and pharmacological evaluation of ferrocene-based acyl ureas and homoleptic cadmium carboxylate derivatives for anti-diabetic potential. Front Pharmacol. 2018;8:1001.
Grewal AS, Kharb R, Prasad DN, Dua JS, Lather V. N-pyridin-2-yl benzamide analogues as allosteric activators of glucokinase: design, synthesis, in vitro, in silico and in vivo evaluation. Chem Biol Drug Des. 2019;93(3):364–72.
Grewal AS, Kharb R, Prasad DN, Dua JS, Lather V. Design, synthesis and evaluation of novel 3,5-disubstituted benzamide derivatives as allosteric glucokinase activators. BMC Chem. 2019;13(1):2.
Singh S, Arora S, Dhalio E, Sharma S, Arora K, Grewal AS. Design and synthesis of newer N-benzimidazol-2yl benzamide analogues as allosteric activators of human glucokinase. Med Chem Res. 2021;30:760–70.
Arora S, Grewal AS, Sharma N, Arora K, Dhalio E, Singh S. Design, synthesis, and evaluation of some novel N-benzothiazol-2-yl benzamide derivatives as allosteric activators of human glucokinase. J Appl Pharm Sci. 2021;11(Suppl 1):038–47.
Filipski KJ, Pfefferkorn JA. A patent review of glucokinase activators and disruptors of the glucokinase–glucokinase regulatory protein interaction: 2011–2014. Expert Opin Ther Pat. 2014;24(8):875–91.
Sarabu R, Berthel SJ, Kester RF, Tilley JW. Novel glucokinase activators: a patent review (2008–2010). Expert Opin Ther Pat. 2011;21(1):13–33.
Pfeferkorn JA. Strategies for the design of hepatoselective glucokinase activators to treat type 2 diabetes. Expert Opin Drug Discov. 2013;8(3):319–30.
Egan A, Vella A. TTP399: an investigational liver-selective glucokinase (GK) activator as a potential treatment for type 2 diabetes. Expert Opin Investig Drugs. 2019;28(9):741–7.
Dransfield PJ, Pattaropong V, Lai S, Fu Z, Kohn TJ, Du X, Cheng A, Xiong Y, Komorowski R, Jin L, Conn M, Tien E, DeWolf WE Jr, Hinklin RJ, Aicher TD, Kraser CF, Boyd SA, Voegtli WC, Condroski KR, et al. Novel series of potent glucokinase activators leading to the discovery of AM-2394. ACS Med Chem Lett. 2016;7(7):714–8.
Efanov AM, Barrett DG, Brenner MB, Briggs SL, Delaunois A, Durbin JD, Giese U, Guo H, Radloff M, Gil GS, Sewing S, Wang Y, Weichert A, Zaliani A, Gromada J. A novel glucokinase activator modulates pancreatic islet and hepatocyte function. Endocrinology. 2005;146:3696–701.
Doliba NM, Fenner D, Zelent B, Bass J, Sarabu R, Matschinsky F. Repair of diverse diabetic defects of β-cells in man and mouse by pharmacological glucokinase activation. Diabetes Obes Metab. 2012;14(Suppl 3):109–19.
Mahmoodi M, Zarei S, Rezaeian M, Arababadi MK, Ghasemi H, Khoramdelazad H, Rezayati N, Hasanshahi G, Hosseini-Zijoud S-M. Persian shallot (Allium hirtifolium Boiss) extract elevates glucokinase (GCK) activity and gene expression in diabetic rats. Am J Plant Sci. 2013;4(7):1393–9.
Ighodaro OM, Akinloye OA, Ugbaja RN, Omotainse SO. Sapium ellipticum (Hochst) Pax ethanol leaf extract modulates glucokinase and glucose-6-phosphatase activities in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2017;7(6):544–8.
Angadi K, Gundampati R, Jagannadham M, Kandru A. Molecular docking studies of guggultetrol from Nymphaea pubescens with target glucokinase (GK) related to type-II diabetes. J Appl Pharm Sci. 2012;3(2):127–31.
Hikino H, Ishiyama M, Suzuki Y, Konno C. Mechanisms of hypoglycemic activity of ganoderan B: a glycan of Ganoderma lucidum fruit bodies. Planta Med. 1989;55(5):423–8.
Kang YJ, Jung UJ, Lee MK, Kim HJ, Jeon SM, Park YB, Chung HG, Baek NI, Lee KT, Jeong TS, Choi MS. Eupatilin, isolated from Artemisia princeps Pampanini, enhances hepatic glucose metabolism and pancreatic beta-cell function in type 2 diabetic mice. Diabetes Res Clin Pract. 2008;82(1):25–32.
Qian-Cutrone J, Ueki T, Huang S, Mookhtiar KA, Ezekiel R, Kalinowski SS, Brown KS, Golik J, Lowe S, Pirnik DM, Hugill R, Veitch JA, Klohr SE, Whitney JL, Manly SP. Glucolipsin a and B, two new glucokinase activators produced by Streptomyces purpurogeniscleroticus and Nocardia vaccinii. J Antibiot. 1999;52(3):245–55.
Grewal AS, Sharma N, Singh S, Arora S. Molecular docking studies of phenolic compounds from Syzygium cumini with multiple targets of type 2 diabetes. J Pharm Technol Res Manag. 2018;6(2):125–33.
Singh AB, Singh N, Akanksha J, Maurya R, Srivastava AK. Coagulanolide modulates hepatic glucose metabolism in C57BL/KsJ-db/db mice. Hum Exp Toxicol. 2012;31(10):1056–65.
Min Q, Cai X, Sun W, Gao F, Li Z, Zhang Q, Wan LS, Li H, Chen J. Identification of mangiferin as a potential glucokinase activator by structure-based virtual ligand screening. Sci Rep. 2017;7:44681.
Jeyabaskar S, Viswanathan T, Mahendran R, Nishandhini M. In silico molecular docking studies to investigate interactions of natural camptothecin molecule with diabetic enzymes. Res J Pharm Technol. 2017;10(9):2917–22.
Grewal AS, Sharma N, Singh S. Molecular docking investigation of compounds from Sapium ellipticum (Hochst) Pax as allosteric activators of human glucokinase. Int J Pharm Qual Assur. 2019;10(4):588–96.
Scheen AJ. Investigational insulin secretagogues for type 2 diabetes. Expert Opin Investig Drugs. 2016;25(4):405–22.
Kamimura H, Ito S, Chijiwa H, Okuzono T, Ishiguro T, Yamamoto Y, Nishinoaki S, Ninomiya SI, Mitsui M, Kalgutkar AS, Yamazaki H, Suemizu H. Simulation of human plasma concentration-time profiles of the partial glucokinase activator PF-04937319 and its disproportionate N-demethylated metabolite using humanized chimeric mice and semi-physiological pharmacokinetic modeling. Xenobiotica. 2017;47(5):382–93.
Tsumura Y, Tsushima Y, Tamura A, Hasebe M, Kanou M, Kato H Kobayashi T. TMG-123, a novel glucokinase activator, exerts durable effects on hyperglycemia without increasing triglyceride in diabetic animal models. PLoS One 2017;12(2):e0172252.
Zhu XX, Zhu DL, Li XY, Li YL, Jin XW, Hu TX, Zhao Y, Li YG, Zhao GY, Ren S, Zhang Y, Ding YH, Chen L. Dorzagliatin (HMS5552), a novel dual-acting glucokinase activator, improves glycaemic control and pancreatic β-cell function in patients with type 2 diabetes: a 28-day treatment study using biomarker-guided patient selection. Diabetes Obes Metab. 2018;20(9):2113–20.
Vella A, Freeman JL, Dunn I, Keller K, Buse JB, Valcarce C. Targeting hepatic glucokinase to treat diabetes with TTP399, a hepatoselective glucokinase activator. Sci Transl Med. 2019;11:475.
Bonadonna RC, Heise T, Arbet-Engels C, Kapitza C, Avogaro A, Grimsby J, Zhi J, Grippo JF, Balena R. Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J Clin Endocrinol Metab. 2010;95(11):5028–36.
Waring MJ, Clarke DS, Fenwick MD, Godfrey L, Groombridge SD, Johnstone J, McKerrecher D, Pike KG, Rayner JW, Robb GR, Wilson I. Property based optimisation of glucokinase activators – discovery of the phase IIb clinical candidate AZD1656. Med Chem Commun. 2012;3:1077–81.
Denney WS, Denham DS, Riggs MR, Amin NB. Glycemic effect and safety of a systemic, partial glucokinase activator, PF-04937319, in patients with type 2 diabetes mellitus inadequately controlled on metformin-a randomized, crossover, active-controlled study. Clin Pharmacol Drug Dev. 2016;5(6):517–27.
Meininger GE, Scott R, Alba M, Shentu Y, Luo E, Amin H, Davies MJ, Kaufman KD, Goldstein BJ. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34(12):2560–6.
Hinklin RJ, Baer BR, Boyd SA, Chicarelli MD, Condroski KR, DeWolf WE Jr, Fischer J, Frank M, Hingorani GP, Lee PA, Neitzel NA, Pratt SA, Singh A, Sullivan FX, Turner T, Voegtli WC, Wallace EM, Williams L, Aicher TD. Discovery and preclinical development of AR453588 as an anti-diabetic glucokinase activator. Bioorg Med Chem. 2020;28(1):115232.
Zhang X, Schneck K, Bue-Valleskey J, Yeo KP, Heathman M, Sinha V. Dose selection using a semi-mechanistic integrated glucose-insulin-glucagon model: designing phase 2 trials for a novel oral glucokinase activator. J Pharmacokinet Pharmacodyn. 2013;40(1):53–65.
Scheen AJ. New hope for glucokinase activators in type 2 diabetes? Lancet Diabetes Endocrinol. 2018;6(8):591–3.
Zheng S, Shao F, Ding Y, Fu Z, Fu Q, Ding S, Xie L, Chen J, Zhou S, Zhang H, Zhou H, Chen Y, Sun C, Zhu J, Zheng X, Yang T. Safety, pharmacokinetics, and pharmacodynamics of globalagliatin, a glucokinase activator, in Chinese patients with type 2 diabetes mellitus: a randomized, phase Ib, 28-day ascending dose study. Clin Drug Investig. 2020;40(12):1155–66.
Matschinsky F. GKAs for diabetes therapy: why no clinically useful drug after two decades of trying? Trends Pharmacol Sci. 2013;34(2):90–9.
Brouwers M, Jacobs C, Bast A, Stehouwer CDA, Schaper NC. Modulation of glucokinase regulatory protein: a double-edged sword? Trends Mol Med. 2015;21(10):583–94.
Tornovsky-Babeay S, Dadon D, Ziv O, Tzipilevich E, Kadosh T, Schyr-Ben Haroush R, Hija A, Stolovich-Rain M, Furth-Lavi J, Granot Z, Porat S, Philipson LH, Herold KC, Bhatti TR, Stanley C, Ashcroft FM, In't Veld P, Saada A, Magnuson MA, et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in beta cells. Cell Metab. 2014;19(1):109–21.
Gao Q, Zhang W, Li T, Yang G, Zhu W, Chen N, Jin H. The efficacy and safety of glucokinase activators for the treatment of type-2 diabetes mellitus: a protocol for systematic review and meta-analysis. Medicine. 2021;100(7):e24873.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, P., Singh, S., Sharma, N. et al. Targeting human Glucokinase for the treatment of type 2 diabetes: an overview of allosteric Glucokinase activators. J Diabetes Metab Disord 21, 1129–1137 (2022). https://doi.org/10.1007/s40200-022-01019-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01019-x